Effect of the Dendrimer Generation Used in the Synthesis of Pt-Ru Nanoparticles Supported on Carbon Nanofibers on the Catalytic Activity towards Methanol Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology and Physical Properties of the Synthesized Pt-Ru/CNF Catalysts
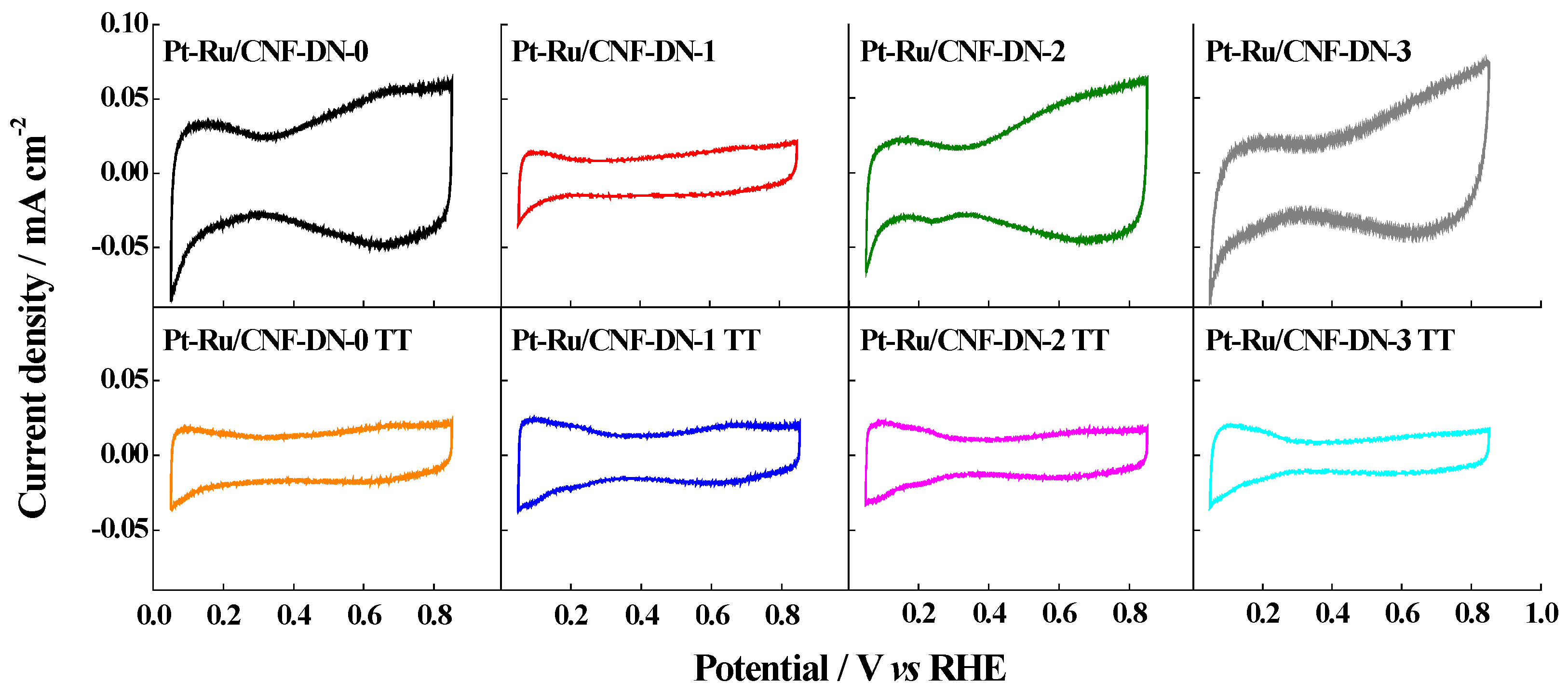
2.2. Activity of Catalysts in the Supporting Electrolyte
2.3. Catalytic Activity towards the Methanol Electrochemical Oxidation
3. Discussion
4. Materials and Methods
4.1. Preparation of Carbon Support
4.2. Pt-Ru/CNF Catalysts Synthesis
4.3. Physicochemical Characterization
4.4. Electrochemical Characterization
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tomalia, D.A.; Naylor, A.; Goddard, W.A. Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms to Macroscopic Matter. Angew. Chem. Int. Ed. Engl. 1967, 29, 138–175. [Google Scholar] [CrossRef]
- Fréchet, J.M.J. Functional Polymers and Dendrimers: Reactivity, Molecular Architecture and Interfacial Energy. Science 1994, 263, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Ardoin, N.; Astruc, D. The Molecular Trees: From Syntheses towards Applications. Bull. Soc. Chim. Fr. 1995, 132, 875–909. [Google Scholar]
- Bosman, A.W.; Janssen, H.M.; Meijer, E.W. About Dendrimers: Structure, Physical Properties and Applications. Chem. Rev. 1999, 99, 1665–1688. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Sun, L.; Crooks, R.M. Preparation of Cu Nanoclusters within Dendrimer Templates. J. Am. Chem. Soc. 1998, 120, 4877–4878. [Google Scholar] [CrossRef]
- Zhao, M.; Crooks, R.M. Homogeneous Hydrogenation Catalysis with Monodisperse, Dendrimer-Encapsulated Pd and Pt Nanoparticles. Angew. Chem. Int. Ed. 1999, 38, 364–366. [Google Scholar] [CrossRef]
- Chechik, V.; Zhao, M.; Crooks, R.M. Self-Assembled Inverted Micelles Prepared from a Dendrimer Template: Phase Transfer of Encapsulated Guests. J. Am. Chem. Soc. 1999, 121, 4910–4911. [Google Scholar] [CrossRef]
- Tokuhisa, H.; Zhao, M.; Baker, L.A. Preparation and Characterization of Dendrimer Monolayers and Dendrimer-Alkanethiolmixed Monolayers Adsorbed to Gold. J. Am. Chem. Soc. 1998, 120, 4492–4501. [Google Scholar] [CrossRef]
- Jansen, J.F.G.A.; de Brabander-van den Berg, E.M.M.; Meijer, E.W. Encapsulation of Guest Molecules into a Dendritic Box. Science 1994, 266, 1226–1229. [Google Scholar] [CrossRef] [PubMed]
- Crooks, R.M.; Zhao, M.; Sun, L.; Chechik, V.; Yeung, L.K. Dendrimer-Encapsulated Metal Nanoparticles: Synthesis, Characterization and Applications to Catalysis. Acc. Chem. Res. 2001, 34, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.; May, R.A.; Iversen, B.L.; Chandler, B.D. Dendrimer-Encapsulated Nanoparticle Precursors to Supported Platinum Catalysts. J. Am. Chem. Soc. 2003, 125, 14832–14836. [Google Scholar] [CrossRef] [PubMed]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters, 1st ed.; Springer: Berlín, Germany, 1995; pp. 203–274. [Google Scholar]
- Curtis, A.C.; Duff, D.G.; Edwards, P.P.; Jefferson, D.A.; Johnson, B.F.G.; Kirkland, A.I.; Wallace, A.S. A Morphology-Selective Copper Organosol. Angew. Chem. Int. Ed. 1988, 27, 1530–1533. [Google Scholar] [CrossRef]
- Lisiecki, I.; Pileni, M.P. Synthesis of Copper Metallic Clusters Using Reverse Micelles as Microreactors. J. Am. Chem. Soc. 1993, 115, 3887–3896. [Google Scholar] [CrossRef]
- Singh, A.; Chandler, B.D. Low-Temperature Activation Conditions for PAMAM Dendrimer Templated Pt Nanoparticles. Langmuir 2005, 21, 10776–10782. [Google Scholar] [CrossRef] [PubMed]
- Beakley, L.W.; Yost, S.E.; Cheng, R.; Chandler, B.D. Nanocomposite Catalysts: Dendrimer Encapsulated Nanoparticles Immobilized in Sol-Gel Silica. Appl. Catal. 2005, 292, 124–129. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Wang, X.; Zhao, B.; Zhang, J.; Li, C. Preparation of Hollow Capsule-Stabilized Gold Nanoparticles through the Encapsulation of the Dendrimer. J. Colloid Interface Sci. 2006, 302, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Crespilho, F.N.; Huguenin, F.; Zucolotto, V.; Olivi, P.; Nart, F.C.; Oliveira, O.N., Jr. Dendrimers as Nanoreactors to Produce Platinum Nanoparticles Embedded in Layer by Layer Films for Methanol-Tolerant Cathodes. Electrochem. Commun. 2006, 8, 348–352. [Google Scholar] [CrossRef]
- Scott, R.W.J.; Datye, A.K.; Crooks, R.M. Bimetallic Palladium-Platinum Dendrimer Encapsulated Catalysts. J. Am. Chem. Soc. 2003, 125, 3708–3709. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.M.; Rhee, H.K. Synthesis and Catalytic Applications of Dendrimer-Templated Bimetallic Nanoparticles. Catal. Surv. Asia 2004, 8, 211–223. [Google Scholar] [CrossRef]
- Niu, Y.; Yeung, L.K.; Crooks, R.M. Size-Selective Hydrogenation of Olefins by Dendrimer-Encapsulated Palladium Nanoparticles. J. Am. Chem. Soc. 2001, 123, 6840–6846. [Google Scholar] [CrossRef]
- García-Martinez, J.C.; Crooks, R.M. Extraction of Au Nanoparticles Having Narrow Size Distributions from within Dendrimer Templates. J. Am. Chem. Soc. 2004, 126, 16170–16178. [Google Scholar] [CrossRef] [PubMed]
- Lemo, J.; Heuzé, K.; Astruc, D. Synthesis and Catalytic Activity of DAB-Dendrimer Encapsulated Pd Nanoparticles for the Suzuki Coupling Reaction. Inorg. Chim. Acta 2006, 359, 4909–4911. [Google Scholar] [CrossRef]
- Peng, X.; Pan, Q.; Rempel, G.L.; Wu, S. Synthesis, Characterization and Application of PdPt and PdRh Bimetallic Nanoparticles Encapsulated within Amine-Terminated Poly(amidoamine) Dendrimers. Catal. Commun. 2009, 11, 62–66. [Google Scholar] [CrossRef]
- Crooks, R.M.; Zhao, M. Dendrimer-Encapsulated Pt Nanoparticles: Synthesis, Characterization and Applications to Catalysis. Adv. Mater. 1999, 11, 217–220. [Google Scholar] [CrossRef]
- Zhang, W.; Li, L.; Du, Y.; Wang, X.; Yang, P. Gold/Platinum Bimetallic Core/Shell Nanoparticles Stabilized by a Frechet Type Dendrimer: Preparation and Catalytic Hydrogenations of Phenylaldehydes and Nitrobenzenes. Catal. Lett. 2009, 127, 429–436. [Google Scholar] [CrossRef]
- Niu, Y.; Sun, L.; Crooks, R.M. Determination of the Intrinsic Proton Binding Constants for Poly(amidoamine) Dendrimers via Potentiometric pH Titration. Macromolecules 2003, 36, 5725–5731. [Google Scholar] [CrossRef]
- Ye, H.; Scott, R.W.J.; Crooks, R.M. Synthesis, Characterization and Surface Immobilization of Platinum and Palladium Nanoparticles Encapsulated within Amine-Terminated Poly(amidoamine) Dendrimers. Langmuir 1999, 20, 2915–2920. [Google Scholar] [CrossRef]
- Calderón, J.C.; García, G.; Calvillo, L.; Rodríguez, J.L.; Lázaro, M.J.; Pastor, E. Electrochemical Oxidation of CO and Methanol on Pt-Ru Catalysts Supported on Carbon Nanofibers: The Influence of Synthesis Method. Appl. Catal. B Environ. 2015, 165, 676–686. [Google Scholar]
- Watanabe, M.; Motoo, S. Electrocatalysis by Ad-Atoms: Part II. Enhancement of the Oxidation of Methanol on Platinum by Ruthenium Ad-Atoms. J. Electroanal. Chem. 1975, 60, 267–273. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Markovic, N.M.; Ross, P.N., Jr. H2 and CO Electrooxidation on Well Characterized Pt, Ru, and Pt-Ru. 1. Rotating Disk Electrode Studies of the Pure Gases Including Temperature Effects. J. Phys. Chem. 1995, 99, 8290–8301. [Google Scholar] [CrossRef]
- Harada, M.; Einaga, H. Preparation of Pt/Rh Bimetallic Colloidal Particles in Polymer Solutions Using Borohydride-Reduction. J. Colloid Interface Sci. 2007, 308, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Nagao, D.; Shimazaki, Y.; Saeki, S.; Kobayashi, Y.; Konno, M. Effect of Ultrasonic Irradiation on Carbon-Supported Pt-Ru Nanoparticles Prepared at High Metal Concentrations. Colloid Surf. 2007, 302, 623–627. [Google Scholar] [CrossRef]
- Gonzalez, E.R.; Ticianelli, E.A.; Pinheiro, A.L.N.; Perez, J. Processo de obtenção de catalisador de platina dispersa ancorada em substrato através da redução por ácido. Brazilian Patent INPI-SP No. 00321, 2 September 1997. [Google Scholar]
- Lizcano-Valbuena, W.H.; Paganin, V.A.; González, E.R. Methanol Electro-Oxidation on Gas Diffusion Electrodes Prepared with Pt-Ru/C Catalysts. Electrochim. Acta 2002, 47, 3715–3722. [Google Scholar] [CrossRef]
- Salgado, J.R.C.; Paganin, V.A.; González, E.R.; Montemor, M.F.; Tacchini, I.; Ansón, A.; Salvador, M.A.; Ferreira, P.; Figueiredo, F.M.L.; Ferreira, M.G.S. Characterization and Performance Evaluation of Pt-Ru Electrocatalysts Supported on Different Carbon Materials for Direct Methanol Fuel Cells. Int. J. Hydrog. Energy 2013, 38, 910–920. [Google Scholar] [CrossRef]
- Dos Santos, L.; Colmati, F.; González, E.R. Preparation and Characterization of Supported Pt-Ru Catalysts with a High Ru Content. J. Power Sources 2006, 159, 869–877. [Google Scholar] [CrossRef]
- Wang, X.; Hsing, I.M. Surfactant Stabilized Pt and Pt Alloy Electrocatalyst for Polymer Electrolyte Fuel Cells. Electrochim. Acta 2002, 47, 2981–2987. [Google Scholar] [CrossRef]
- Yan, S.; Sun, G.; Tian, J.; Jiang, L.; Qi, J.; Xin, Q. Polyol Synthesis of Highly Active PtRu/C Catalyst with High Metal Loading. Electrochim. Acta 2006, 52, 1692–1696. [Google Scholar] [CrossRef]
- Liu, Z.; Hong, L. Electrochemical Characterization of the Electrooxidation of Methanol, Ethanol and Formic Acid on Pt/C and PtRu/C Electrodes. J. Appl. Electrochem. 2007, 37, 505–510. [Google Scholar] [CrossRef]
- Kanninen, P.; Borghei, M.; Ruiz, V.; Kauppinen, E.I.; Kallio, T.; Yi, J. The Effect of Nafion Content in a Graphitized Carbon Nanofiber-Based Anode for the Direct Methanol Fuel Cell. Int. J. Hydrog. Energy 2012, 37, 19082–19091. [Google Scholar] [CrossRef]
- Sebastián, D.; Suelves, I.; Pastor, E.; Moliner, R.; Lázaro, M.J. The Effect of Carbon Nanofiber Properties as Support for Pt-Ru Nanoparticles on the Electrooxidation of Alcohols. Appl. Catal. B Environ. 2013, 132, 13–21. [Google Scholar] [CrossRef]
- Alegre, C.; Gálvez, M.E.; Moliner, R.; Baglio, V.; Stassi, A.; Aricò, A.S.; Lázaro, M.J. Platinum Ruthenium Catalysts Supported on Carbon Xerogel for Methanol Electro-Oxidation: Influence of the Catalyst Synthesis Method. ChemCatChem 2013, 5, 3770–3780. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, G.; Hu, X.F.; Chen, D.A.; Hansen, T.; Zur Loye, H.C.; Ploehn, H.J. PAMAM-Stabilized Pt-Ru Nanoparticles for Methanol Electro-Oxidation. J. Power Sources 2010, 195, 425–434. [Google Scholar] [CrossRef]
- Calderón, J.C.; Calvillo, L.; Lázaro, M.J.; Pastor, E. Use of Dendrimers during the Synthesis of Pt-Ru Electrocatalysts for PEM Fuel Cells: Effects on the Physical and Electrochemical Properties. Int. J. Electrochem. 2011. [Google Scholar] [CrossRef]
- Calderón, J.C.; Calvillo, L.; Lázaro, M.J.; Rodríguez, J.L.; Pastor, E. CO Electrochemical Oxidation on Dendrimers-Synthesized Pt-Ru Catalysts Supported on Carbon Nanofibers: A Kinetic Study. Ciênc. Tecnol. Mater. 2012, 24, 176–179. [Google Scholar]
- McBreen, J.; Olender, H.; Srinivasan, S.; Kordesch, K. Carbon Supports for Phosphoric Acid Fuel Cell Electrocatalysts: Alternative Materials and Method of Evaluations. J. Appl. Electrochem. 1981, 11, 787–796. [Google Scholar] [CrossRef]
- Uchida, M.; Aoyama, Y.; Tanabe, M.; Yanagihara, N.; Eda, N.; Ohta, A. Influences of both Carbon Supports and Heat-Treatment of Supported Catalyst on Electrochemical Oxidation of Methanol. J. Electrochem. Soc. 1995, 142, 2572–2576. [Google Scholar] [CrossRef]
- Pantea, D.; Darmstadt, H.; Kaliaguine, S.; Sümmchen, L.; Roy, C. Electrical Conductivity of Thermal Carbon Blacks: Influence of Surface Chemistry. Carbon 2001, 39, 1147–1158. [Google Scholar] [CrossRef]
- Jun, S.; Joo, S.H.; Ryoo, R.; Kruk, M.; Jaroniec, M.; Liu, Z.; Ohsuna, T.; Terasaki, O. Synthesis of New Nanoporous Carbon with Hexagonally Ordered Mesostructure. J. Am. Chem. Soc. 2000, 122, 10712–10713. [Google Scholar] [CrossRef]
- Chang, H.; Joo, S.H.; Pak, C. Synthesis and Characterization of Mesoporous Carbons for Fuel Cells Applications. J. Mater. Chem. 2007, 17, 3078–3088. [Google Scholar] [CrossRef]
- Calvillo, L.; Lázaro, M.J.; Bordejé, E.G.; Moliner, R.; Cabot, P.L.; Esparbé, I.; Pastor, E.; Quintana, J.J. Platinum Supported on Functionalized Ordered Mesoporous Carbon as Electrocatalyst for Direct Methanol Fuel Cells. J. Power Sources 2007, 169, 59–64. [Google Scholar] [CrossRef]
- Alegre, C.; Gálvez, M.E.; Baquedano, E.; Moliner, R.; Pastor, E.; Lázaro, M.J. Oxygen-Functionalized Highly Mesoporous Carbon Xerogel Based Catalysts for Direct Methanol Fuel Cell Anodes. J. Phys. Chem. C 2013, 117, 13045–13058. [Google Scholar] [CrossRef]
- Park, I.S.; Park, K.W.; Choi, J.H.; Park, C.R.; Sung, Y.E. Electrocatalytic Enhancement of Methanol Oxidation by Graphite Nanofibers with a High Loading of PtRu Alloy Nanoparticles. Carbon 2007, 45, 28–33. [Google Scholar] [CrossRef]
- Cohen, J.L.; Volpe, D.J.; Abruña, H.D. Electrochemical Determination of Activation Energies for Methanol Oxidation on Polycrystalline Platinum in Acidic and Alkaline Electrolytes. Phys. Chem. Chem. Phys. 2007, 9, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Drazic, D.M.; Drazic, V. Nature of the Rest Potential of Platinum Electrodes in Alkaline Alcohol Solutions. Electrochim. Acta 1966, 11, 1235–1241. [Google Scholar] [CrossRef]
- Chu, D.; Gilman, S. Methanol Electro-Oxidation on Unsupported Pt-Ru Alloys at Different Temperatures. J. Electrochem. Soc. 1996, 143, 1685–1690. [Google Scholar] [CrossRef]
- Tremiliosi-Filho, G.; Kim, H.; Chrzanowski, W.; Wieckowski, A.; Grzybowska, B.; Kulesza, P. Reactivity and Activation Parameters in Methanol Oxidation on Platinum Single Crystal Electrodes ‘Decorated’ by Ruthenium Adlayers. J. Electroanal. Chem. 1999, 467, 143–156. [Google Scholar] [CrossRef]
- Madden, T.H.; Stuve, E.M. Mechanisms of Elevated Temperature Methanol Electro-Oxidation and Poisoning on Pt/C-Nafion Catalyst Layers. J. Electrochem. Soc. 2003, 150, E571–E577. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Ross, P.N., Jr.; Cairns, E.J. LEIS and AES on Sputtered and Annealed Polycrystalline Pt-Ru Bulk Alloys. Surf. Sci. 1993, 293, 67–80. [Google Scholar] [CrossRef]
- Radmilovic, V.; Gasteiger, H.A.; Ross, P.N. Structure and Chemical Composition of a Supported Pt-Ru Electrocatalyst for Methanol Oxidation. J. Catal. 1995, 154, 98–106. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Grant, N.M. X-ray Diffraction: A Practical Approach, 1st ed.; Plenum Press: New York, NY, USA, 1998. [Google Scholar]
- Jiqiao, L.; Baiyun, H. Particle Size Characterization of Ultrafine Tungsten Powder. Int. J. Refract. Met. Hard Mater. 2001, 19, 89–99. [Google Scholar] [CrossRef]
- Carmo, M.; dos Santos, A.R.; Poco, J.G.R.; Linardi, M. Physical and Electrochemical Evaluation of Commercial Carbon Black as Electrocatalysts Supports for DMFC Applications. J. Power Sources 2007, 173, 860–866. [Google Scholar] [CrossRef]
- Bigall, N.C.; Eychmüller, A. Synthesis of Noble Metal Nanoparticles and their Non-Ordered Superstructures. Philos. Trans. R. Soc. A 2010, 368, 1385–1404. [Google Scholar] [CrossRef] [PubMed]
- Tripkovic, A.V.; Strbac, S.; Popovi, K.D. Effect of Temperature on the Methanol Oxidation at Supported Pt and PtRu Catalysts in Alkaline Solution. Electrochem. Commun. 2003, 5, 484–490. [Google Scholar] [CrossRef]
- Guillén-Villafuerte, O.; García, G.; Guil-López, R.; Nieto, E.; Rodríguez, J.L.; Fierro, J.L.G.; Pastor, E. Carbon Monoxide and Methanol Oxidations on Pt/X@MoO3/C (X = Mo2C, MoO2, Mo0) Electrodes at Different Temperatures. J. Power Sources 2013, 231, 163–172. [Google Scholar] [CrossRef]
- Poelsema, B.; Verheij, L.K.; Comsa, G. He-Scattering Investigation of CO Migration on Pt (111). Phys. Rev. Lett. 1982, 49, 1731–1735. [Google Scholar] [CrossRef]
- Reutt-Robey, J.E.; Doren, D.J.; Chabal, Y.J.; Christman, S.B. CO Diffusion on Pt (111) with Time-Resolved Infrared-Pulsed Molecular Beam Methods: Critical Tests and Analysis. J. Chem. Phys. 1990, 93, 9113–9129. [Google Scholar] [CrossRef]
- Croci, M.; Félix, C.; Vandoni, G.; Harbich, W.; Monot, R. Measurement of Macroscopic Diffusion of CO on Pt (111) by Thermal Helium Scattering. Surf. Sci. Lett. 1993, 290, L667–L672. [Google Scholar]
- Calderón, J.C.; García, G.; Querejeta, A.; Alcaide, F.; Calvillo, L.; Lázaro, M.J.; Rodríguez, J.L.; Pastor, E. Carbon Monoxide and Methanol Oxidations on Carbon Nanofibers Supported Pt–Ru Electrodes at Different Temperatures. Electrochim. Acta 2015, 186, 359–368. [Google Scholar]
- Sebastián, D.; Lázaro, M.J.; Moliner, R.; Suelves, I.; Aricò, A.S.; Baglio, V. Oxidized Carbon Nanofibers Supporting PtRu Nanoparticles for Direct Methanol Fuel Cells. Int. J. Hydrog. Energy 2014, 39, 5414–5423. [Google Scholar] [CrossRef]
- Velázquez-Palenzuela, A.; Centellas, F.; Garrido, J.A.; Arias, C.; Rodríguez, R.M.; Brillas, E.; Cabot, P.L. Kinetic Analysis of Carbon Monoxide and Methanol Oxidation on High Performance Carbon-Supported Pt-Ru Electrocatalyst for Direct Methanol Fuel Cells. J. Power Sources 2011, 196, 3503–3512. [Google Scholar] [CrossRef]
- Suelves, I.; Lázaro, M.J.; Moliner, R.; Corbella, B.M.; Palacios, J.M. Hydrogen Production by Thermo Catalytic Decomposition of Methane on Ni-Based Catalysts: Influence of Operating Conditions on Catalyst Deactivation and Carbon Characteristics. Int. J. Hydrog. Energy 2005, 30, 1555–1567. [Google Scholar] [CrossRef]
- Suelves, I.; Lázaro, M.J.; Moliner, R.; Echegoyen, Y.; Palacios, J.M. Characterization of NiAl and NiCuAl Catalysts Prepared by Different Methods for Hydrogen Production by Thermo Catalytic Decomposition of Methane. Catal. Today 2006, 116, 271–280. [Google Scholar] [CrossRef]
- Calvillo, L.; Lázaro, M.J.; Suelves, I.; Echegoyen, Y.; Bordejé, E.G.; Moliner, R. Study of the Surface Chemistry of Modified Carbon Nanofibers by Oxidation Treatments in Liquid Phase. J. Nanosci. Nanotechnol. 2009, 9, 4164–4169. [Google Scholar] [CrossRef] [PubMed]
- Trassatti, S.; Petrii, O.A. Real Surface Area Measurements in Electrochemistry. Pure Appl. Chem. 1991, 63, 711–734. [Google Scholar] [CrossRef]

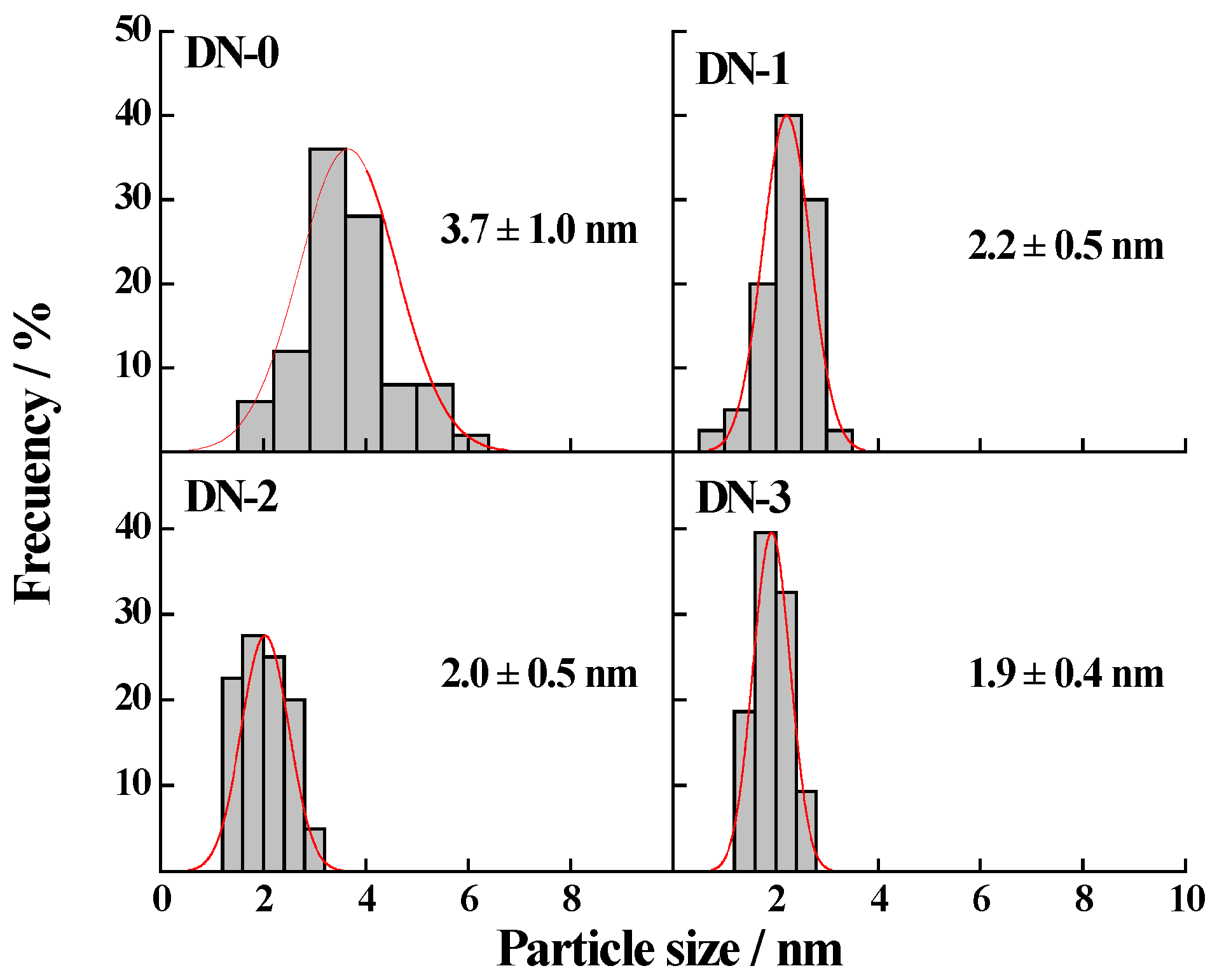
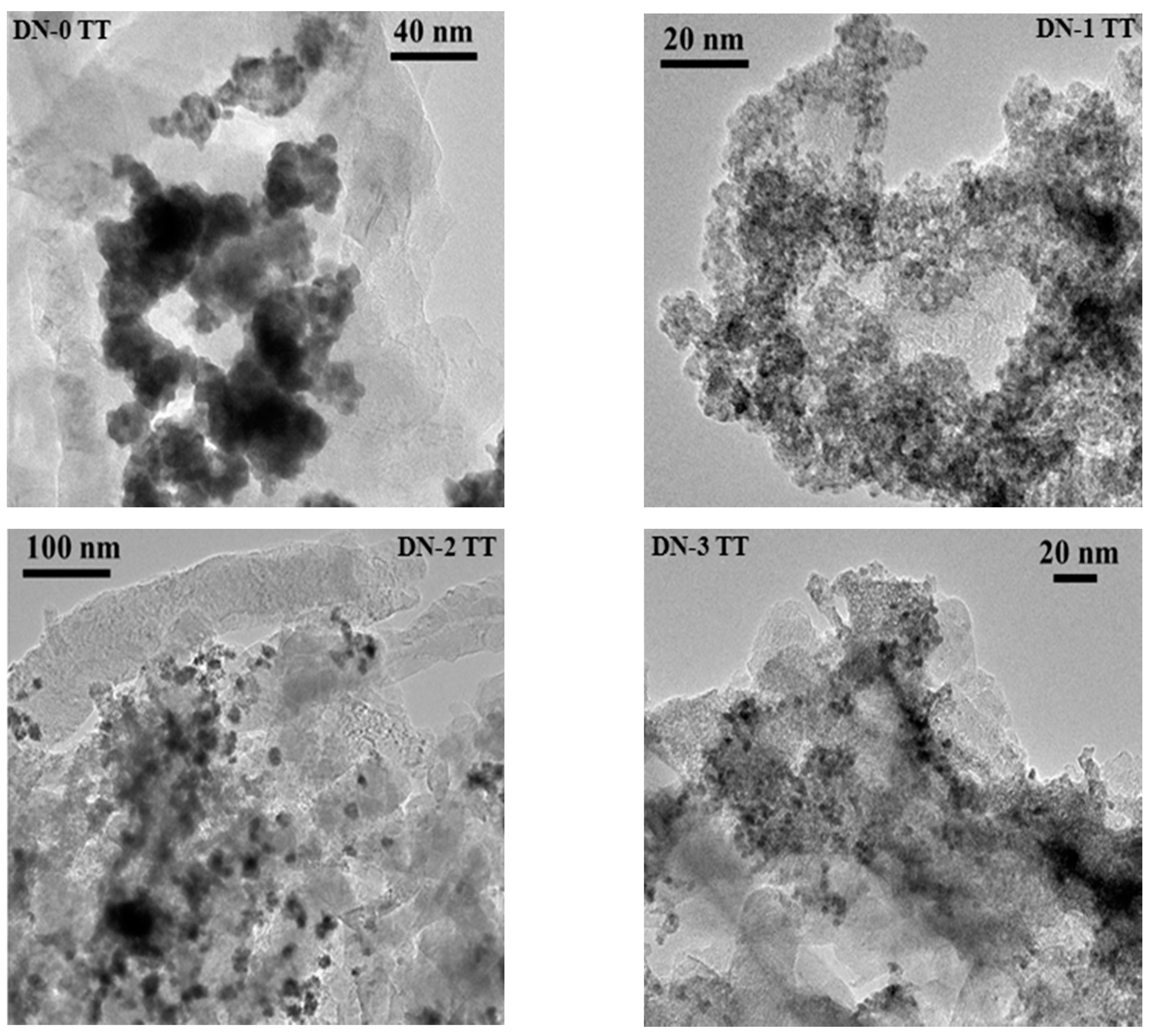
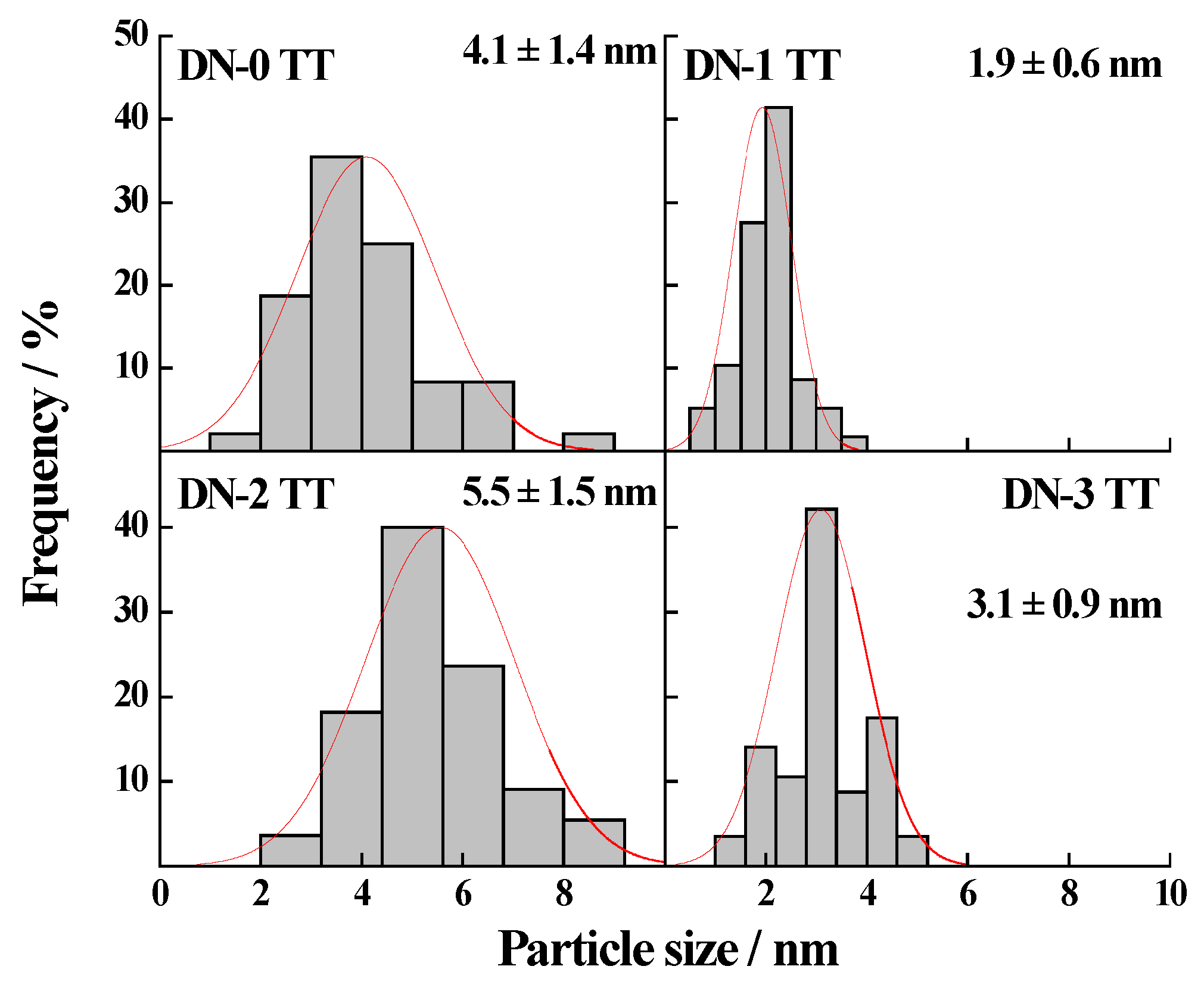

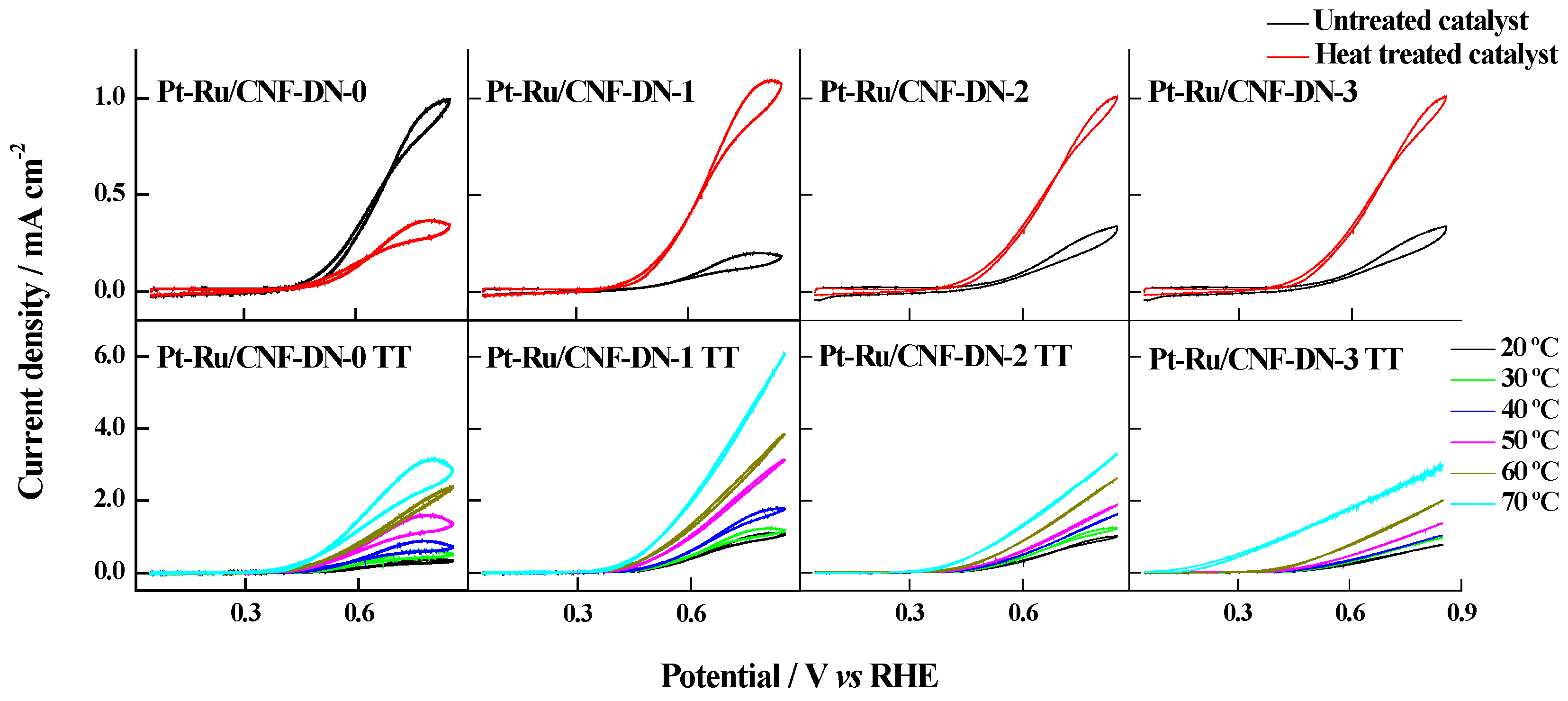

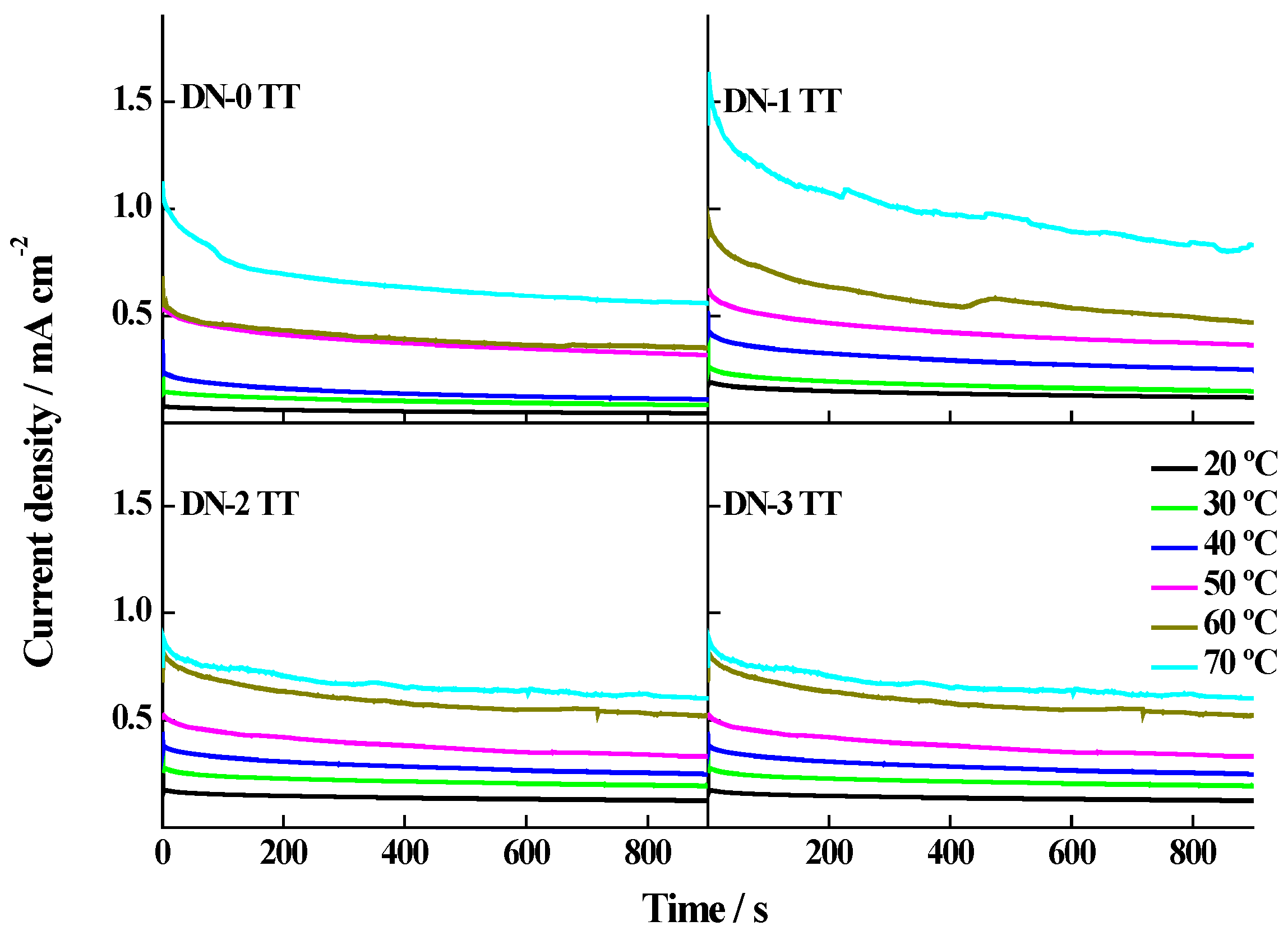
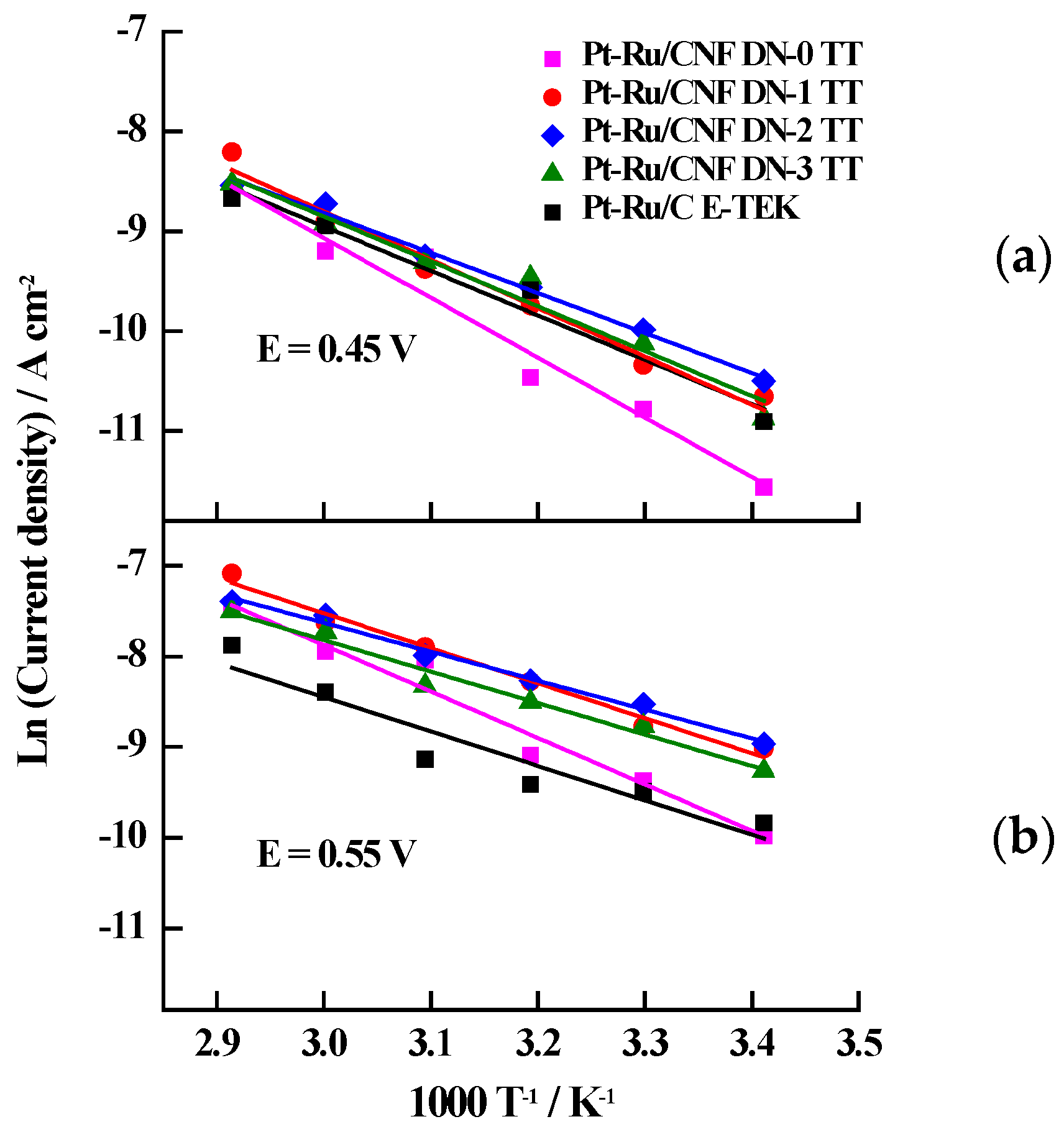
| Catalyst | Metal Loading (wt. %) | Atomic Ratio Pt:Ru | Particle Size/nm XRD TEM | Metal Surface Area/m2·g−1 | ECSA/m2·g−1Metal | |
|---|---|---|---|---|---|---|
| Pt-Ru/CNF-DN-0 | 12 | 58:42 | 3.3 | 3.7 ± 1.0 | 103 | 28 |
| Pt-Ru/CNF-DN-0 TT | 18 | 49:51 | 4.1 | 4.1 ± 1.4 | 87 | 21 |
| Pt-Ru/CNF-DN-1 | 20 | 59:41 | 3.8 | 2.2 ± 0.5 | 89 | 32 |
| Pt-Ru/CNF-DN-1 TT | 17 | 52:48 | 3.6 a | 1.9 ± 0.6 | 98 | 19 |
| Pt-Ru/CNF-DN-2 | 20 | 44:56 | 3.0 a | 2.0 ± 0.5 | 123 | 13 |
| Pt-Ru/CNF-DN-2 TT | 19 | 40:60 | 3.8 a | 5.5 ± 1.5 | 99 | 28 |
| Pt-Ru/CNF-DN-3 | 19 | 47:53 | 3.0 | 1.9 ± 0.4 | 121 | 7 |
| Pt-Ru/CNF-DN-3 TT | 21 | 41:59 | 2.9 a | 3.1 ± 0.9 | 129 | 41 |
| Pt-Ru/C E-TEK | 20 | 45:55 | 4.4 | 4.5 b | 76 | 42 |
| Catalyst | Maximum Current Densities/mA·cm−2 | |||||
|---|---|---|---|---|---|---|
| 20 °C | 30 °C | 40 °C | 50 °C | 60 °C | 70 °C | |
| Pt-Ru/CNF-DN-0 TT | 0.39 | 0.65 | 0.90 | 1.63 | 2.41 | 3.19 |
| Pt-Ru/CNF-DN-1 TT | 1.15 | 1.25 | 1.83 | 3.19 | 3.92 | 6.16 |
| Pt-Ru/CNF-DN-2 TT | 1.04 | 1.29 | 1.64 | 1.92 | 2.67 | 3.35 |
| Pt-Ru/CNF-DN-3 TT | 0.81 | 1.01 | 1.09 | 1.39 | 2.02 | 2.03 |
| Pt-Ru/C E-TEK | 0.29 | 0.43 | 0.40 | 0.48 | 1.08 | 1.73 |
| Catalyst | Stationary Current Densities/mA·cm−2 | Eap/kJ·mol−1 | |||||
|---|---|---|---|---|---|---|---|
| 20 °C | 30 °C | 40 °C | 50 °C | 60 °C | 70 °C | ||
| Pt-Ru/CNF-DN-0 TT | 0.01 | 0.02 | 0.03 | 0.10 | 0.10 | 0.18 | 49.8 |
| Pt-Ru/CNF-DN-1 TT | 0.02 | 0.03 | 0.06 | 0.09 | 0.14 | 0.27 | 40.2 |
| Pt-Ru/CNF-DN-2 TT | 0.03 | 0.05 | 0.07 | 0.10 | 0.16 | 0.20 | 33.4 |
| Pt-Ru/CNF-DN-3 TT | 0.02 | 0.04 | 0.08 | 0.09 | 0.13 | 0.20 | 37.4 |
| Pt-Ru/C E-TEK | 0.02 | ----- | 0.07 | ----- | 0.13 | 0.17 | 38.6 |
| Catalyst | Stationary Current Densities/mA·cm−2 | Eap/kJ·mol−1 | |||||
|---|---|---|---|---|---|---|---|
| 20 °C | 30 °C | 40 °C | 50 °C | 60 °C | 70 °C | ||
| Pt-Ru/CNF-DN-0 TT | 0.05 | 0.09 | 0.11 | 0.33 | 0.36 | 0.56 | 42.6 |
| Pt-Ru/CNF-DN-1 TT | 0.13 | 0.16 | 0.26 | 0.37 | 0.50 | 0.85 | 32.1 |
| Pt-Ru/CNF-DN-2 TT | 0.13 | 0.20 | 0.26 | 0.34 | 0.53 | 0.62 | 26.5 |
| Pt-Ru/CNF-DN-3 TT | 0.09 | 0.15 | 0.23 | 0.23 | 0.43 | 0.55 | 28.9 |
| Pt-Ru/C E-TEK | 0.05 | 0.08 | 0.09 | 0.11 | 0.22 | 0.41 | 31.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón, J.C.; Calvillo, L.; Lázaro, M.J.; Rodríguez, J.L.; Pastor, E. Effect of the Dendrimer Generation Used in the Synthesis of Pt-Ru Nanoparticles Supported on Carbon Nanofibers on the Catalytic Activity towards Methanol Oxidation. Energies 2017, 10, 159. https://doi.org/10.3390/en10020159
Calderón JC, Calvillo L, Lázaro MJ, Rodríguez JL, Pastor E. Effect of the Dendrimer Generation Used in the Synthesis of Pt-Ru Nanoparticles Supported on Carbon Nanofibers on the Catalytic Activity towards Methanol Oxidation. Energies. 2017; 10(2):159. https://doi.org/10.3390/en10020159
Chicago/Turabian StyleCalderón, Juan Carlos, Laura Calvillo, María Jesús Lázaro, José Luis Rodríguez, and Elena Pastor. 2017. "Effect of the Dendrimer Generation Used in the Synthesis of Pt-Ru Nanoparticles Supported on Carbon Nanofibers on the Catalytic Activity towards Methanol Oxidation" Energies 10, no. 2: 159. https://doi.org/10.3390/en10020159







