Using a Reactive Transport Simulator to Simulate CH4 Production from Bear Island Basin in the Barents Sea Utilizing the Depressurization Method†
Abstract
:1. Introduction
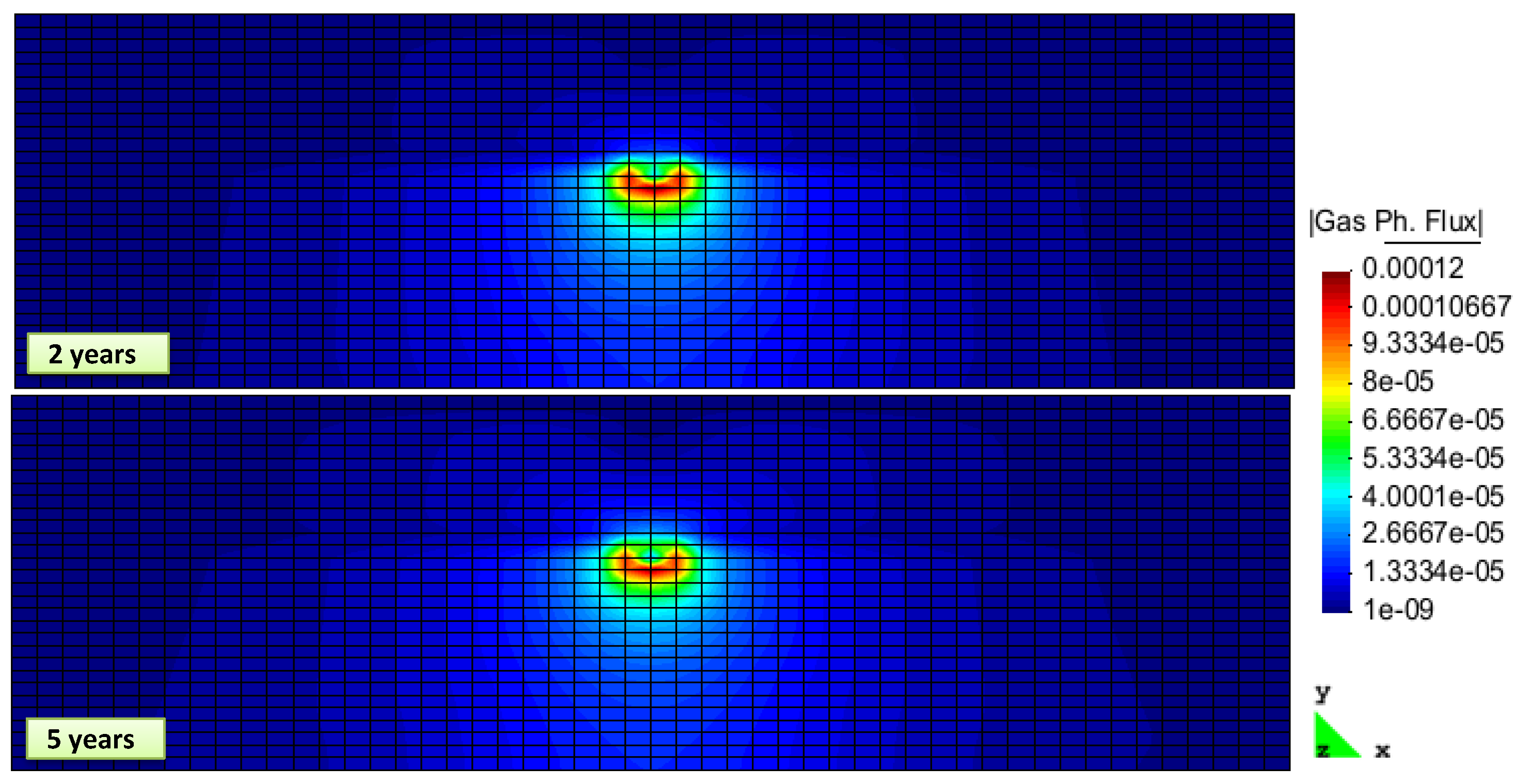
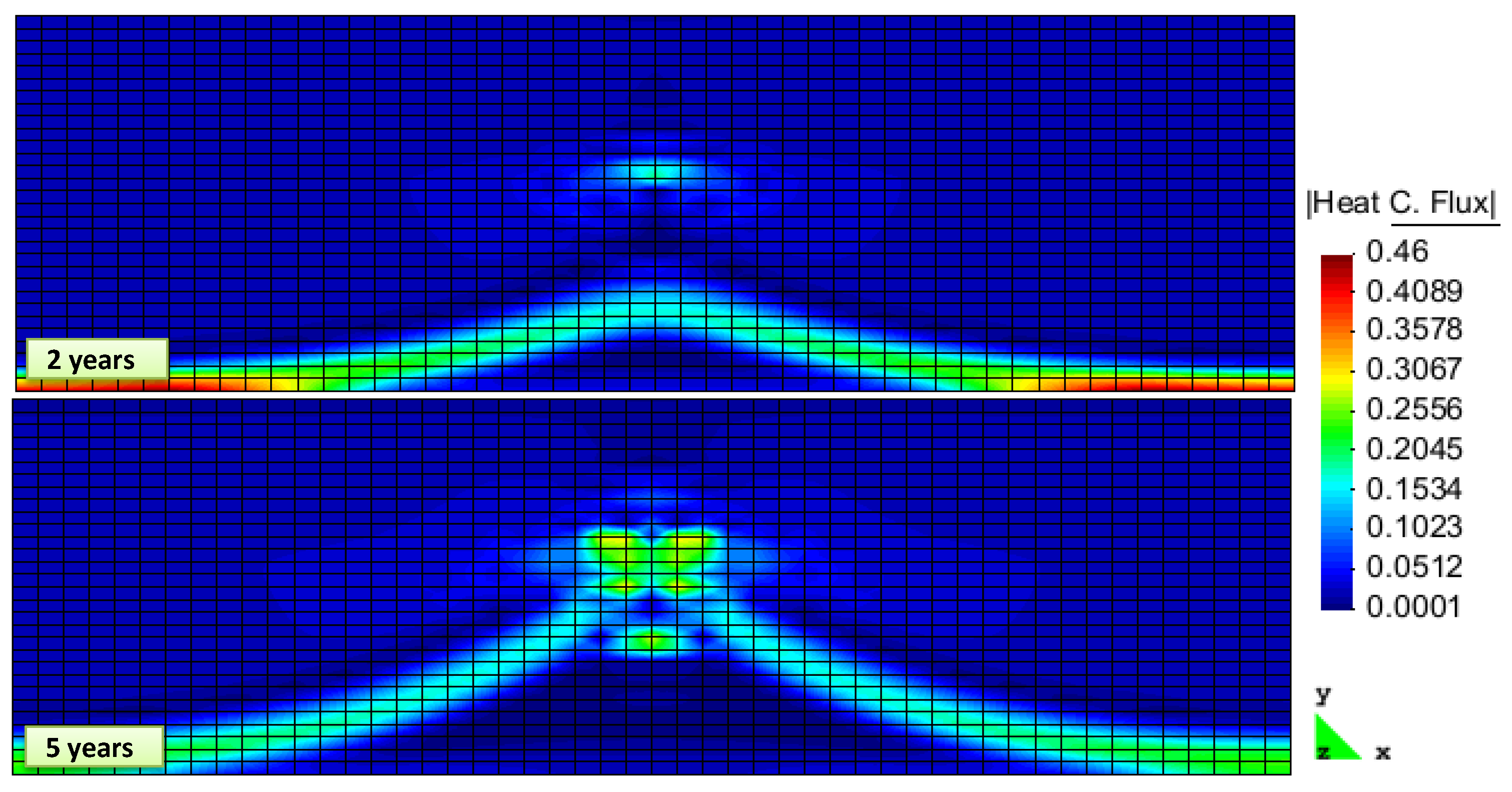
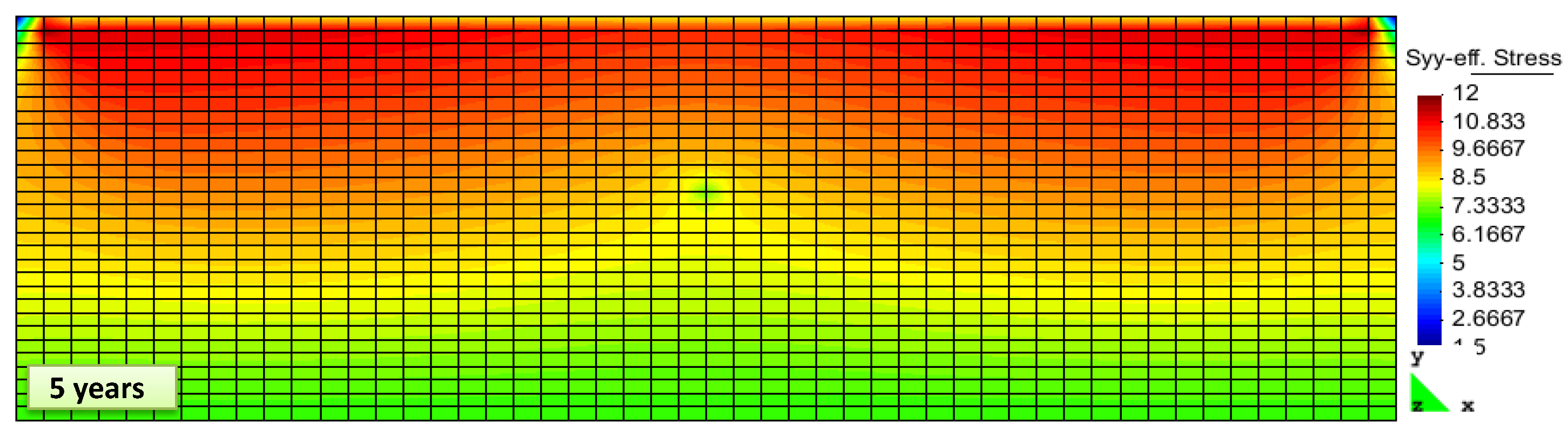
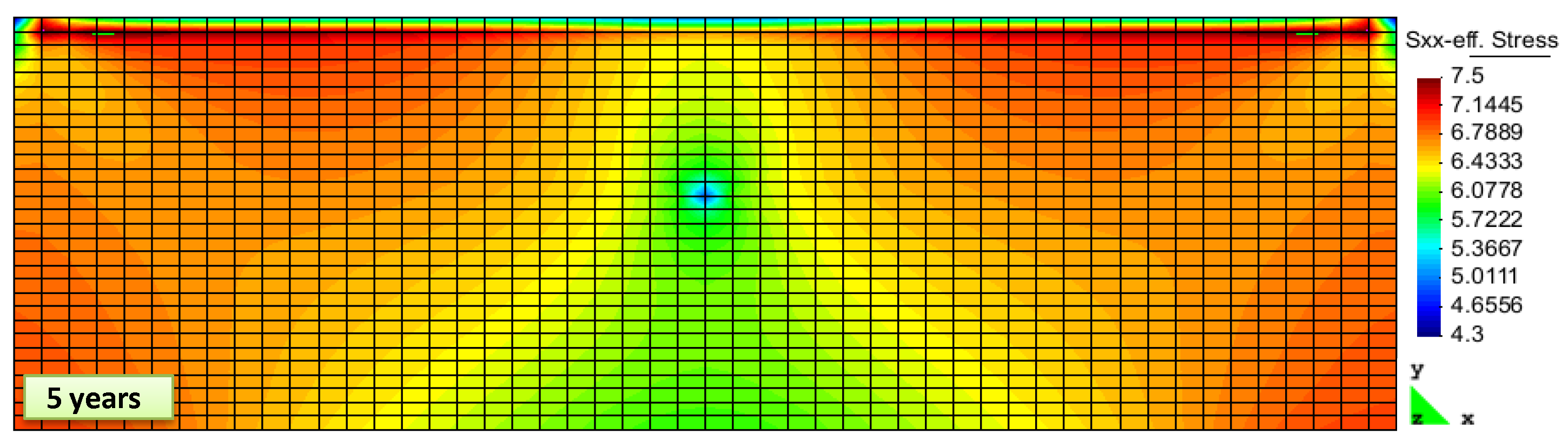
2. Results and Discussion
Simulation Setup
3. Methods and Procedures
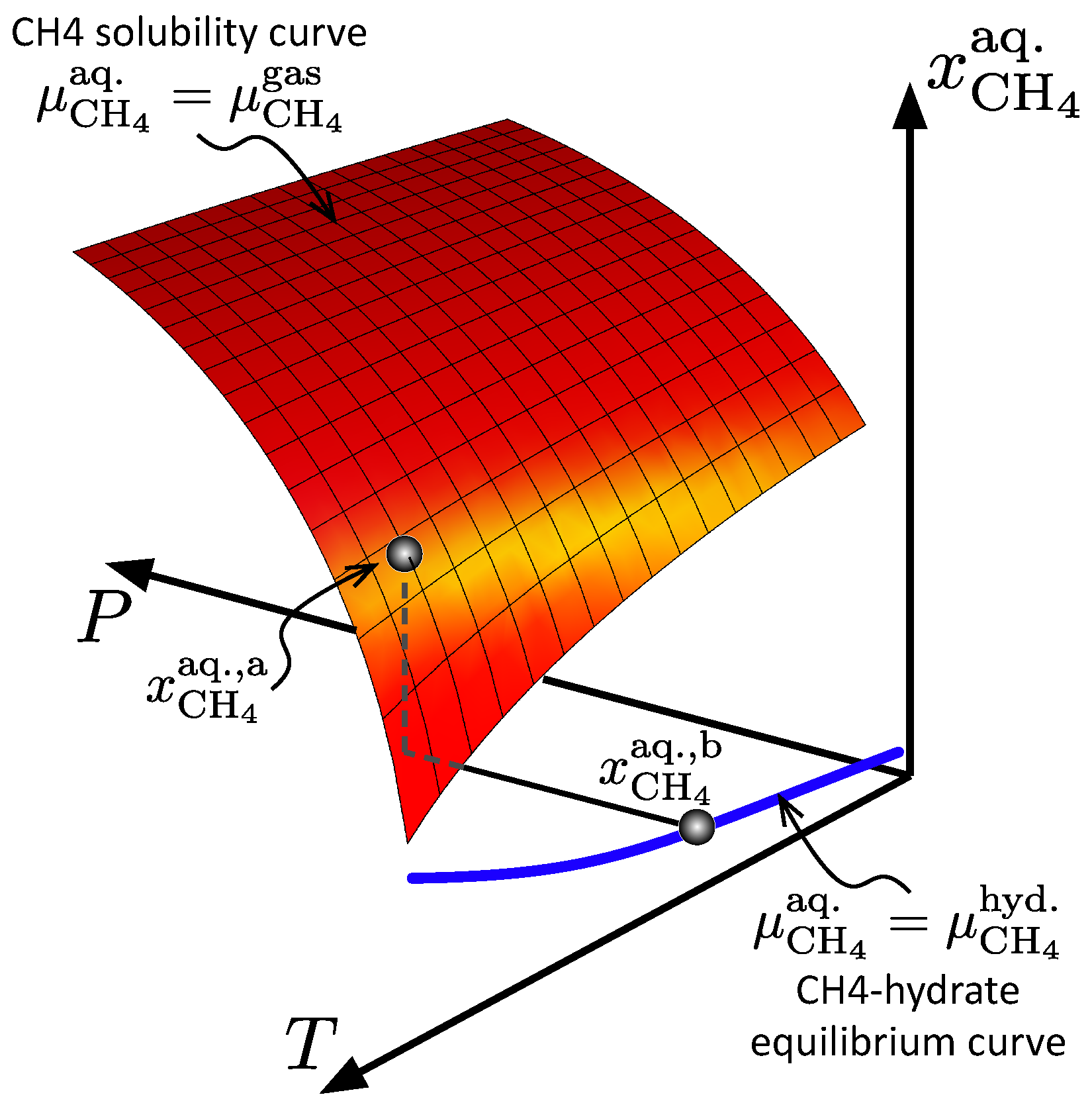
Theory
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| D (m/s) | Diffusion coefficient |
| P (MPa) | Pressure |
| R (mol/(ms)) | Rate |
| T (K) | Temperature |
| (1) | Mole-fraction |
| z (nm) | Distance |
| H | Hydrate phase |
| β (mol/kJ) | Inverse temperature 1/ |
| (mol/m) | Molar density |
| μ (kJ/mol) | Chemical potential |
| G (kJ/mol) | Gibbs free energy |
Appendix A. RCB Simulator Package
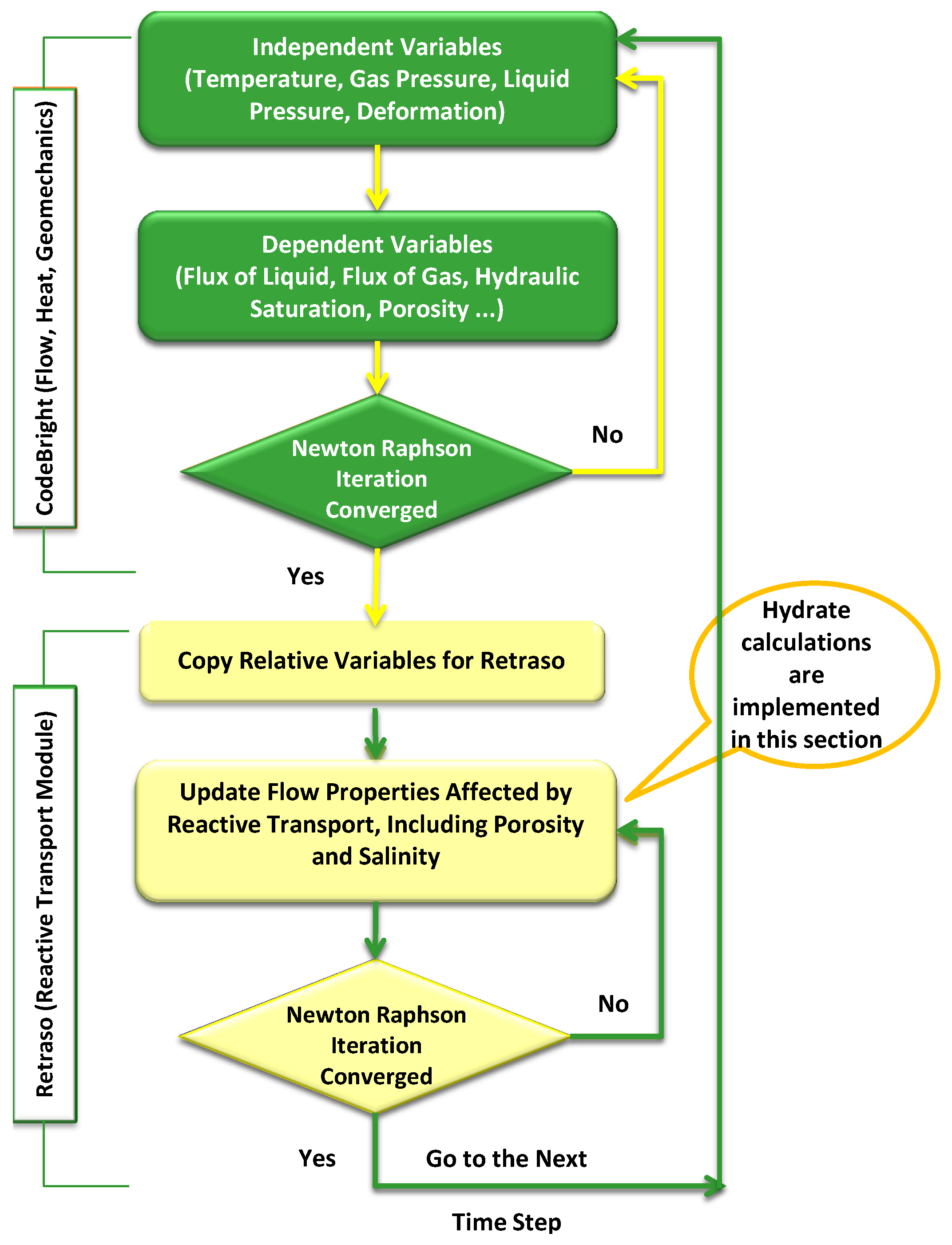
References
- Laberg, J.S.; Andreassen, K. Gas hydrate and free gas indications within the Cenozoic succession of the Bjørnøya Basin, western Barents Sea. Mar. Pet. Geol. 1996, 13, 921–940. [Google Scholar] [CrossRef]
- Kvenvolden, K. Gas hydrates as a potential energy resource—A review of their methane content. U. S. Geol. Surv. Prof. Pap. (U. S.) 1993, 1570, 555–561. [Google Scholar]
- Sloan, E.; Koh, C. Clathrate Hydrates of Natural Gases; Chemical Rubber Company (CRC) Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Graue, A.; Kvamme, B.; Baldwin, B.; Stevens, J.; Howard, J.J.; Aspenes, E.; Ersland, G.; Husebo, J.; Zornes, D. MRI visualization of spontaneous methane production from hydrates in sandstone core plugs when exposed to CO2. SPE J. 2008, 13, 146–152. [Google Scholar] [CrossRef]
- Singh, S.; Arora, A. Techniques for exploitation of gas hydrate (clathrates) an untapped resource of methane gas. J. Microb. Biochem. Technol. 2015, 7, 108–111. [Google Scholar]
- Chabert, A.; Minshull, T.A.; Westbrook, G.K.; Berndt, C.; Thatcher, K.E.; Sarkar, S. Characterization of a stratigraphically constrained gas hydrate system along the western continental margin of Svalbard from ocean bottom seismometer data. J. Geophys. Res. 2011, 116. [Google Scholar] [CrossRef] [Green Version]
- Chand, S.; Mienert, J.; Andreassen, K.; Knies, J.; Plassen, L.; Fotland, B. Gas hydrate stability zone modelling in areas of salt tectonics and pockmarks of the Barents Sea suggests an active hydrocarbon venting system. Mar. Pet. Geol. 2008, 25, 625–636. [Google Scholar] [CrossRef]
- Saaltink, M.; Ayora, C.; Olivella, S. User’s Guide for RetrasoCodeBright (RCB); Institute of Earth Sciences Jauma Almera, Spanish Research Council (CSIC): Barcelona, Spain, 2005. [Google Scholar]
- Qorbani, K.; Kvamme, B. Non-equilibrium simulation of CH4 production from gas hydrate reservoirs through the depressurization method. J. Nat. Gas Sci. Eng. 2016, 35, 1544–1554. [Google Scholar] [CrossRef]
- Chejara, A.; Kvamme, B.; Vafaei, M.T.; Jemai, K. Simulations of long term methane hydrate dissociation by pressure reduction using an extended RetrasoCodeBright simulator. Energy Convers. Manag. 2013, 68, 313–323. [Google Scholar] [CrossRef]
- Kvamme, B. Kinetics of Hydrate Formation From Nucleation Theory; International Society of Offshore and Polar Engineers: Mountain View, CA, USA, 2002. [Google Scholar]
- Kvamme, B. Droplets of Dry Ice and Cold Liquid CO2 for Self-Transport of CO2 to Large Depths; International Society of Offshore and Polar Engineers: Mountain View, CA, USA, 2003. [Google Scholar]
- Kvamme, B.; Kuznetsova, T.; Kivelæ, P.H.; Bauman, J. Can hydrate form in carbon dioxide from dissolved water? Phys. Chem. Chem. Phys. 2013, 15, 2063–2074. [Google Scholar] [CrossRef] [PubMed]
- Kvamme, B.; Qasim, M.; Baig, K.; Kivelä, P.H.; Bauman, J. Hydrate phase transition kinetics from Phase Field Theory with implicit hydrodynamics and heat transport. Int. J. Greenh. Gas Control 2014, 29, 263–278. [Google Scholar] [CrossRef]
- Laberg, J.S.; Andreassen, K.; Knutsen, S.M. Inferred gas hydrate on the Barents Sea shelf—A model for its formation and a volume estimate. Geo-Mar. Lett. 1998, 18, 26–33. [Google Scholar] [CrossRef]
- Knutsen, S.M.; Vorren, T.O. Early Cenozoic sedimentation in the Hammerfest Basin. Mar. Geol. 1991, 101, 31–48. [Google Scholar] [CrossRef]
- Løvø, V.; Elverhøi, A.; Antonsen, P.; Solheim, A.; Liestøl, O. Submarine Permafrost and Gas Hydrates in the Northern Barents Sea; Technical Report; Norsk Polarinstitutt: Tromsø, Norway, 1990. [Google Scholar]
- Waite, W.F.; Stern, L.A.; Kirby, S.H.; Winters, W.J.; Mason, D.H. Simultaneous determination of thermal conductivity, thermal diffusivity and specific heat in sI methane hydrate. Geophys. J. Int. 2007, 169, 767–774. [Google Scholar] [CrossRef]
- Winters, W.J.; Pecher, I.A.; Waite, W.F.; Mason, D.H. Physical properties and rock physics models of sediment containing natural and laboratory-formed methane gas hydrate. Am. Mineral. 2004, 89, 1221–1227. [Google Scholar] [CrossRef]
- Waite, W.F.; Santamarina, J.C.; Cortes, D.D.; Dugan, B.; Espinoza, D.N.; Germaine, J.; Jang, J.; Jung, J.W.; Kneafsey, T.J.; Shin, H.; et al. Physical properties of hydrate-bearing sediments. Rev. Geophys. 2009, 47. [Google Scholar] [CrossRef]
- Olivella, S.; Carrera, J.; Gens, A.; Alonso, E.E. Nonisothermal multiphase flow of brine and gas through saline media. Transp. Porous Med. 1994, 15, 271–293. [Google Scholar] [CrossRef]
- Olivella, S.; Gens, A.; Carrera, J.; Alonso, E. Numerical formulation for a simulator (CODE_BRIGHT) for the coupled analysis of saline media. Eng. Comput. 1996, 13, 87–112. [Google Scholar] [CrossRef]
- Olivella, S.; Gens, A.; Carrera, J. CodeBright User’s Guide; Universitat Politecnicade Catalunya and Institutode Ciencias de la Tierra, CSIS: Barcelona, Spain, 1997. [Google Scholar]
- Saaltink, M.W.; Batlle, F.; Ayora, C.; Carrera, J.; Olivella, S. RETRASO, a code for modeling reactive transport in saturated and unsaturated porous media. Geol. Acta 2004, 2, 235–251. [Google Scholar]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Kozeny, J. Ueber kapillare leitung des wassers im boden. Akad. Wiss. 1927, 136, 271–306. [Google Scholar]
- Soave, G. Equilibrium constants from a modified Redlich-Kwong equation of state. Chem. Eng. Sci. 1972, 27, 1197–1203. [Google Scholar] [CrossRef]
- Vafaei, M.; Kvamme, B.; Chejara, A.; Jemai, K. A new reservoir simulator for studying hydrate dynamics in reservoir. Int. J. Greenh. Gas Control 2014, 23, 12–21. [Google Scholar] [CrossRef]
- International Center for Numerical Methods in Engineering. GID—The Personal Pre and Post Processor. Available online: http://www.gidhome.com/ (accessed on 31 December 2016).
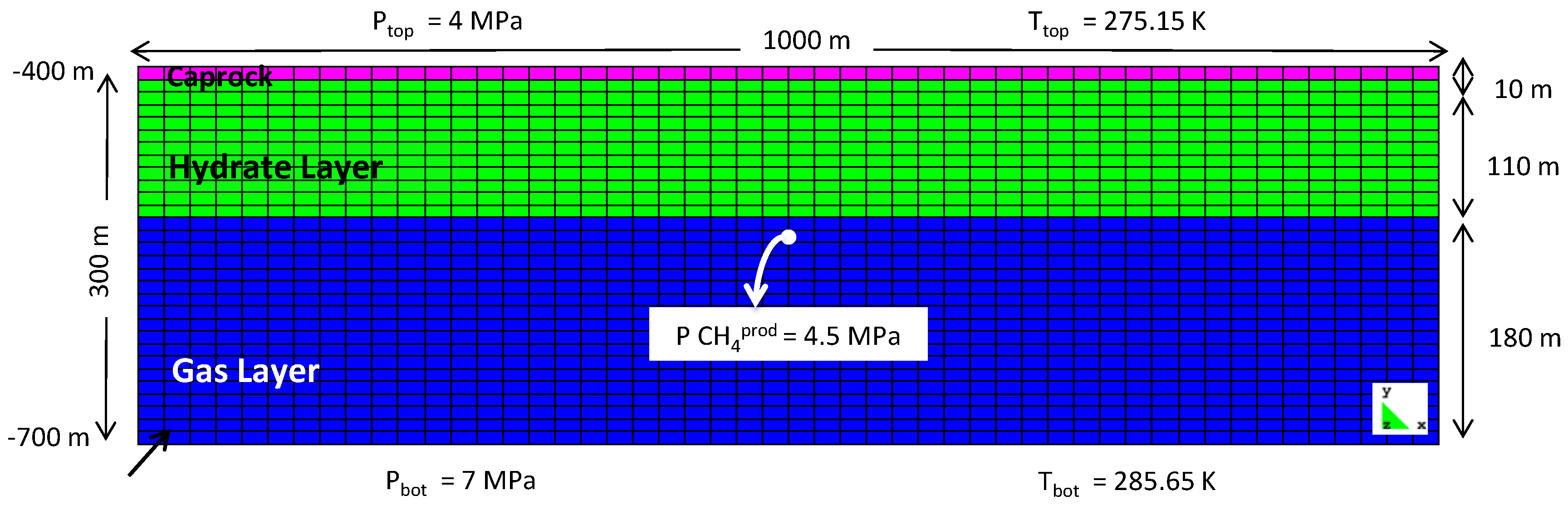
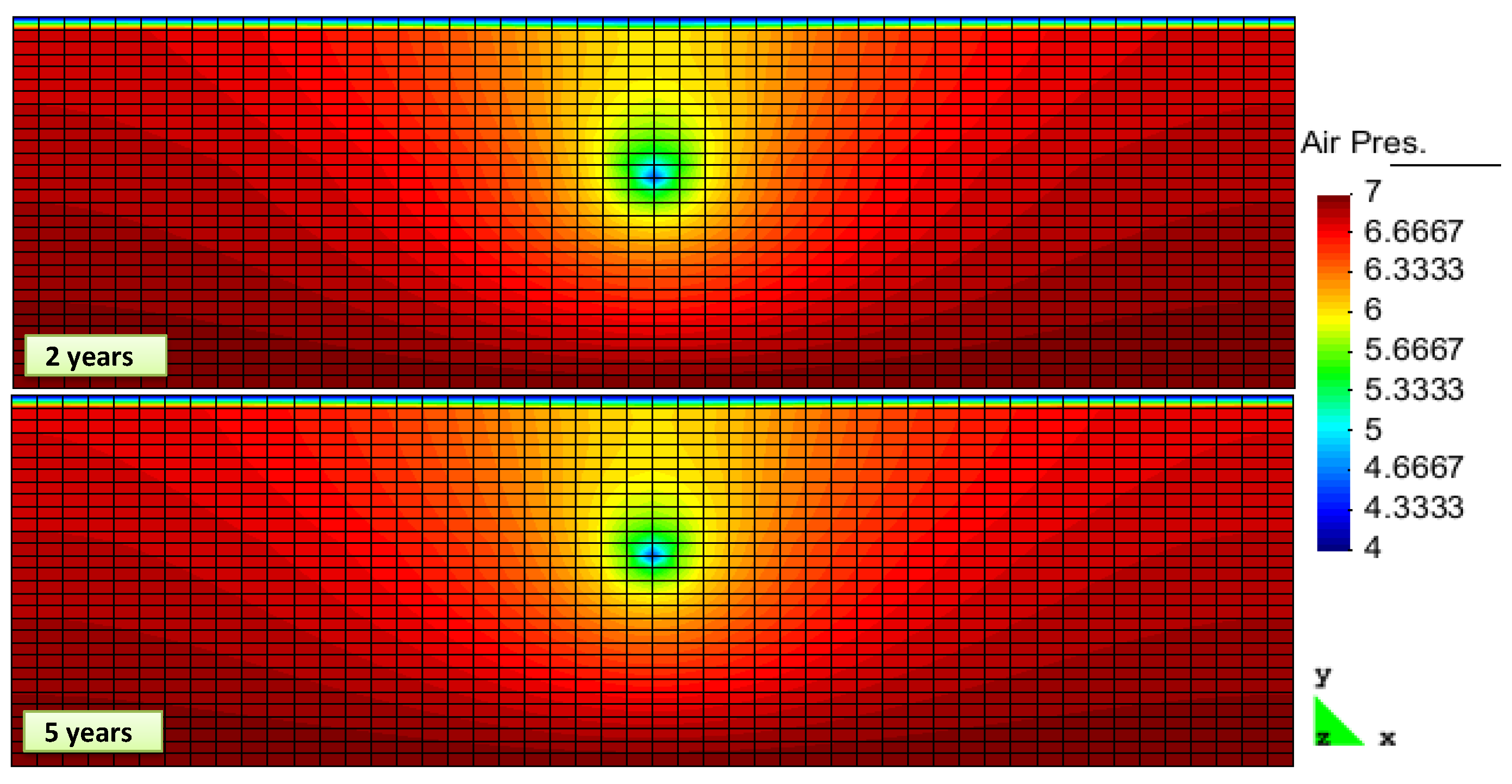
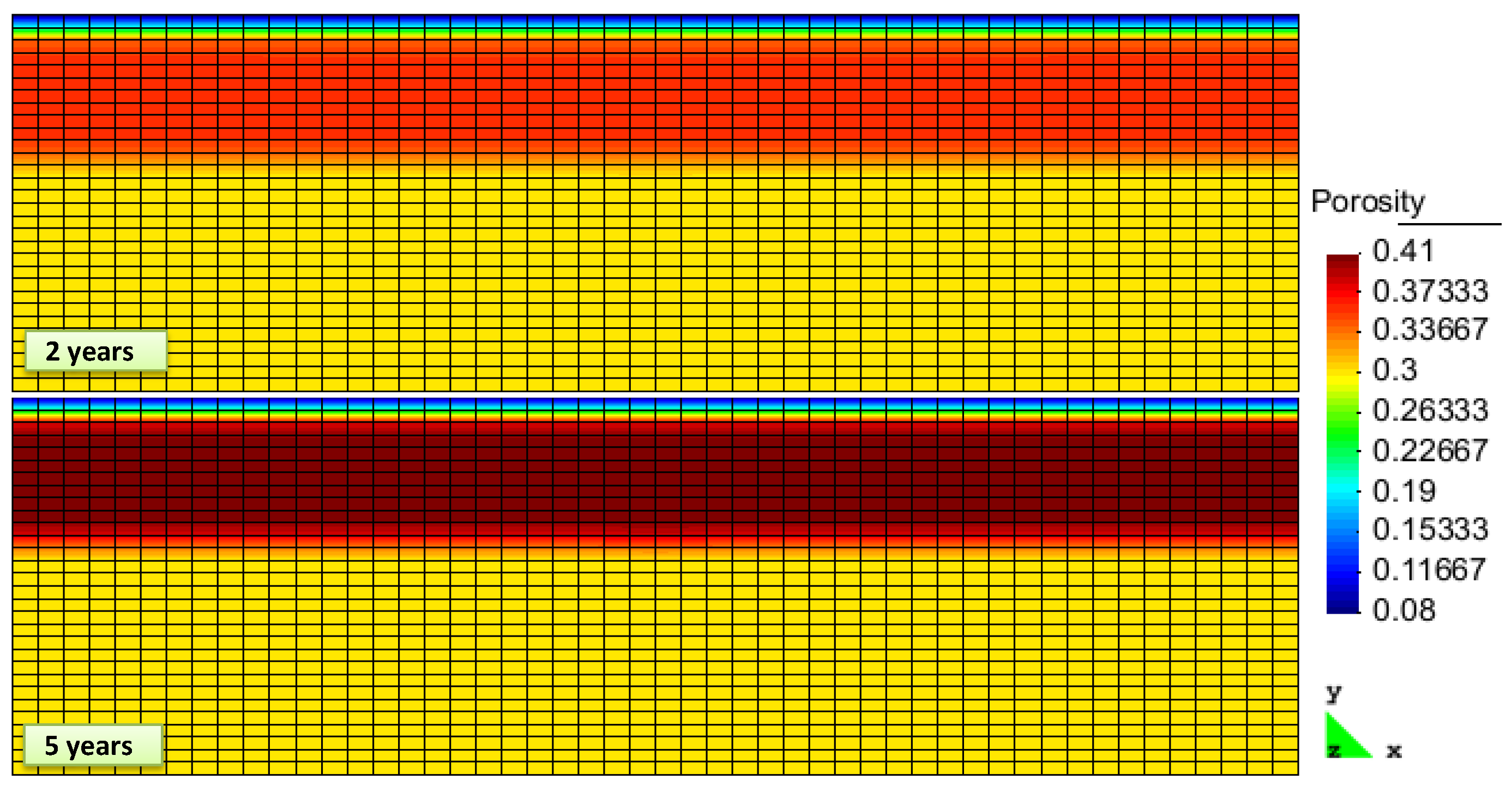

| Property | Gas Layer | Hydrate Layer | Caprock |
|---|---|---|---|
| Young’s modulus (GPa) | 0.5 | 0.5 | 0.5 |
| Poisson’s ratio | 0.25 | 0.25 | 0.25 |
| Zero stress porosity | 0.3 | 0.3 | 0.08 |
| Zero stress permeability (m) | 2 × | 3 × | 10 |
| Van Genuchten’s gas entry pressure (at zero stress) (MPa) | 0.0196 | 0.0196 | 0.196 |
| Van Genuchten’s exponent (m) | 0.457 | 0.457 | 0.457 |
| Longitude dispersion factor (m) | 11 | 11 | 11 |
| Molecular diffusion (m) | 10 | 10 | 10 |
| Property | Value | Unit |
|---|---|---|
| Thermal conductivity of saturated medium | 2.18 | W/(m·K) |
| Thermal conductivity of dry medium | 2.0 | W/(m·K) |
| Solid Phase Density | 2150 | Kg/m |
| Specific heat of rock | 874 | J/(kg·K) |
| CH-hydrate molecular weight | 119.5 | g/mol |
| CH-hydrate density | 907.40 | kg/m |
| CH-hydrate specific heat | 2200 | J/(kg·K) [18] |
| CH-hydrate reaction enthalpy | 53.24 | kJ/mol |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qorbani, K.; Kvamme, B.; Kuznetsova, T. Using a Reactive Transport Simulator to Simulate CH4 Production from Bear Island Basin in the Barents Sea Utilizing the Depressurization Method†. Energies 2017, 10, 187. https://doi.org/10.3390/en10020187
Qorbani K, Kvamme B, Kuznetsova T. Using a Reactive Transport Simulator to Simulate CH4 Production from Bear Island Basin in the Barents Sea Utilizing the Depressurization Method†. Energies. 2017; 10(2):187. https://doi.org/10.3390/en10020187
Chicago/Turabian StyleQorbani, Khadijeh, Bjørn Kvamme, and Tatiana Kuznetsova. 2017. "Using a Reactive Transport Simulator to Simulate CH4 Production from Bear Island Basin in the Barents Sea Utilizing the Depressurization Method†" Energies 10, no. 2: 187. https://doi.org/10.3390/en10020187






