An Optimized Energy Management Strategy for Preheating Vehicle-Mounted Li-ion Batteries at Subzero Temperatures
Abstract
:1. Introduction
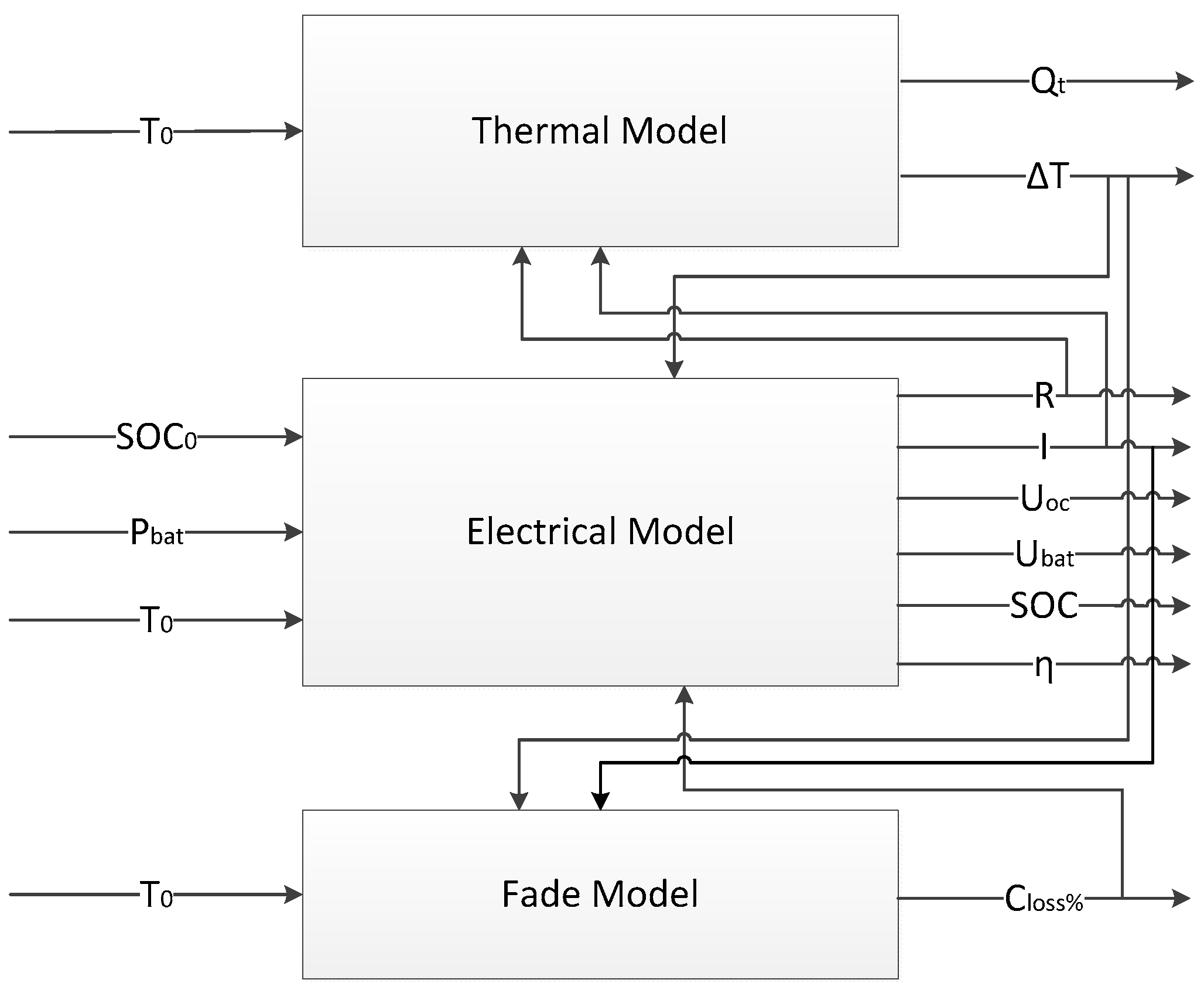
2. Battery Model Consisting of Electro-Thermal and Degradation Models
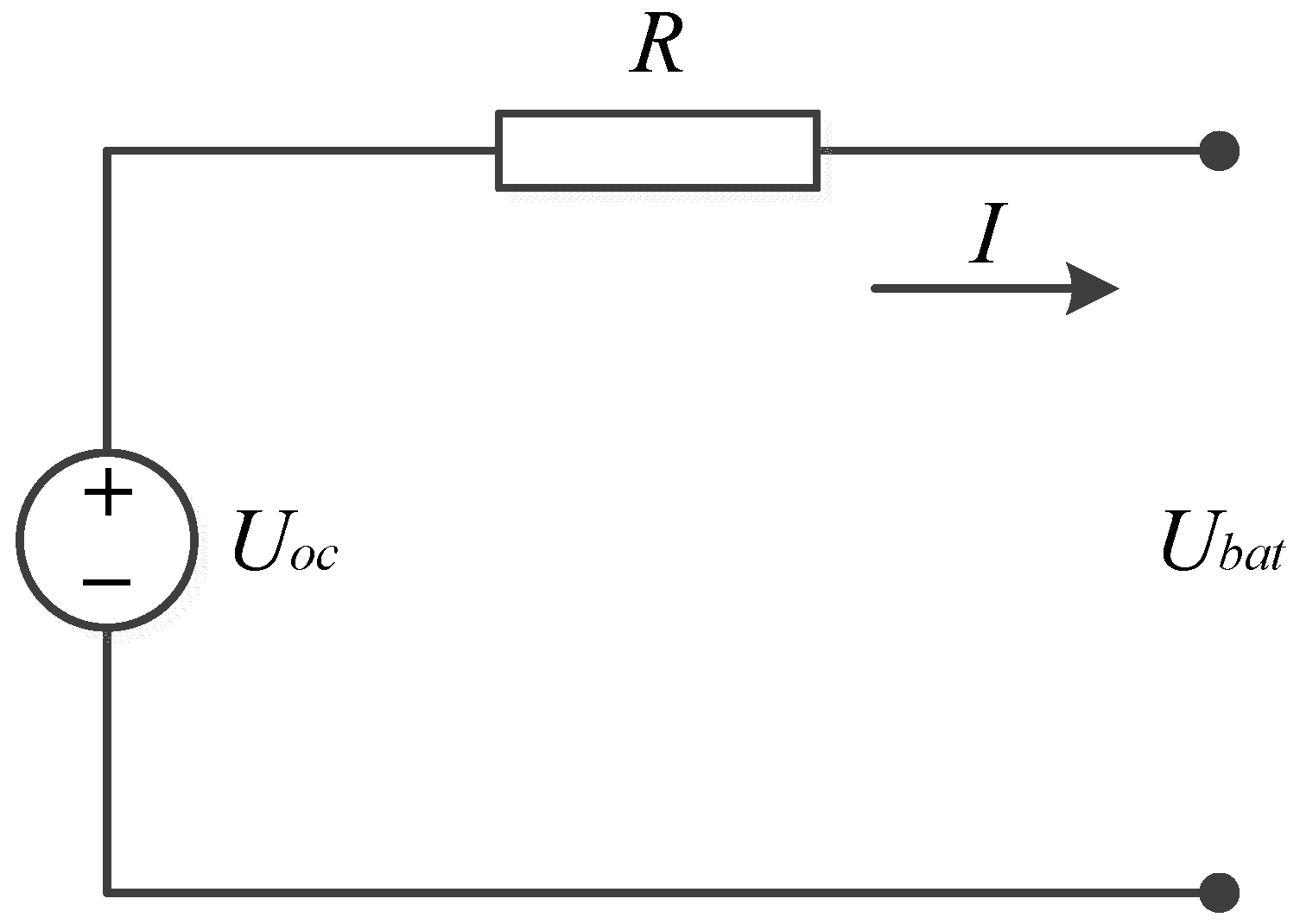
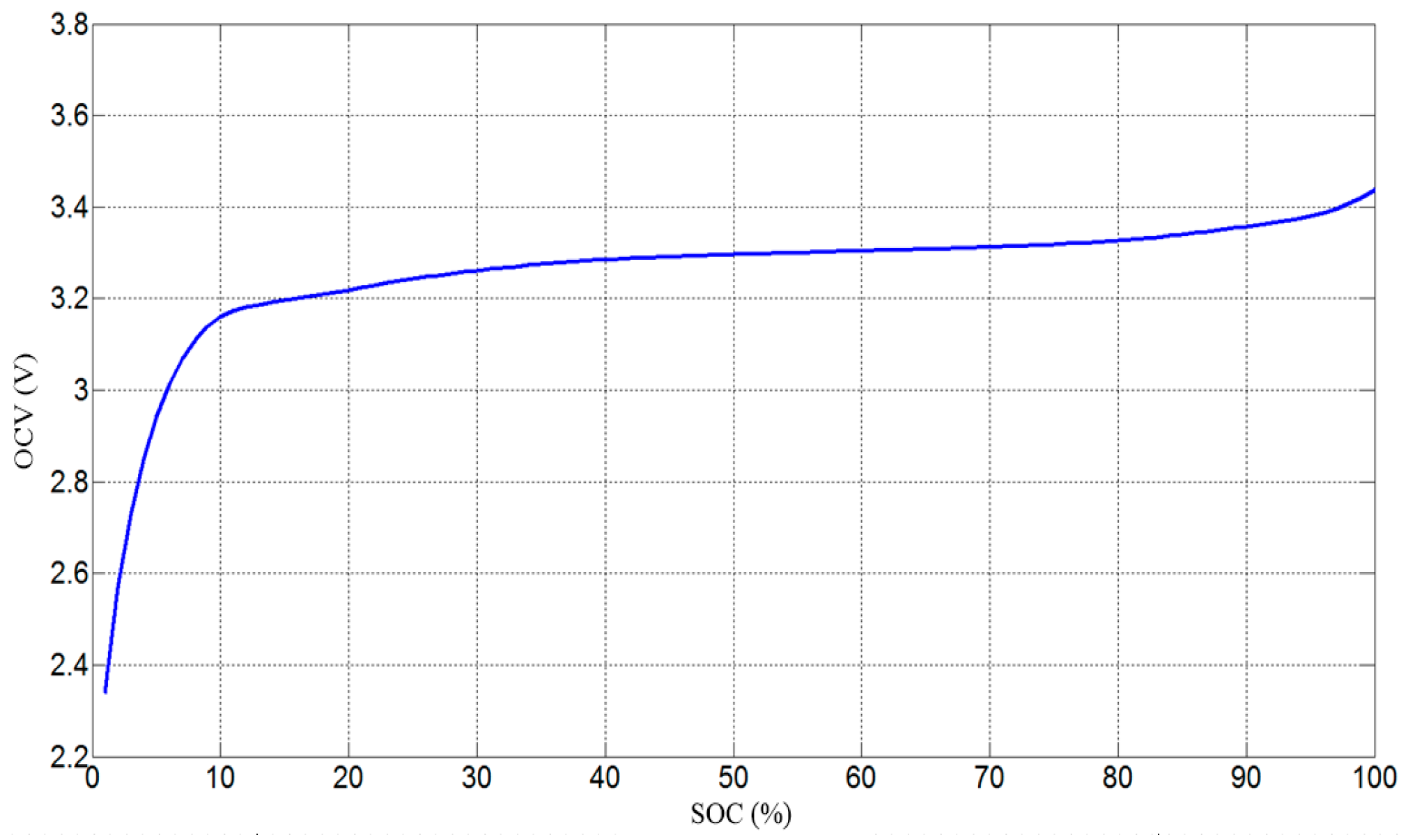
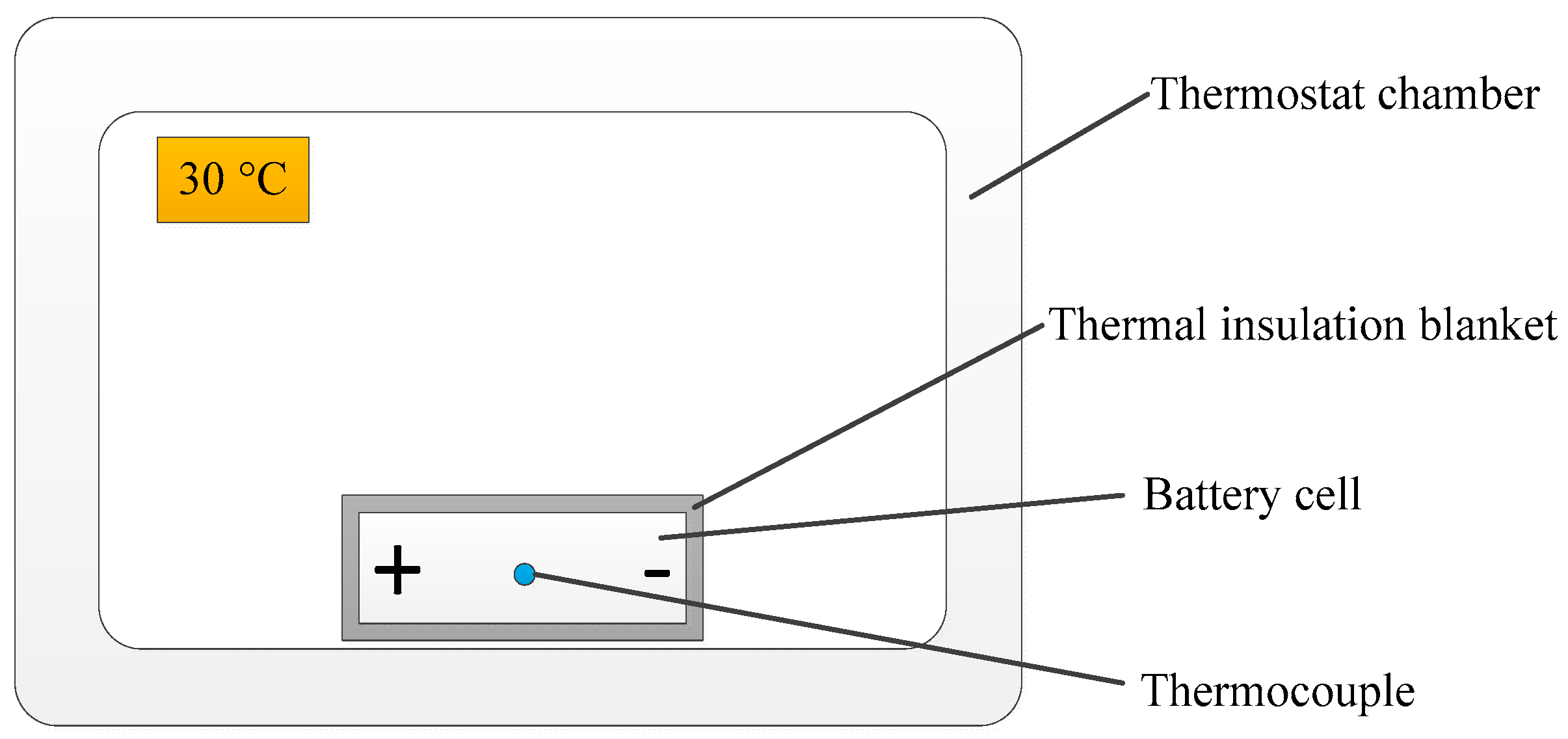
2.1. Battery Electrical Model and Parameters Characterization
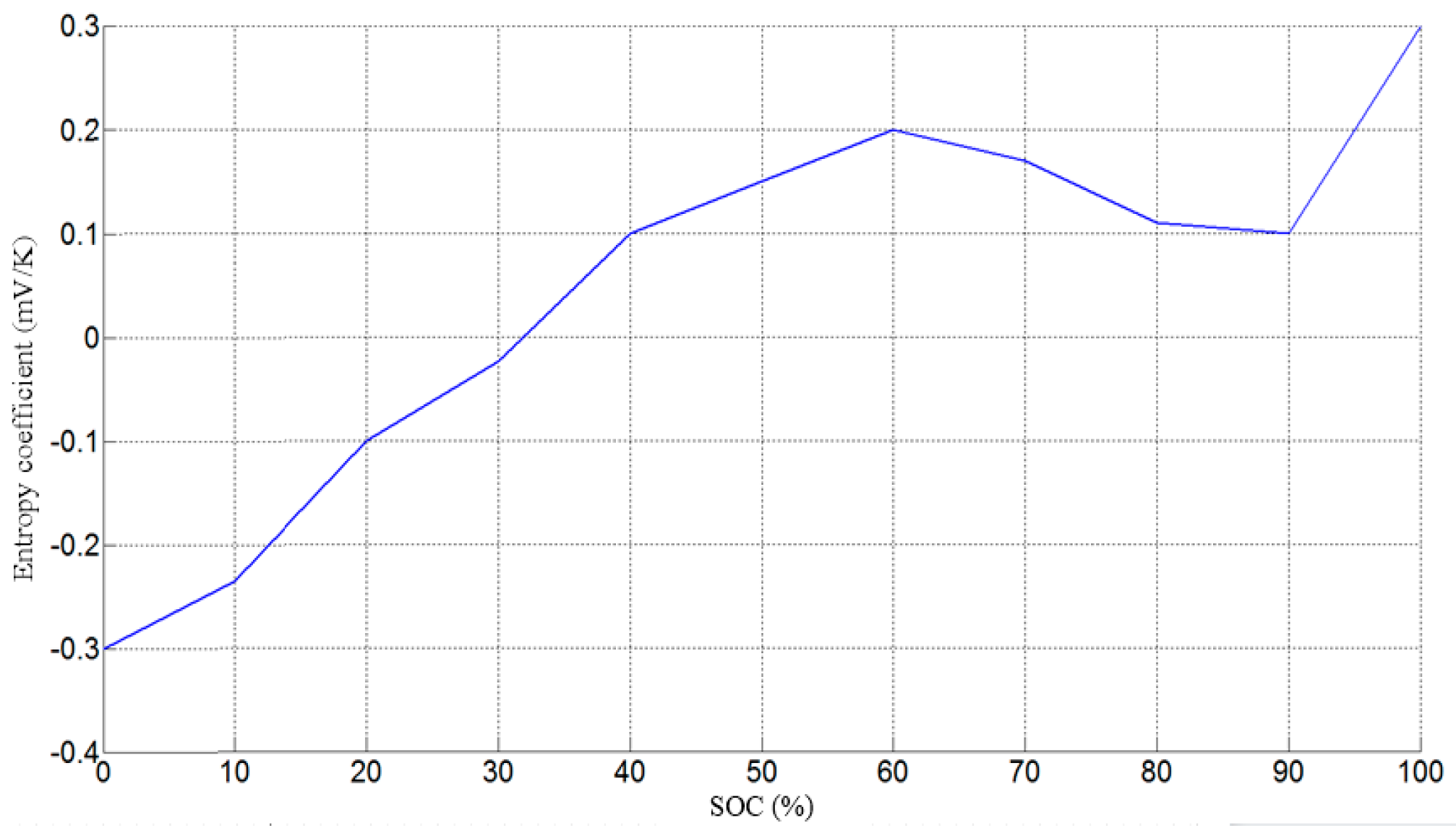
2.2. Battery Heat Generation Mechanism and Thermal Model
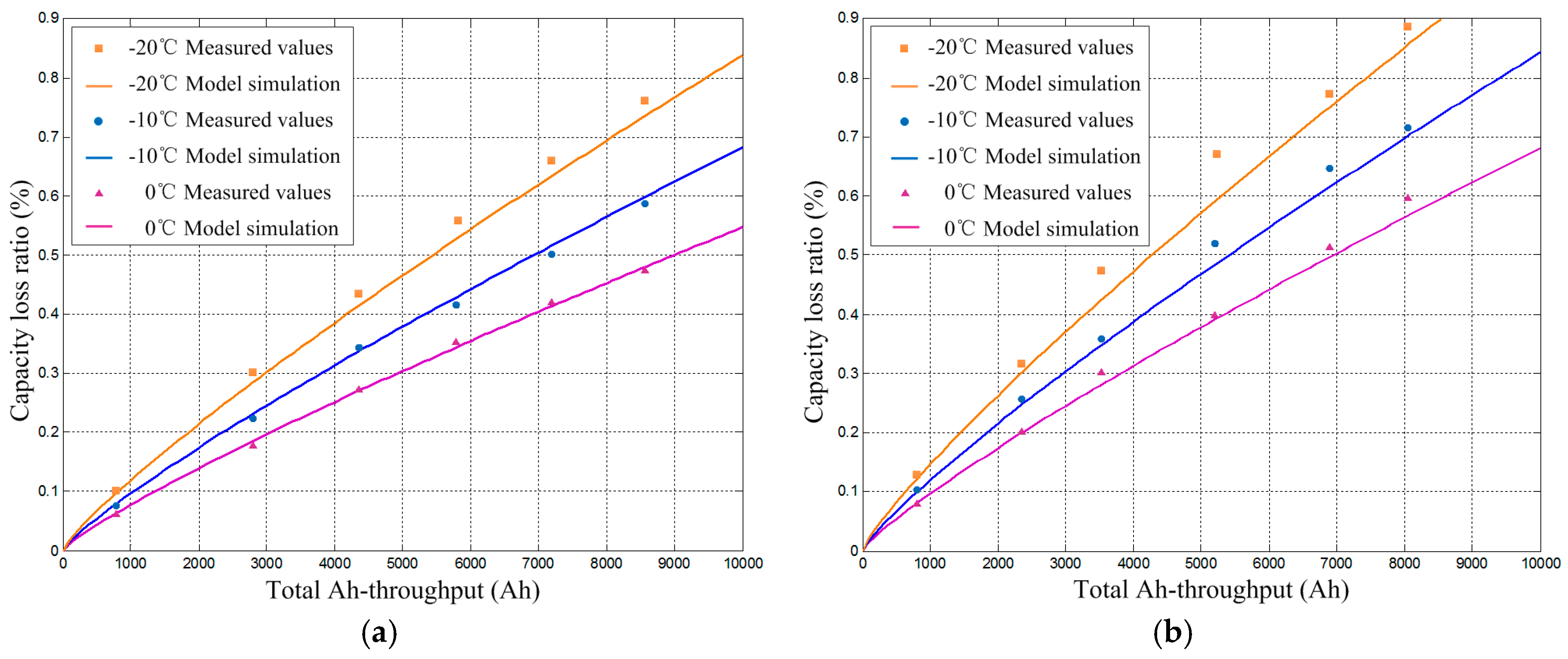
2.3. Battery Degradation Model
2.4. Battery Coupling Model Composed of Electro-Thermal and Degradation Models
3. Deduction of Total Vehicle Operation Cost
3.1. Vehicle Electricity Consumption Cost
3.1.1. Preheating Energy Consumption ()
3.1.2. Charging Energy Loss ()
3.1.3. Vehicle Running Energy Consumption ()
3.2. Battery Fade Cost
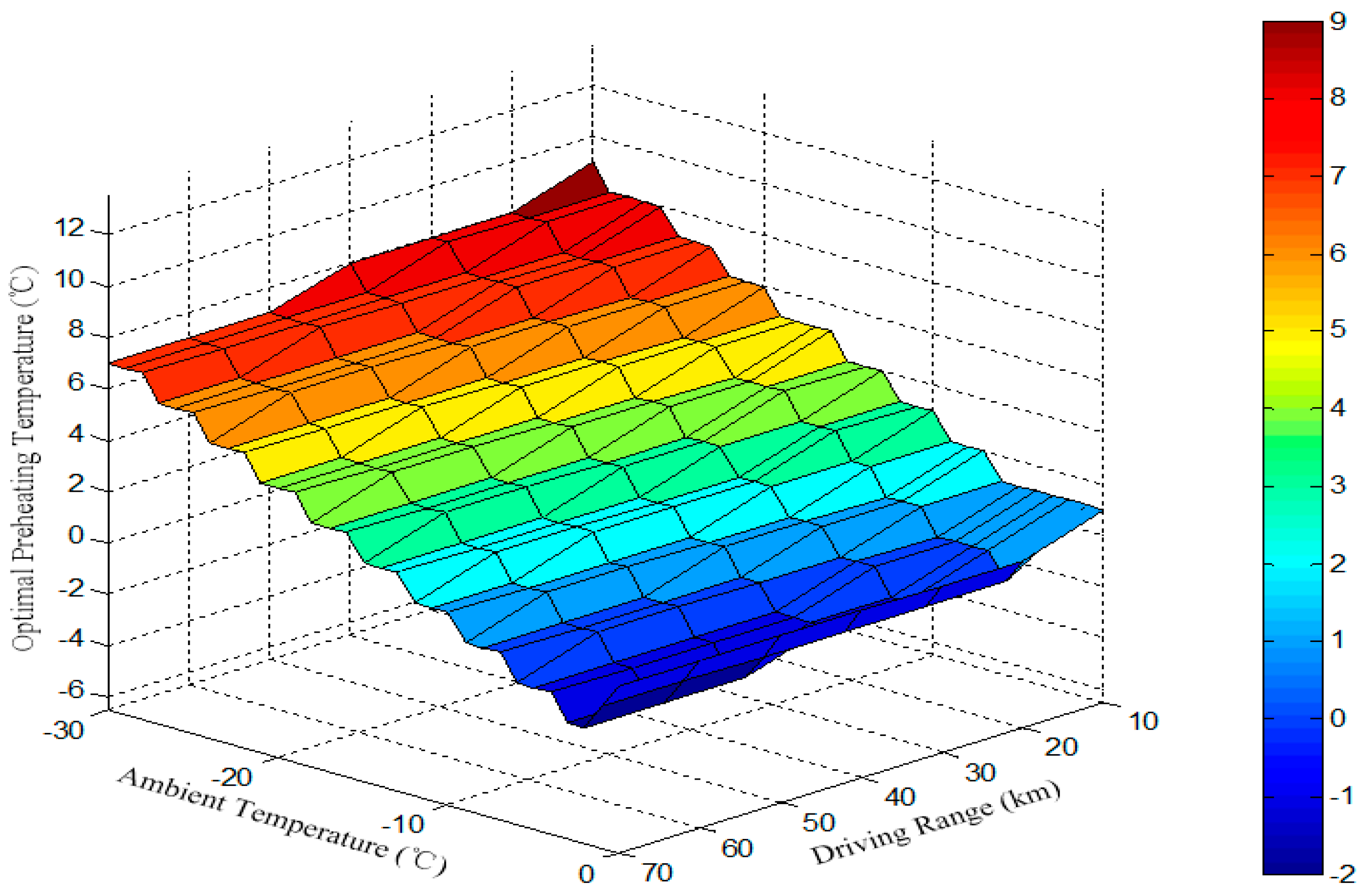
4. Determination of Preheating Target Temperatures
5. Implementation and Control of Preheating System
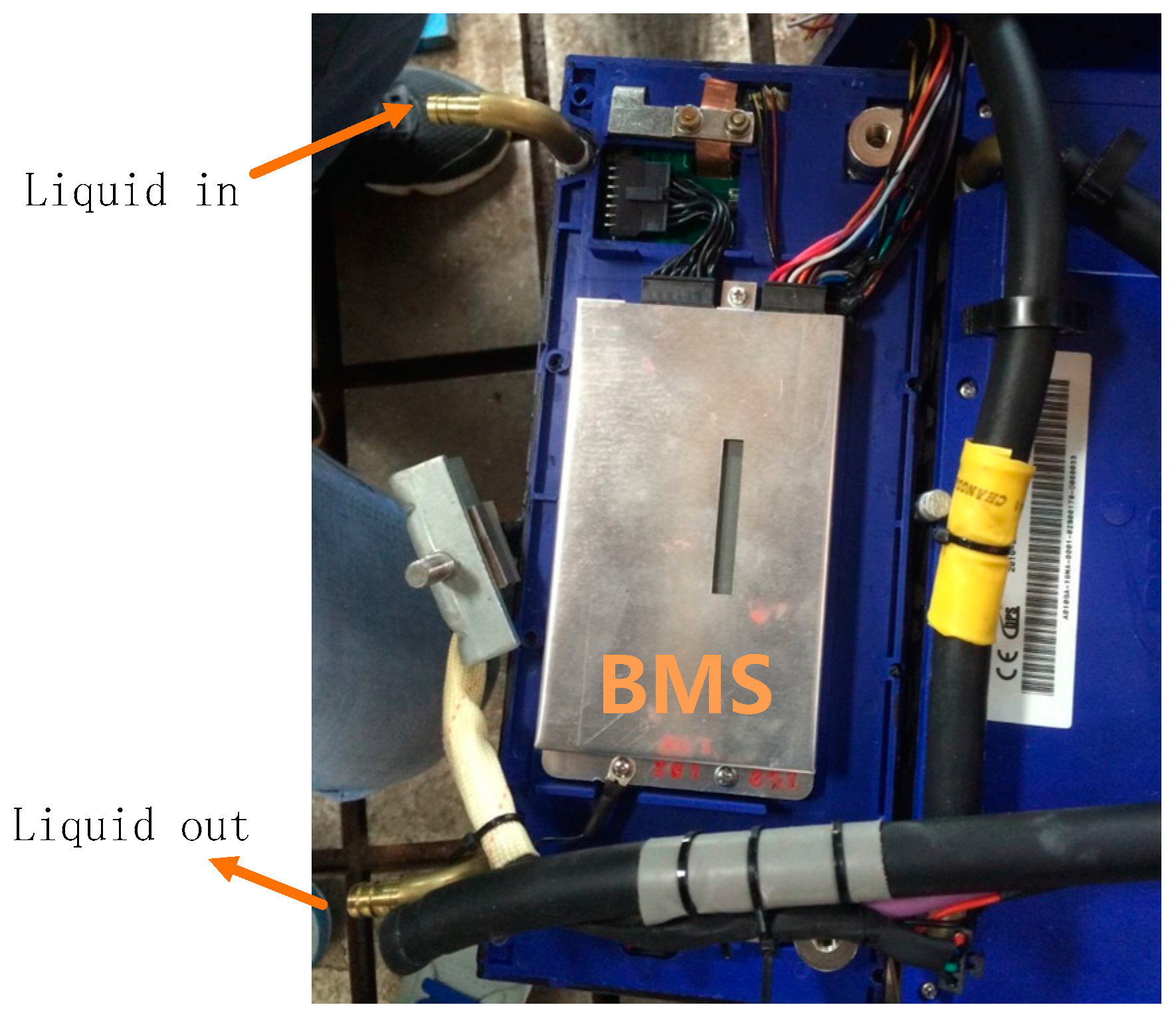
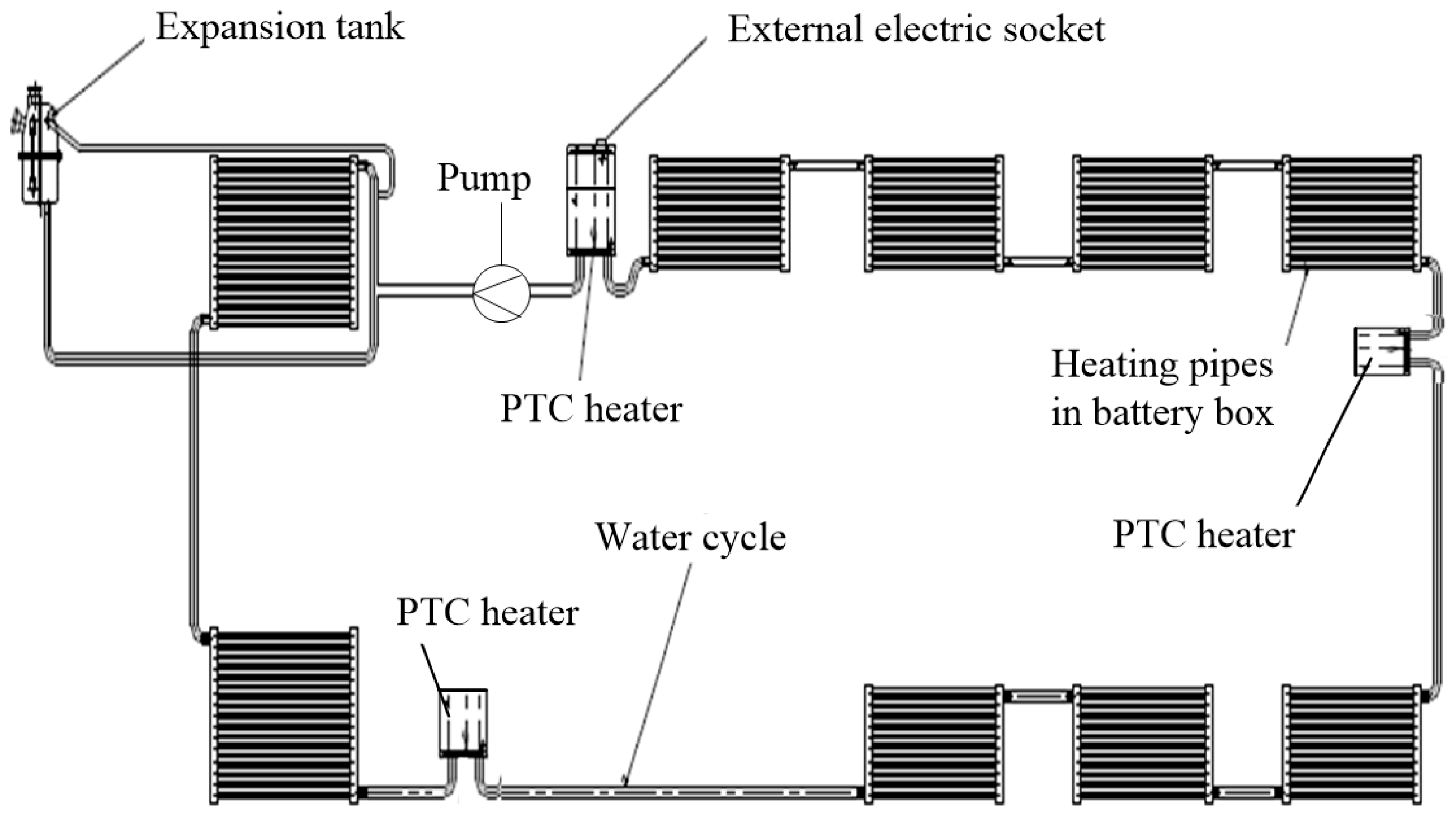
5.1. Selection and Implementation of Preheating System
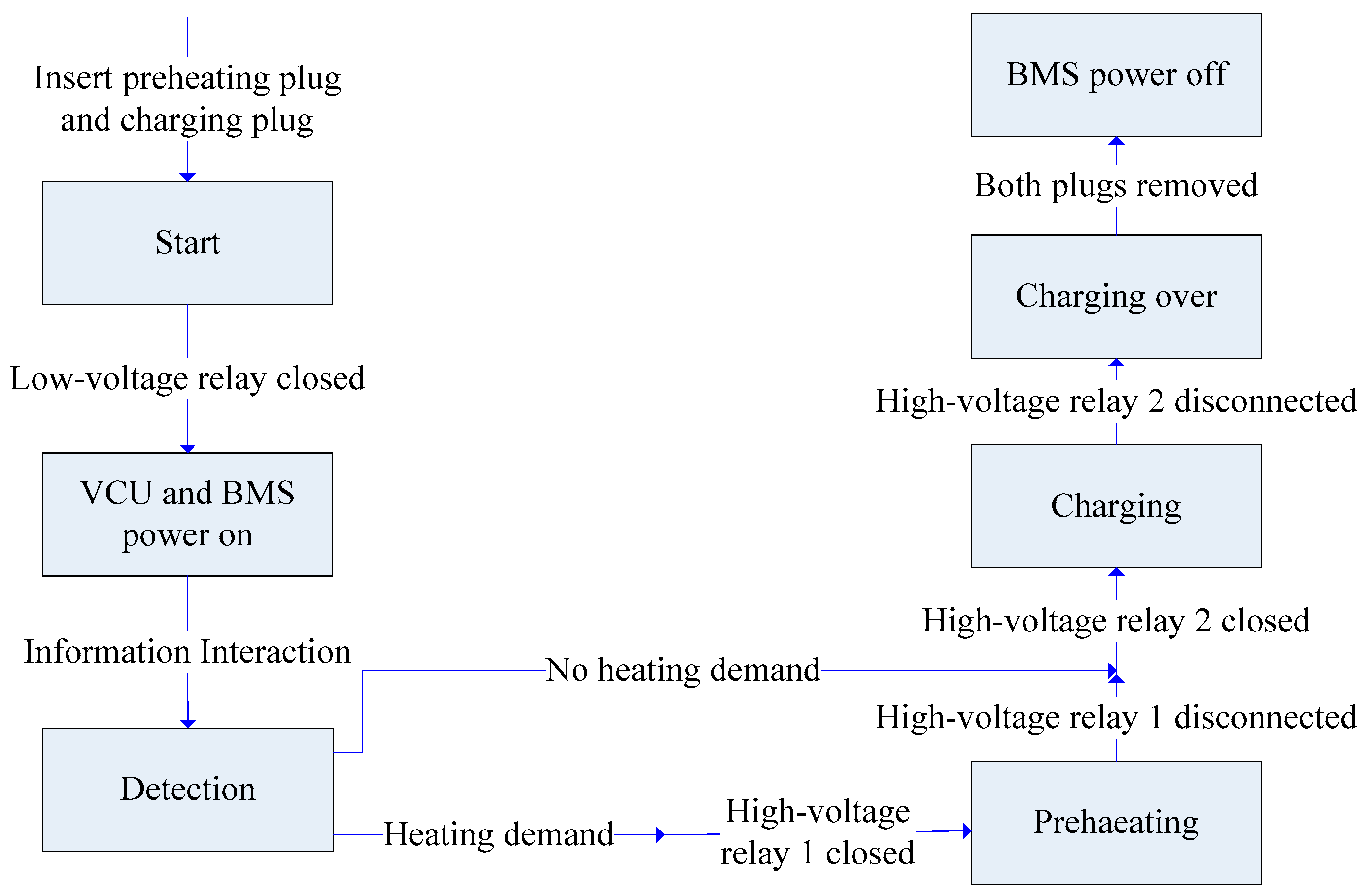
5.2. Control Strategy for Coordinating the Process of Preheating and Charging
5.3. Performance of the Preheating System
- (1)
- Power source: The preheating system is powered by charging piles. Compared with battery self-heating whose power supply is the battery itself, this preheating system could save the energy inside the battery, which indirectly prolongs the vehicle’s driving range;
- (2)
- System cost: Three PTC heaters, an external electric socket and several relays were added on the basis of original cooling system, so it is very cost-saving;
- (3)
- Temperature uniformity: To some extent, temperature imbalance among different cells is unavoidable because the water circulation loop is too long. However, exactly because of this reason, three distributive PTC heaters were arranged at different positions in the water cycle (Figure 13) to reverse this trend. According to the information collected by BMS, if these three heaters were given same power, the biggest temperature difference among different cells is 3.1 °C.
- (4)
- System efficiency: Here the heating efficiency is defined as:The heating efficiency was calculated with the ambient temperature being −10 °C and the results showed an average efficiency of 78%.
- (5)
- Heating time: The heating time was tested with the ambient temperature being −10 °C, the target temperature being 2 °C and the power of each heater being 8 kW (max power). The tested preheating time is 1157 s. Compared with other preheating methods such as self-heating, this preheating time is much longer. As a consequence, the utilization of the electric bus should be scheduled carefully. The long heating time is a serious disadvantage of liquid heating.
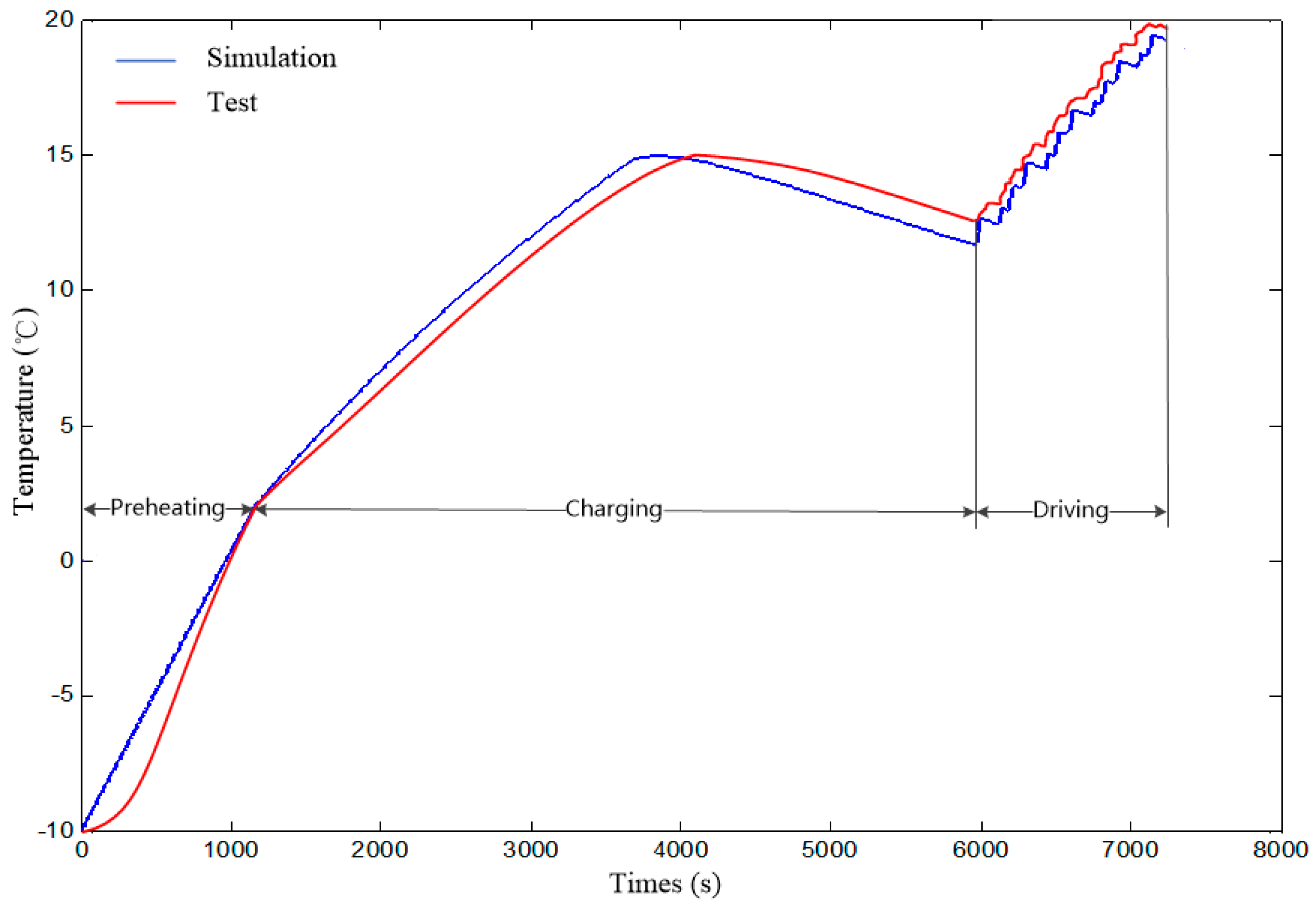
6. Vehicle Test
- (1)
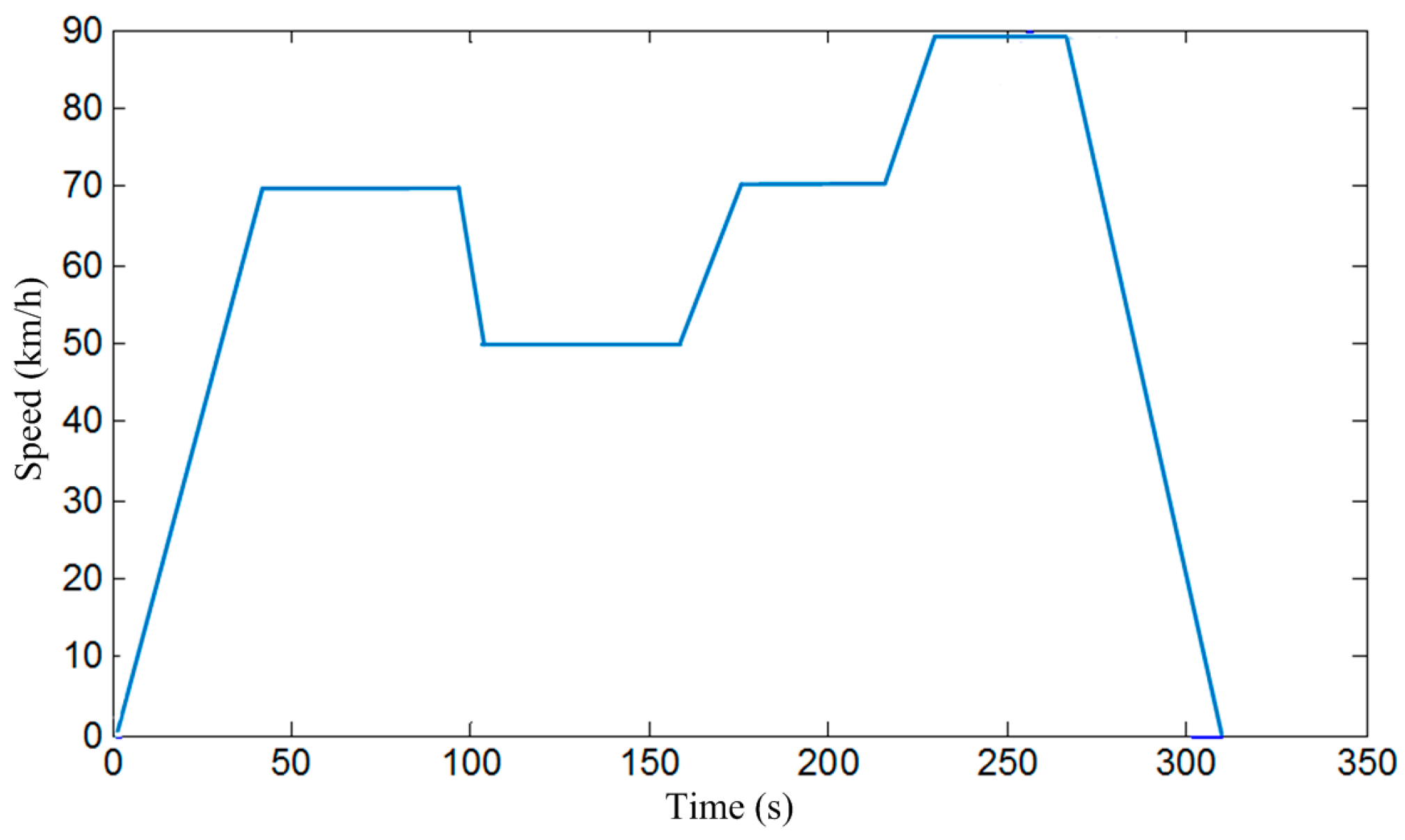
- Battery without preheating. Keep battery SOC at 20% through charging or discharging, and then cool the battery to ambient temperature. After this, fully charge the battery without preheating. Then drive the electric bus for 20 km in accordance with the driving cycles in Figure 9.
- (2)
- Battery heated to 2 °C (optimal target temperature given by the simulation). Keep SOC of battery at 20% through charging or discharging, and then cool the battery to ambient temperature. After this, preheat the battery to 20 °C before charging. Then fully charge battery and drive the electric bus for 20 km in accordance with the driving cycles in Figure 9.
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| Increment of battery Ah-throughput (Ah) | |
| Increment of battery capacity loss ratio | |
| Battery temperature change (K) | |
| Battery entropy coefficient | |
| Surface area of battery () | |
| Surface area of cell () | |
| Ah | Battery Ah-throughput, Ah= (cycle number) × (DOD) × (full battery capacity) (Ah) |
| Vehicle front area () | |
| B | Pre-exponential factor |
| Battery capacity loss ratio | |
| Special heat capacity of battery component i (J/(kg·K)) | |
| Battery initial capacity (Ah) | |
| Air drag coefficient | |
| Vehicle electricity consumption cost ($) | |
| Battery fade cost ($) | |
| Battery specific heat capacity (J/(kg·K)) | |
| Battery current rate (C) | |
| f | Rolling resistance coefficient |
| Total braking force of vehicle (N) | |
| Vehicle mechanical braking force (N) | |
| Braking force of motor (N) | |
| Braking force of rear axle (N) | |
| g | Gravity acceleration () |
| h | Coefficient of heat transfer between battery and outside air () |
| I | Battery current (A) |
| Battery current during charge (A) | |
| Battery current of the charging pile during preheating process (A) | |
| Battery current during discharge (A) | |
| Gearbox ratio | |
| Final drive ratio | |
| Battery mass (kg) | |
| Cell mass (kg) | |
| Vehicle mass (kg) | |
| n | Rotational speed of motor (r/min) |
| Battery output power (kW) | |
| Battery power when vehicle is braking (kW) | |
| Battery power when vehicle is driving (kW) | |
| Motor power (kW) | |
| Power demand of vehicle (kW) | |
| Battery heat generated during charge (J) | |
| Energy loss of charging (J) | |
| Energy consumption of driving cycles (J) | |
| Energy consumption of driving cycles when vehicle is braking (J) | |
| Energy consumption of driving cycles when vehicle is driving (J) | |
| Battery heat generated during discharge (J) | |
| Battery heat dissipated (W) | |
| Vehicle electricity consumption (J) | |
| Battery Joule heat generation (W) | |
| Energy loss during discharge or charge (J) | |
| Battery polarization reaction heat generation (W) | |
| Energy consumption of preheating (J) | |
| Battery reaction heat generation (W) | |
| Battery side reaction heat generation (W) | |
| Battery heat generation (W) | |
| r | Wheel radius (m) |
| R | Battery equivalent resistance (Ω) |
| t | Time (s) |
| Battery surface temperature (K) | |
| Battery ambient temperature (K) | |
| Battery preheating target temperature (K) | |
| Temperature of thermostat chamber (K) | |
| Battery finial temperature in measurement process (K) | |
| Battery initial temperature in measurement process (K) | |
| u | Vehicle speed (km/h) |
| Battery terminal voltage (V) | |
| Battery terminal voltage during charge (V) | |
| Voltage of the charging pile during preheating process (V) | |
| Battery open circuit voltage (OCV) (V) | |
| Volume of battery component i () | |
| z | Vehicle braking intensity |
| Correction coefficient of rotating mass | |
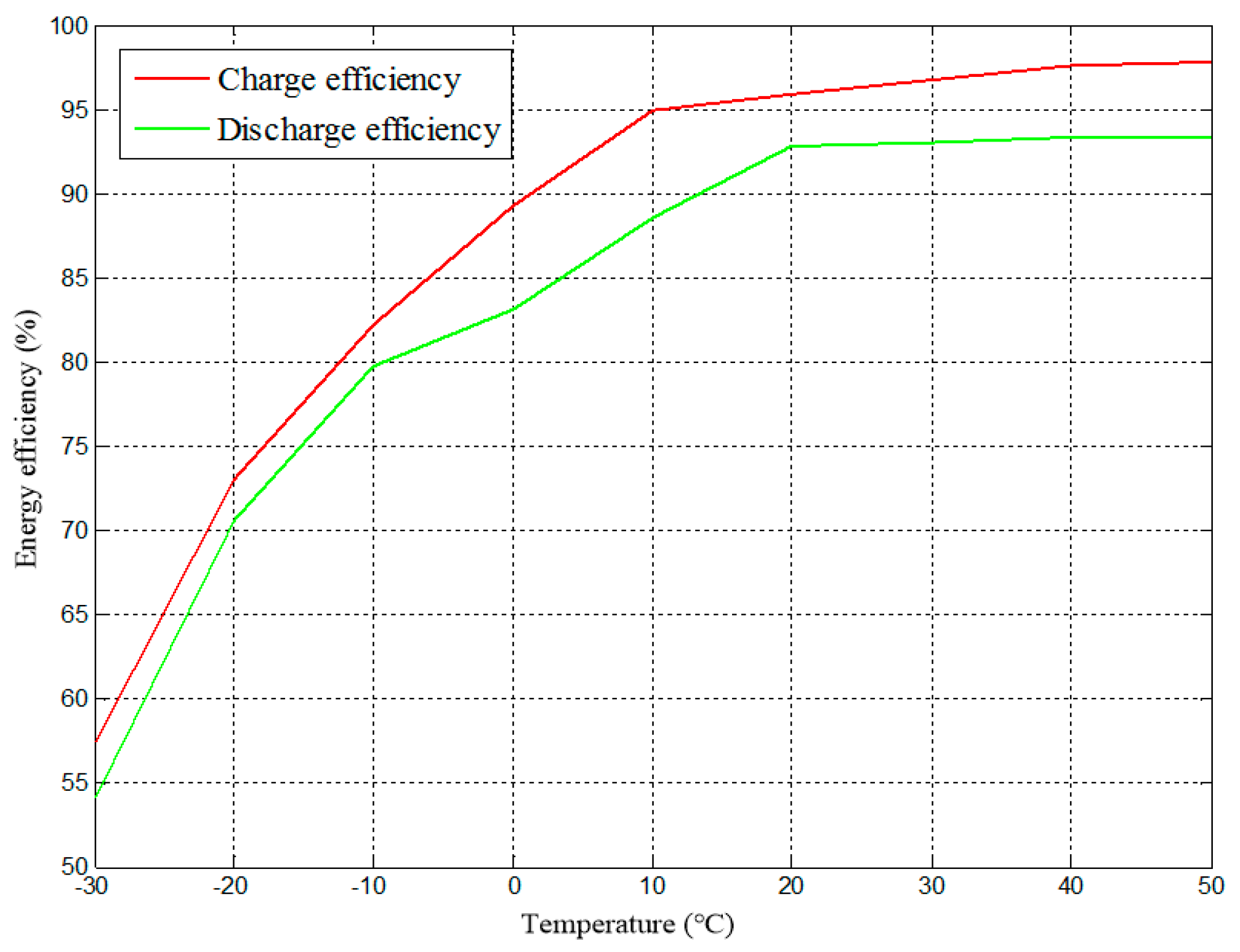
| η | Battery energy efficiency |
| Battery charge efficiency | |
| Battery discharge efficiency | |
| Motor inverter efficiency | |
| Motor efficiency | |
| Preheating system efficiency | |
| Transmission efficiency | |
| λ | Coefficient between and |
| Density of battery component i (kg/) |
References
- Doughty, D.H.; Butler, P.C.; Jungst, R.G.; Roth, E.P. Lithium battery thermal models. J. Power Sources 2002, 110, 357–363. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Lee, J.; Cho, B.H. State-of-charge and capacity estimation of lithium-ion battery using a new open-circuit voltage versus state-of-charge. J. Power Sources 2008, 185, 1367–1373. [Google Scholar] [CrossRef]
- Yang, J.; Cai, S. Battery Pack Thermal Management System and Method. U.S. Patent 865,829,9, 25 February 2011. [Google Scholar]
- Lake, B.J.; Ziehr, L.; Tagliapietra, L.C. Controller for Heating in Reversible Air Conditioning and Heat Pump HVAC System for Electric Vehicles. U.S. Patent 611,809,9, 12 Sepetember 2000. [Google Scholar]
- Chakib, A. Electric Vehicle Energy Management System. Ph.D. Thesis, University of Massachusetts Lowell, Lowell, MA, USA, 2001. [Google Scholar]
- Apfelbeck, R.; Barthel, F. Heating comfort and range perfectly combined-heating systems for vehicles with alternative drive system. Prospects and challenges of biofuel-operated water and air heaters. In Proceedings of the Society of Automotive Engineers (SAE) 2013 World Congress & Exhibition, Detroit, MI, USA, 16–18 April 2013.
- Lin, C.; Xu, S.; Li, Z.; Li, B.; Chang, G.; Liu, J. Thermal analysis of large-capacity LiFePO4 power batteries for electric vehicles. J. Power Sources 2015, 294, 633–642. [Google Scholar] [CrossRef]
- Jaguemont, J.; Boulon, L.; Dubé, Y. A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures. Appl. Energy 2016, 164, 99–114. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Z.; Wang, X.; Jia, L.; Yang, L. A pseudo three-dimensional electrochemical–thermal model of a prismatic LiFePO4 battery during discharge process. Energy 2015, 80, 303–317. [Google Scholar] [CrossRef]
- Kang, J.; Rizzoni, G. Study of relationship between temperature and thermal energy, operating conditions as well as environmental factors in large-scale lithium-ion batteries. Energy Res. 2014, 38, 1994–2002. [Google Scholar] [CrossRef]
- Agarwal, V.; Uthaichana, K.; Decarlo, R.A.; Tsoukalas, L.H. Development and validation of a battery model useful for discharging and charging power control and lifetime estimation. IEEE Trans. Energy Convers. 2010, 25, 821–835. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Kang, X.; Shrestha, S.O.B. Effects of temperature on internal resistances of lithium-ion batteries. J. Energy Resour. Technol. 2015, 137. [Google Scholar] [CrossRef]
- Samadani, E.; Farhad, S.; Scott, W.; Mastali, M.; Gimenez, L.E.; Fowler, M.; Fraser, R.A. Empirical modeling of lithium-ion batteries based on electrochemical impedance spectroscopy tests. Electrochim. Acta 2015, 160, 169–177. [Google Scholar] [CrossRef]
- Samadani, E.; Mastali, M.; Farhad, S.; Fraser, R.A.; Fowler, M. Li-ion battery performance and degradation in electric vehicles under different usage scenarios. Int. J. Energy Res. 2016, 40, 379–392. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Key, B.; Chen, H.; Best, A.S.; Hollenkamp, A.F.; Grey, C.P. In situ NMR observation of the formation of metallic lithium microstructures in lithium batteries. Nat. Mater. 2010, 9, 504–510. [Google Scholar] [CrossRef]
- Petzl, M.; Kasper, M.; Danzer, M.A. Lithium plating in a commercial lithium-ion battery—A low-temperature aging study. J. Power Sources 2015, 275, 799–807. [Google Scholar] [CrossRef]
- Kwon, O.J.; Fang, W.; Wang, C. Lithium deposition in the anode of an automotive Li-ion battery: Experiments and modeling. In Proceedings of the 15th International Meeting on Lithium Batteries, Montreal, QC, Canada, 27 June–2 July 2010.
- Sarasketa-Zabala, E.; Gandiaga, I.; Martinez-Laserna, E.; Rodriguez-Martinez, L.M.; Villarreal, I. Calendar ageing analysis of a LiFePO4/graphite cell with dynamic model validations: Towards realistic lifetime predictions. J. Power Sources 2014, 275, 573–587. [Google Scholar] [CrossRef]
- Groot, J.; Swierczynski, M.; Stan, A.I.; Kær, S.K. On the complex ageing characteristics of high-power LiFePO4/graphite battery cells cycled with high charge and discharge currents. J. Power Sources 2015, 286, 475–487. [Google Scholar] [CrossRef]
- Waldmann, T.; Wilka, M.; Kasper, M.; Fleischhammer, M.; Wohlfahrt-Mehrens, M. Temperature dependent ageing mechanisms in lithium-ion batteries—A post-mortem study. J. Power Sources 2014, 262, 129–135. [Google Scholar] [CrossRef]
- Wang, J.; Liu, P.; Hicks-Garner, J.; Sherman, E.; Soukiazian, S.; Verbrugge, M.; Tataria, H.; Musser, J.; Finamore, P. Cycle-life model for graphite-LiFePO4 cells. J. Power Sources 2011, 196, 3942–3948. [Google Scholar] [CrossRef]
- Wang, J.; Purewal, J.; Liu, P.; Hicks-Garner, J.; Soukazian, S.; Sherman, E.; Sorenson, A.; Vu, L.; Tataria, H.; Verbrugge, M.W. Degradation of lithium ion batteries employing graphite negatives and nickel-cobalt- manganese oxide + spinel manganese oxide positives: Part 1, aging mechanisms and life estimation. J. Power Sources 2014, 269, 937–948. [Google Scholar] [CrossRef]
- Rao, Z.; Wang, S. A review of power battery thermal energy management. Renew. Sustain. Energy Rev. 2011, 15, 4554–4571. [Google Scholar] [CrossRef]
- Chen, Y.; Evans, J.W. Heat transfer phenomena in lithium/polymer-electrolyte batteries for electric vehicle application. J. Electrochem. Soc. 1993, 140, 1833–1837. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, C. Heating strategies for Li-ion batteries operated from subzero temperatures. Electrochim. Acta 2013, 107, 664–674. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, H.; Li, Z.; Ding, Z. Internal heating of lithium-ion batteries using alternating current based on the heat generation model in frequency domain. J. Power Sources 2015, 273, 1030–1037. [Google Scholar] [CrossRef]
- Pesaran, A.A.; Vlahinos, A.; Stuart, T.A. Cooling and preheating of batteries in hybrid electric vehicles. In Proceedings of the 6th ASME-JSME Thermal Engineering Joint Conference, Honolulu, HI, USA, 13–17 March 2003.
- Wang, Q.; Jiang, B.; Xue, Q.; Sun, H.; Li, B.; Zou, H.; Yan, Y. Experimental investigation on EV battery cooling and heating by heat Pipes. Appl. Therm. Eng. 2015, 88, 54–60. [Google Scholar] [CrossRef]
- Song, Z.; Hofmann, H.; Li, J.; Hou, J.; Zhang, X.; Ouyang, M. The optimization of a hybrid energy storage system at subzero temperatures: Energy management strategy design and battery heating requirement analysis. Appl. Energy 2015, 159, 576–588. [Google Scholar] [CrossRef]
- Bauer, S.; Suchaneck, A.; León, F.P. Thermal and energy battery management optimization in electric vehicles using Pontryagin’s maximum principle. J. Power Sources 2014, 246, 808–818. [Google Scholar] [CrossRef]
- Rao, R.; Virudhula, S.; Rakhmatov, D. Battery models for energy aware system design. Computer 2003, 36, 77–87. [Google Scholar]
- Zhe, L.; Han, X.; Lu, L.; Ouyang, M. Temperature characteristics of power LiFePO4 batteries. J. Mech. Eng. 2011, 47, 115–120. [Google Scholar]
- Gao, H.; Lv, F.; Wei, X.; Dai, H. Research of Li-ion battery’s energy efficiency. Chin. J. Power Sources 2013, 12, 2132–2134. [Google Scholar]
- Bernardi, D.; Pawlikowski, E.; Newman, J. A general energy balance for battery systems. J. Electrochem. Soc. 1985, 132, 5–12. [Google Scholar] [CrossRef]
- Damay, N.; Forgez, C.; Bichat, M.P.; Friedrich, G. Thermal modeling of large prismatic LiFePO4/graphite battery. Coupled thermal and heat generation models for characterization and simulation. J. Power Sources 2015, 283, 37–45. [Google Scholar] [CrossRef]
- Johnson, V.H. Battery performance models in ADVISOR. J. Power Sources 2002, 110, 321–329. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T. A critical review of thermal issues in lithium-ion batteries. J. Electrochem. Soc. 2011, 158, 1–25. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, J.; Li, Z.; Wu, B.; Nie, Z.; Sun, Y.; An, F.; Wu, N. Comparison and validation of methods for estimating heat generation rate of large-format lithium-ion batteries. J. Therm. Anal. Calorim. 2014, 117, 447–461. [Google Scholar] [CrossRef]
- Steffke, K.W.; Spigno, C.; Bezzina, C. Li-ion air-cooled battery system interactions with the vehicle HVAC system. In Proceedings of the SAE 2013 World Congress & Exhibition, Detroit, MI, USA, 16–18 April 2013.
- Pesaran, A.; Keyser, M.; Burch, S. An approach for designing thermal management systems for electric and hybrid vehicle battery packs. In Proceedings of the 1999 Vehicle Thermal Management Systems Conference, London, UK, 24–27 May 1999.
- Hallaj, S.A.; Maleki, H.; Hong, J.S.; Selman, J.R. Thermal modeling and design considerations of lithium-ion batteries. J. Power Sources 1999, 83, 1–8. [Google Scholar] [CrossRef]
- Jaguemont, J.; Boulon, L.; Venet, P.; Dube, Y. Lithium-ion battery aging experiments at subzero temperatures and model development for capacity fade estimation. IEEE Trans. Vehar. Technol. 2016, 65, 4328–4343. [Google Scholar] [CrossRef]
- Ehsani, M.; Gao, Y.; Emadi, A. Modern Electric, Hybrid Electric, and Fuel Cell Vehicles Fundamentals, Theory, and Design, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 89–362. [Google Scholar]
- Deb, K.; Pratap, A.; Agarwal, S.; Meyarivan, T. A fast and elitist multi-objective genetic algorithm: NSGA-II. IEEE Trans. Evol. Comput. 2002, 6, 182–197. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, J.; Li, J. Study on the special thermal management system of extended-range EV. Auto Electr. Parts 2013, 12, 5–8. [Google Scholar]
- Yokoyama, A.; Osaka, T.; Imanishi, Y.; Sekiya, S. Thermal management system for electric vehicles. SAE Int. J. Mater. Manuf. 2011, 4, 1277–1285. [Google Scholar] [CrossRef]
- Zheng, Y.; Ouyang, M.; Lu, L.; Li, J. Understanding aging mechanisms in lithium-ion battery packs: From cell capacity loss to pack capacity evolution. J. Power Sources 2015, 278, 287–295. [Google Scholar] [CrossRef]






| Parameters | Value or Specification |
|---|---|
| Nominal voltage | 3.2 V |
| Cathode material | LiFePO4 |
| Anode material | Graphite |
| Nominal capacity | 180 Ah, 576 Wh |
| Size | 282 mm × 182 mm × 71 mm |
| Mass | 5.8 kg |
| Charge cut-off voltage | 3.8 V |
| Discharge cut-off voltage | 2.8 V |
| Operating temperature | −30~60 ℃ |
| Specific heat capacity | 854 J/(kg·K) |
| Parameters | Value |
|---|---|
| Vehicle mass, | 13,946 kg |
| Vehicle size | 11983 mm × 2250 mm × 3720 mm |
| Final drive ratio, | 3.7 |
| Gearbox ratio, | 3.757/1.969 |
| Wheel radius, | 0.506 m |
| Gravity acceleration, | 9.8 |
| Rolling resistance coefficient, | 0.013 |
| Air drag coefficient, | 0.7 |
| Front area, | 9.5 |
| Motor efficiency, | 85% |
| Transmission efficiency, | 88% |
| Motor inverter efficiency | 90% |
| Test Items | Test 1 Battery without Heating (Test/Simulation) | Test 2 Battery Heated to 2 °C (Test/Simulation) |
|---|---|---|
| Initial SOC | 20%/20% | 20%/20% |
| SOC after charging | 100%/100% | 100%/100% |
| SOC after driving | 72.07%/74.75% | 73.71%/75.75% |
| Energy consumption of preheating () | 0/0 | 14.38/13.29 kWh |
| Energy loss of charging () | 12.67/12.55 kWh | 5.31/5.24 kWh |
| Energy consumption of driving cycles () | 28.96/26.18 kWh | 27.26/25.14 kWh |
| Battery fade ratio () | 0.023%/0.022% | 0.015%/0.014% |
| Vehicle operation cost () | 143.49/141.00 $ | 93.78/88.74 $ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, T.; Min, H.; Yu, Y.; Zhao, Z.; Xu, T.; Chen, Y.; Li, X.; Zhang, C. An Optimized Energy Management Strategy for Preheating Vehicle-Mounted Li-ion Batteries at Subzero Temperatures. Energies 2017, 10, 243. https://doi.org/10.3390/en10020243
Zhu T, Min H, Yu Y, Zhao Z, Xu T, Chen Y, Li X, Zhang C. An Optimized Energy Management Strategy for Preheating Vehicle-Mounted Li-ion Batteries at Subzero Temperatures. Energies. 2017; 10(2):243. https://doi.org/10.3390/en10020243
Chicago/Turabian StyleZhu, Tao, Haitao Min, Yuanbin Yu, Zhongmin Zhao, Tao Xu, Yang Chen, Xinyong Li, and Cong Zhang. 2017. "An Optimized Energy Management Strategy for Preheating Vehicle-Mounted Li-ion Batteries at Subzero Temperatures" Energies 10, no. 2: 243. https://doi.org/10.3390/en10020243






