Optimization of a Bubbling Fluidized Bed Plant for Low-Temperature Gasification of Biomass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gasification Equipment
2.2. Biomass Characterization
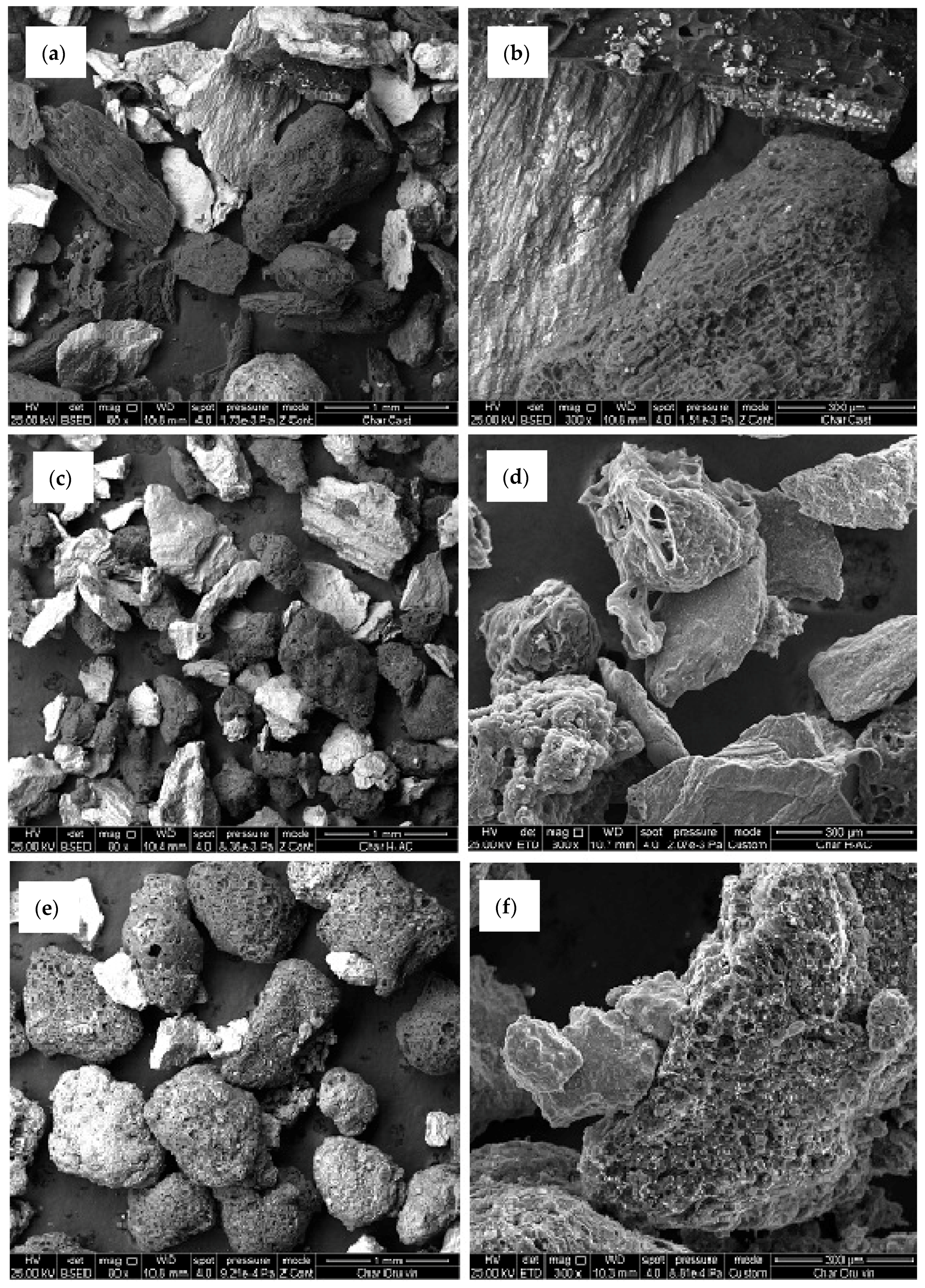
2.3. Char Samples
3. Results and Discussion
3.1. Biomass Analysis
3.2. Preliminary Selection of Operation Conditions
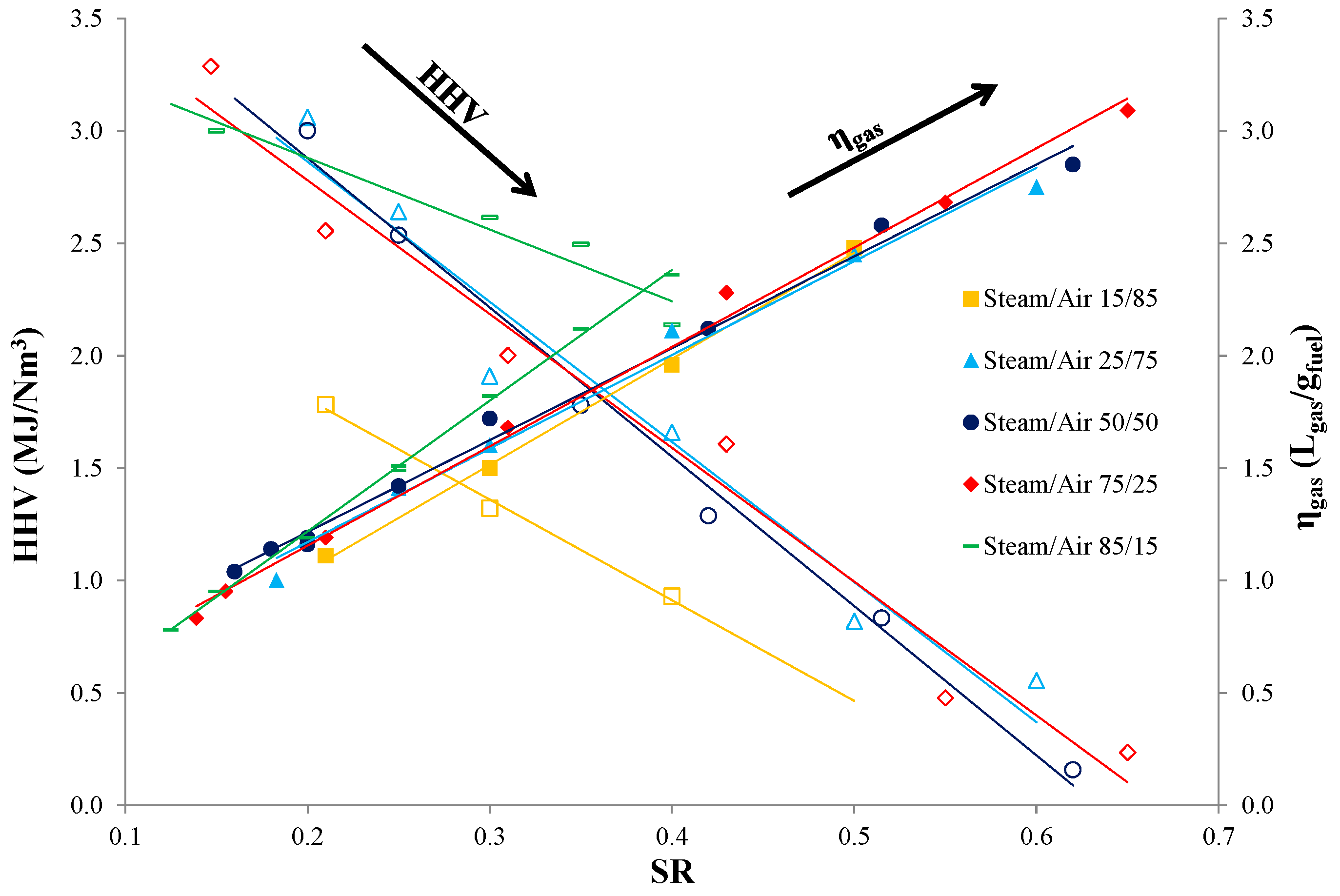
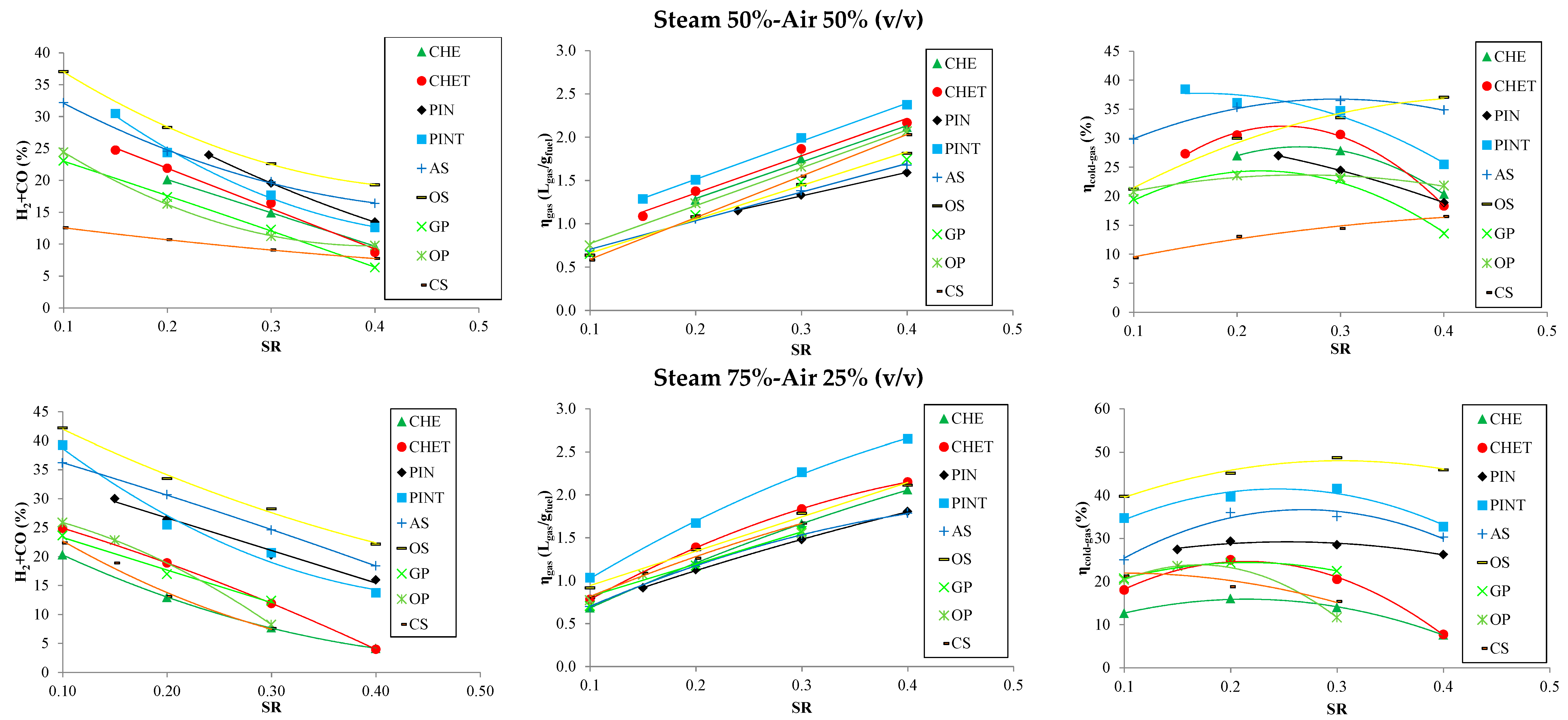
3.3. Effect of SR and Steam/Air Ratio on the Product Gas Properties
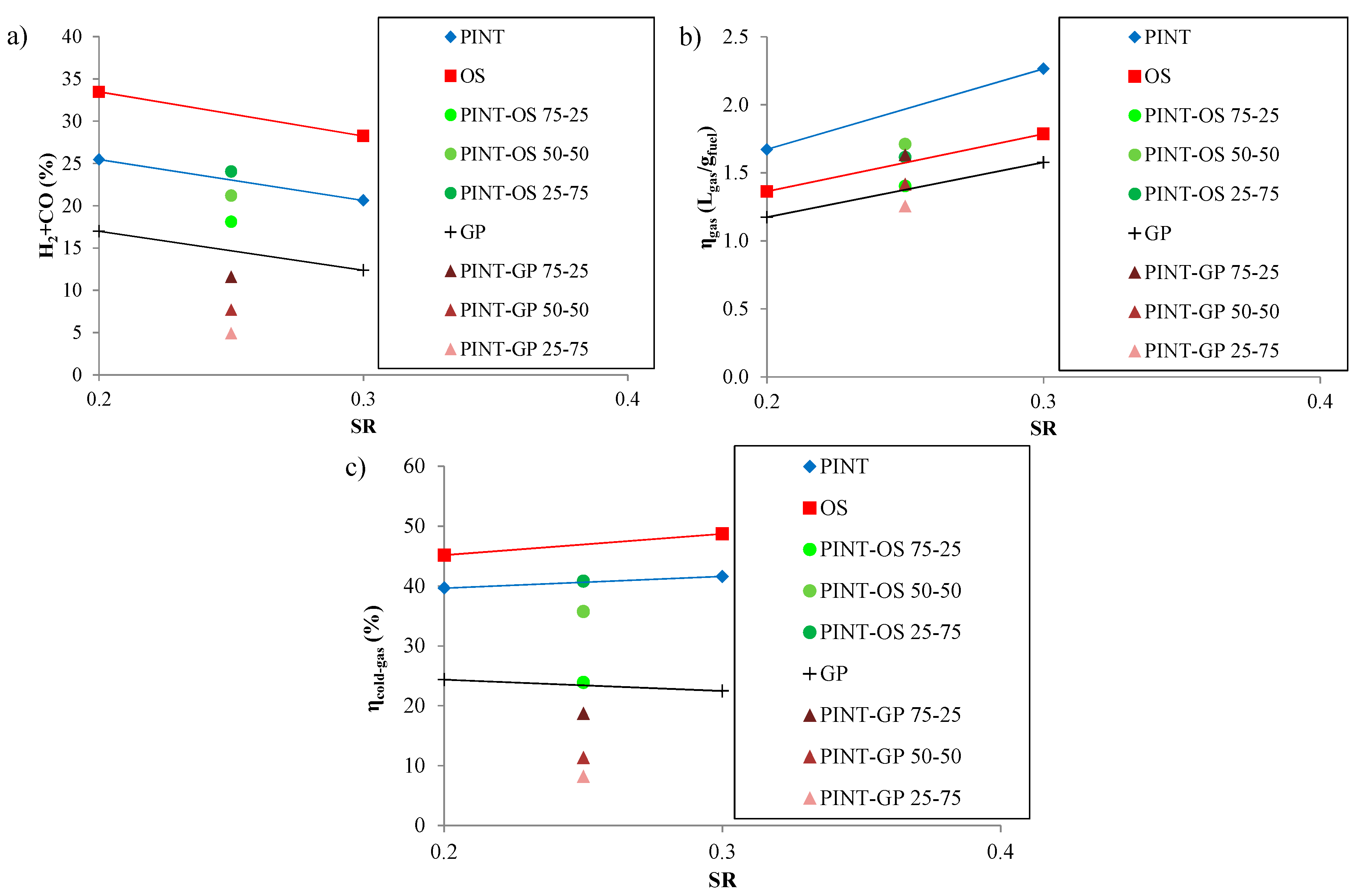
3.4. Selection of Optimum Conditions and Biomass Mixtures
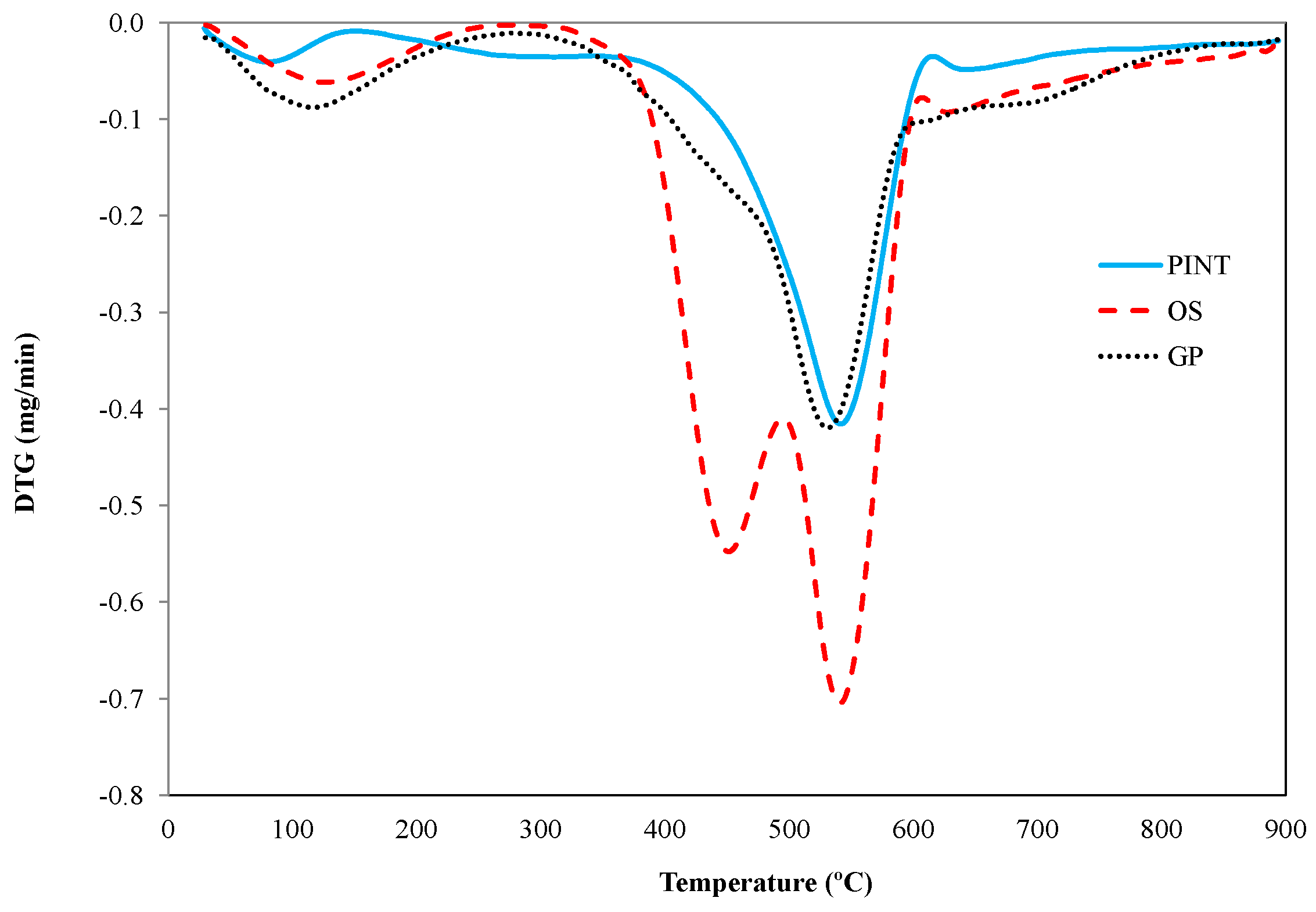
3.5. Chars Study
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- European Commission. Communication from the Commission to the European Parliament and the Council; EC: London, UK, 2015. [Google Scholar]
- Pacesila, M.; Burcea, S.G.; Colesca, S.E. Analysis of renewable energies in European Union. Renew. Sustain. Energy Rev. 2016, 56, 156–170. [Google Scholar] [CrossRef]
- Bartolini, F.; Angelini, L.G.; Brunori, G.; Gava, O. Impacts of the CAP 2014–2020 on the agroenergy sector in Tuscany, Italy. Energies 2015, 8, 1058–1079. [Google Scholar] [CrossRef]
- International Energy Agency. Energy Policies of IEA Countries. 2015. Available online: http://www.iea.org/countries/membercountries/spain/ (accessed on 3 February 2017).
- Siedlecki, M.; de Jong, W.; Verkooijen, A.H.M. Fluidized bed gasification as a mature and reliable technology for the production of bio-syngas and applied in the production of liquid transportation fuels—A review. Energies 2011, 4, 389–434. [Google Scholar] [CrossRef]
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Characterization of Spanish biomass wastes for energy use. Bioresour. Technol. 2012, 103, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Mudhoo, A.; Kumar, S. Effects of heavy metals as stress factors on anaerobic digestion processes and biogas production from biomass. Int. J. Environ. Sci. Technol. 2013, 10, 1383–1398. [Google Scholar] [CrossRef]
- Wu, T.Y.; Hay, J.X.W.; Kong, L.B.; Juan, J.C.; Jahim, J.M. Recent advances in reuse of waste material as substrate to produce biohydrogen by purple non-sulfur (PNS) bacteria. Renew. Sustain. Energy Rev. 2012, 16, 3117–3122. [Google Scholar] [CrossRef]
- Meng, X.; de Jong, W.; Fu, N.; Verkooijen, A.H.M. Biomass gasification in a 100 kWth steam-oxygen blown circulating fluidized bed gasifier: Effects of operational conditions on product gas distribution and tar formation. Biomass Bioenergy 2011, 35, 2910–2924. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical biomass gasification: A review of the current status of the technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Alipour Moghadam, R.; Yusup, S.; Azlina, W.; Nehzati, S.; Tavasoli, A. Investigation on syngas production via biomass conversion through the integration of pyrolysis and air–steam gasification processes. Energy Convers. Manag. 2014, 87, 670–675. [Google Scholar] [CrossRef]
- Dudyński, M.; van Dyk, J.C.; Kwiatkowski, K.; Sosnowska, M. Biomass gasification: Influence of torrefaction on syngas production and tar formation. Fuel Process. Technol. 2015, 131, 203–212. [Google Scholar] [CrossRef]
- Wan Ab Karim Ghani, W.A.; Moghadam, R.A.; Mohd Salleh, M.A.; Alias, A.B. Air gasification of agricultural waste in a fluidized bed gasifier: Hydrogen production performance. Energies 2009, 2, 258–268. [Google Scholar] [CrossRef]
- Skoulou, V.; Koufodimos, G.; Samaras, Z.; Zabaniotou, A. Low temperature gasification of olive kernels in a 5-kW fluidized bed reactor for H2-rich producer gas. Int. J. Hydrog. Energy 2008, 33, 6515–6524. [Google Scholar] [CrossRef]
- Hernández, J.J.; Aranda, G.; Barba, J.; Mendoza, J.M. Effect of steam content in the air–steam flow on biomass entrained flow gasification. Fuel Process. Technol. 2012, 99, 43–55. [Google Scholar] [CrossRef]
- Balat, M.; Balat, M.; Kirtay, E.; Balat, H. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 2: Gasification systems. Energy Convers. Manag. 2009, 50, 3158–3168. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S. Interpretation of biomass gasification yields regarding temperature intervals under nitrogen–steam atmosphere. Fuel Process. Technol. 2007, 88, 417–425. [Google Scholar] [CrossRef]
- Sharma, A.M.; Kumar, A.; Huhnke, R.L. Effect of steam injection location on syngas obtained from an air–steam gasifier. Fuel 2014, 116, 388–394. [Google Scholar] [CrossRef]
- Guizani, C.; Jeguirim, M.; Gadiou, R.; Escudero Sanz, F.J.; Salvador, S. Biomass char gasification by H2O, CO2 and their mixture: Evolution of chemical, textural and structural properties of the chars. Energy 2016, 112, 133–145. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Wu, W.; Chen, W.-H. Gasification performances of raw and torrefied biomass in a downdraft fixed bed gasifier using thermodynamic analysis. Fuel 2014, 117, 1231–1241. [Google Scholar] [CrossRef]
- Chen, W.H.; Chen, C.J.; Hung, C.I.; Shen, C.H.; Hsu, H.W. A comparison of gasification phenomena among raw biomass, torrefied biomass and coal in an entrained-flow reactor. Appl. Energy 2013, 112, 421–430. [Google Scholar] [CrossRef]
- Zaccariello, L.; Mastellone, M.L. Fluidized-bed gasification of plastic waste, wood, and their blends with coal. Energies 2015, 8, 8052–8068. [Google Scholar] [CrossRef]
- Makwana, J.P.; Joshi, A.K.; Athawale, G.; Singh, D.; Mohanty, P. Air gasification of rice husk in bubbling fluidized bed reactor with bed heating by conventional charcoal. Bioresour. Technol. 2015, 178, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Rapagnà, S.; Latif, A. Steam gasification of almond shells in a fluidised bed reactor: The influence of temperature and particle size on product yield and distribution. Biomass Bioenergy 1997, 12, 281–288. [Google Scholar] [CrossRef]
- Kulkarni, A.; Baker, R.; Abdoulmomine, N.; Adhikari, S.; Bhavnani, S. Experimental study of torrefied pine as a gasification fuel using a bubbling fluidized bed gasifier. Renew. Energy 2016, 93, 460–468. [Google Scholar] [CrossRef]
- Mohd Salleh, M.A.; Kisiki, N.H.; Yusuf, H.M.; Ab Karim Ghani, W.A.W. Gasification of Biochar from Empty Fruit Bunch in a Fluidized Bed Reactor. Energies 2010, 3, 1344–1352. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.N.; Carolino, C.; Miranda, M.; Abelha, P.; Direito, D.; Dohrup, J.; Sørensen, H.R.; Girio, F. Effects of experimental conditions and of addition of natural minerals on syngas production from lignin by oxy-gasification: Comparison of bench- and pilot scale gasification. Fuel 2015, 140, 62–72. [Google Scholar] [CrossRef]
- Gil, J.; Corella, J.; Aznar, M.P.; Caballero, M.A. Biomass gasification in atmospheric and bubbling fluidized bed: Effect of the type of gasifying agent on the product distribution. Biomass Bioenergy 1999, 17, 389–403. [Google Scholar] [CrossRef]
- Lim, Y.; Lee, U.-D. Quasi-equilibrium thermodynamic model with empirical equations for air–steam biomass gasification in fluidized-beds. Fuel Process. Technol. 2014, 128, 199–210. [Google Scholar] [CrossRef]
- Gucho, E.M.; Shahzad, K.; Bramer, E.A.; Akhtar, N.A.; Brem, G. Experimental study on dry torrefaction of beech wood and miscanthus. Energies 2015, 8, 3903–3923. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lu, K.-M.; Tsai, C.-M. An experimental analysis on property and structure variations of agricultural wastes undergoing torrefaction. Appl. Energy 2012, 100, 318–325. [Google Scholar] [CrossRef]
- Chew, J.J.; Doshi, V. Recent advances in biomass pretreatment—Torrefaction fundamentals and technology. Renew. Sustain. Energy Rev. 2011, 15, 4212–4222. [Google Scholar] [CrossRef]
- Arias, B.; Pevida, C.; Fermoso, J.; Plaza, M.G.; Rubiera, F.; Pis, J.J. Influence of torrefaction on the grindability and reactivity of woody biomass. Fuel Process. Technol. 2008, 89, 169–175. [Google Scholar] [CrossRef]
- Tapasvi, D.; Khalil, R.; Skreiberg, Ø.; Tran, K.Q.; Grønli, M. Torrefaction of Norwegian birch and spruce: An experimental study using macro-TGA. Energy Fuels 2012, 26, 5232–5240. [Google Scholar] [CrossRef]
- Chen, W.-H.; Kuo, P.-C. A study on torrefaction of various biomass materials and its impact on lignocellulosic structure simulated by a thermogravimetry. Energy 2010, 35, 2580–2586. [Google Scholar] [CrossRef]
- Gil, M.V.; García, R.; Pevida, C.; Rubiera, F. Grindability and combustion behavior of coal and torrefied biomass blends. Bioresour. Technol. 2015, 191, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Narváez, I.; Orío, A.; Aznar, M.P.; Corella, J. Biomass gasification with air in an atmospheric bubbling fluidized bed. Ind. Eng. Chem. Res. 1996, 35, 2110–2120. [Google Scholar] [CrossRef]
- Telmo, C.; Lousada, J.; Moreira, N. Proximate analysis, backwards stepwise regression between gross calorific value, ultimate and chemical analysis of wood. Bioresour. Technol. 2010, 101, 3808–3815. [Google Scholar] [CrossRef] [PubMed]
- Obernberger, I.; Brunner, T.; Bärnthaler, G. Chemical properties of solid biofuels—Significance and impact. Biomass Bioenergy 2006, 30, 973–982. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, M.; Jin, B.; Huang, Y.; Zhou, H. High-temperature air/steam-blown gasification of coal in a pressurized spout-fluid bed. Energy Fuels 2006, 20, 715–720. [Google Scholar] [CrossRef]
- Weiland, F.; Nordwaeger, M.; Olofsson, I.; Wiinikka, H.; Nordin, A. Entrained flow gasification of torrefied wood residues. Fuel Process. Technol. 2014, 125, 51–58. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Patil, K.; Bellmer, D.; Wang, D.; Yuan, W.; Huhnke, R.L. Effects of biomass feedstocks and gasification conditions on the physiochemical properties of char. Energies 2013, 6, 3972–3986. [Google Scholar] [CrossRef]
- Gómez-Barea, A.; Arjona, R.; Ollero, P. Pilot-plant gasification of olive stone: A technical assessment. Energy Fuels 2005, 19, 598–605. [Google Scholar] [CrossRef]
- De Micco, G.; Fouga, G.G.; Bohé, A.E. Coal gasification studies applied to H2 production. Int. J. Hydrogen Energy 2010, 35, 6012–6018. [Google Scholar] [CrossRef]
- Zambon, I.; Colosimo, F.; Monarca, D.; Cecchini, M.; Gallucci, F.; Proto, A.; Lord, R.; Colantoni, A. An innovative agro-forestry supply chain for residual biomass: Physicochemical characterisation of biochar from olive and hazelnut pellets. Energies 2016, 9, 526. [Google Scholar] [CrossRef] [Green Version]
- Bouraoui, Z.; Jeguirim, M.; Guizani, C.; Limousy, L.; Dupont, C.; Gadiou, R. Thermogravimetric study on the influence of structural, textural and chemical properties of biomass chars on CO2 gasification reactivity. Energy 2015, 88, 703–710. [Google Scholar] [CrossRef]

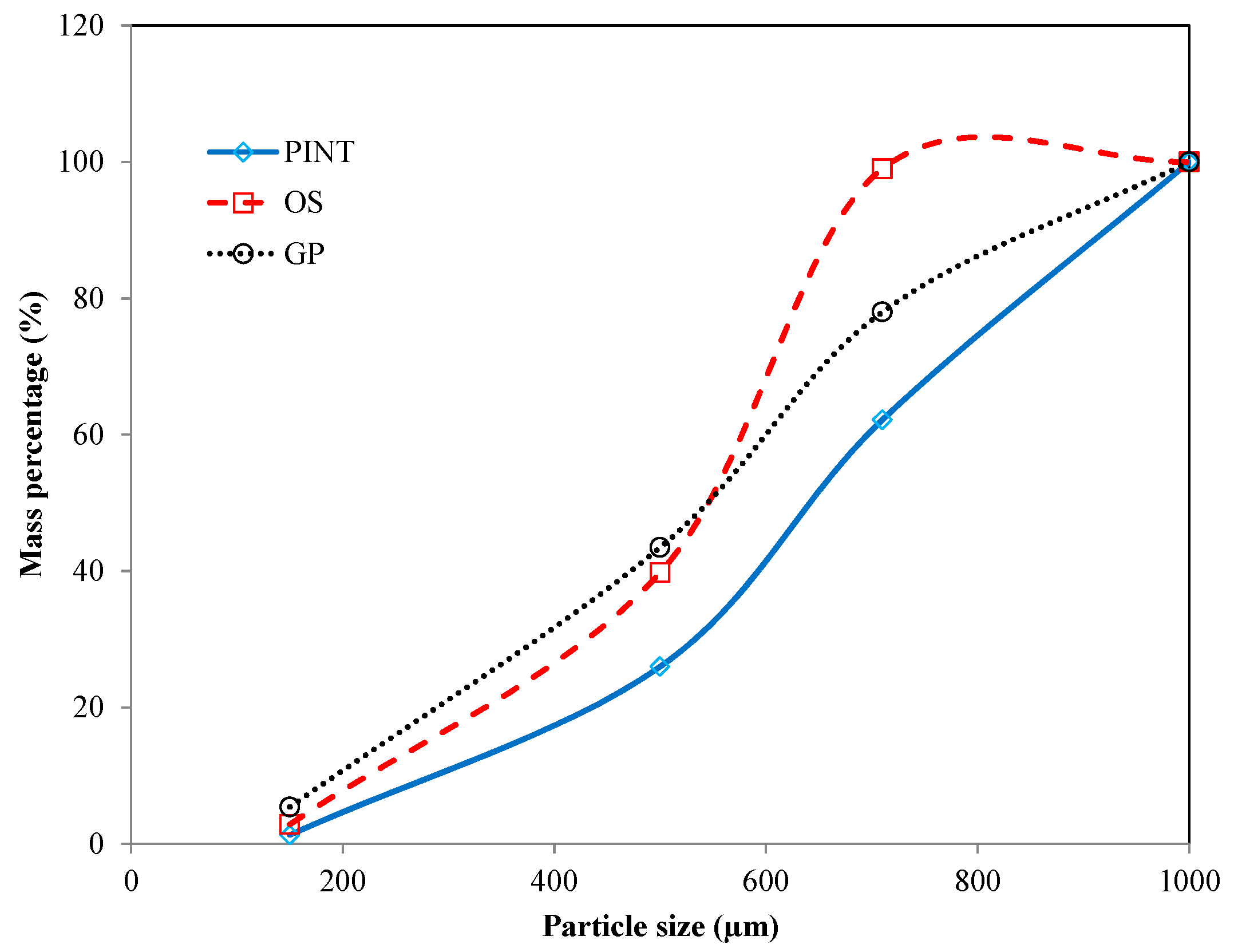
| Samples | Ultimate Analysis (wt %, db) | Proximate Analysis (wt %, db) | HHV (MJ/kg, db) | BD (kg/m3) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | N | H | S | O | Ash | VM * | FC | |||
| CHE | 50.2 | 0.3 | 5.6 | 0.01 | 43.4 | 0.5 | 81.2 | 18.3 | 19.1 | 296 |
| CHET | 51.3 | 0.4 | 5.4 | 0.02 | 42.6 | 0.3 | 80.0 | 19.7 | 19.6 | 374 |
| PIN | 51.0 | 0.3 | 6.0 | 0.02 | 42.2 | 0.4 | 85.1 | 14.5 | 19.9 | 226 |
| PINT | 56.1 | 0.4 | 5.7 | 0.01 | 37.4 | 0.5 | 77.1 | 22.4 | 22.0 | 265 |
| AS | 49.4 | 0.3 | 5.9 | 0.05 | 42.9 | 1.5 | 78.9 | 19.6 | 19.6 | 655 |
| OS | 51.2 | 0.3 | 6.0 | 0.03 | 41.9 | 0.6 | 81.5 | 17.9 | 20.5 | 781 |
| GP | 45.5 | 1.8 | 5.1 | 0.17 | 34.7 | 12.7 | 67.6 | 19.7 | 18.7 | 772 |
| OP | 49.4 | 1.6 | 5.4 | 0.12 | 37.4 | 6.2 | 72.5 | 21.3 | 20.3 | 772 |
| CS | 48.0 | 2.7 | 5.9 | 0.21 | 35.3 | 7.9 | 70.4 | 21.7 | 19.1 | 490 |
| Samples | 50% Steam-50% Air (v/v) | 75% Steam-25% Air (v/v) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SR | SBR | CO | CH4 | H2 | HHV | SR | SBR | CO | CH4 | H2 | HHV | ||
| CHE | 0.1 | 1.1 | 11.4 | 2.4 | 8.9 | 3.5 | |||||||
| 0.2 | 0.7 | 11.1 | 3.8 | 9.0 | 4.1 | 0.2 | 2.2 | 6.9 | 2.2 | 6.0 | 2.5 | ||
| 0.3 | 1.1 | 8.2 | 2.9 | 6.8 | 3.0 | 0.3 | 3.2 | 4.3 | 1.6 | 3.4 | 1.6 | ||
| 0.4 | 1.4 | 5.7 | 1.5 | 4.0 | 1.8 | 0.4 | 4.3 | 3.7 | 0.5 | 0.4 | 0.7 | ||
| CHET | 0.15 | 0.5 | 14.4 | 4.5 | 10.3 | 4.9 | 0.1 | 1.1 | 10.9 | 3.4 | 14.0 | 4.5 | |
| 0.2 | 0.7 | 12.6 | 3.9 | 9.2 | 4.3 | 0.2 | 2.2 | 9.4 | 2.8 | 9.5 | 3.5 | ||
| 0.3 | 1.1 | 9.5 | 2.9 | 6.9 | 3.2 | 0.3 | 3.3 | 5.9 | 1.7 | 6.0 | 2.2 | ||
| 0.4 | 1.5 | 5.5 | 1.4 | 3.3 | 1.7 | 0.4 | 4.4 | 2.6 | 0.5 | 1.4 | 0.7 | ||
| PIN | 0.15 | 1.1 | 15.4 | 5.4 | 14.6 | 6.0 | |||||||
| 0.24 | 0.6 | 13.4 | 4.1 | 10.6 | 4.7 | 0.2 | 1.5 | 13.4 | 4.6 | 13.0 | 5.2 | ||
| 0.3 | 0.8 | 10.5 | 2.9 | 9.1 | 3.6 | 0.3 | 2.3 | 10.0 | 3.2 | 10.3 | 3.8 | ||
| 0.4 | 1.0 | 7.2 | 1.6 | 6.3 | 2.4 | 0.4 | 3.0 | 7.7 | 2.2 | 8.3 | 2.9 | ||
| PINT | 0.15 | 0.6 | 18.5 | 6.2 | 11.9 | 6.3 | 0.1 | 1.2 | 21.0 | 5.3 | 18.2 | 7.1 | |
| 0.2 | 0.8 | 14.4 | 4.9 | 9.9 | 5.1 | 0.2 | 2.3 | 11.9 | 4.4 | 13.6 | 5.0 | ||
| 0.3 | 1.2 | 10.1 | 3.6 | 7.5 | 3.7 | 0.3 | 3.5 | 9.4 | 3.1 | 11.3 | 3.9 | ||
| 0.4 | 1.6 | 7.2 | 1.7 | 5.4 | 2.3 | 0.4 | 4.7 | 7.9 | 2.2 | 5.8 | 2.6 | ||
| AS | 0.1 | 0.2 | 19.7 | 11.1 | 12.5 | 8.5 | 0.1 | 0.7 | 16.1 | 6.1 | 20.1 | 7.0 | |
| 0.2 | 0.5 | 14.3 | 8.6 | 10.3 | 6.5 | 0.2 | 1.4 | 13.3 | 5.1 | 17.4 | 5.9 | ||
| 0.3 | 0.7 | 11.2 | 6.7 | 8.5 | 5.2 | 0.3 | 2.2 | 10.4 | 3.5 | 14.2 | 4.5 | ||
| 0.4 | 1.0 | 9.3 | 5.0 | 7.1 | 4.1 | 0.4 | 2.9 | 7.6 | 2.5 | 10.8 | 3.3 | ||
| OS | 0.1 | 0.3 | 25.0 | 5.3 | 12.0 | 6.8 | 0.1 | 0.8 | 23.5 | 8.9 | 18.7 | 8.9 | |
| 0.2 | 0.5 | 18.6 | 5.3 | 9.7 | 5.7 | 0.2 | 1.5 | 17.0 | 6.4 | 16.4 | 6.8 | ||
| 0.3 | 0.8 | 14.5 | 4.7 | 8.1 | 4.7 | 0.3 | 2.3 | 14.2 | 5.0 | 14.0 | 5.6 | ||
| 0.4 | 1.0 | 12.2 | 4.4 | 7.1 | 4.2 | 0.4 | 3.1 | 10.6 | 4.1 | 11.5 | 4.5 | ||
| GP | 0.1 | 0.3 | 11.1 | 6.7 | 12.0 | 5.6 | 0.1 | 0.9 | 9.2 | 6.3 | 14.4 | 5.5 | |
| 0.2 | 0.6 | 7.9 | 4.5 | 9.4 | 4.0 | 0.2 | 1.8 | 6.8 | 4.3 | 10.2 | 3.9 | ||
| 0.3 | 0.9 | 5.8 | 3.3 | 6.5 | 2.9 | 0.3 | 2.8 | 5.4 | 2.7 | 7.0 | 2.7 | ||
| 0.4 | 1.2 | 3.6 | 1.6 | 2.7 | 1.5 | ||||||||
| OP | 0.1 | 0.3 | 12.5 | 6.3 | 11.9 | 5.6 | 0.1 | 1.0 | 12.0 | 5.2 | 13.9 | 5.4 | |
| 0.2 | 0.7 | 8.6 | 4.5 | 7.7 | 3.9 | 0.15 | 1.6 | 9.9 | 4.0 | 12.9 | 4.5 | ||
| 0.3 | 1.1 | 6.4 | 3.2 | 4.8 | 2.7 | 0.3 | 3.2 | 5.0 | 1.2 | 3.2 | 1.5 | ||
| 0.4 | 1.4 | 6.2 | 2.3 | 3.6 | 2.1 | ||||||||
| CS | 0.1 | 0.3 | 6.7 | 3.7 | 5.9 | 3.1 | 0.1 | 1.1 | 8.3 | 5.5 | 14.1 | 5.0 | |
| 0.2 | 0.7 | 6.0 | 2.3 | 4.6 | 2.3 | 0.15 | 1.6 | 6.7 | 4.3 | 12.2 | 4.1 | ||
| 0.3 | 1.1 | 5.5 | 1.6 | 3.6 | 1.8 | 0.2 | 2.1 | 4.7 | 3.0 | 8.4 | 2.8 | ||
| 0.4 | 1.4 | 4.8 | 1.4 | 2.9 | 1.6 | 0.3 | 3.2 | 2.8 | 2.0 | 4.8 | 1.8 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Vázquez, M.P.; García, R.; Pevida, C.; Rubiera, F. Optimization of a Bubbling Fluidized Bed Plant for Low-Temperature Gasification of Biomass. Energies 2017, 10, 306. https://doi.org/10.3390/en10030306
González-Vázquez MP, García R, Pevida C, Rubiera F. Optimization of a Bubbling Fluidized Bed Plant for Low-Temperature Gasification of Biomass. Energies. 2017; 10(3):306. https://doi.org/10.3390/en10030306
Chicago/Turabian StyleGonzález-Vázquez, María Pilar, Roberto García, Covadonga Pevida, and Fernando Rubiera. 2017. "Optimization of a Bubbling Fluidized Bed Plant for Low-Temperature Gasification of Biomass" Energies 10, no. 3: 306. https://doi.org/10.3390/en10030306







