Thermal Stability of Modified Insulation Paper Cellulose Based on Molecular Dynamics Simulation
Abstract
:1. Introduction
2. Model Building and Parameter Setting
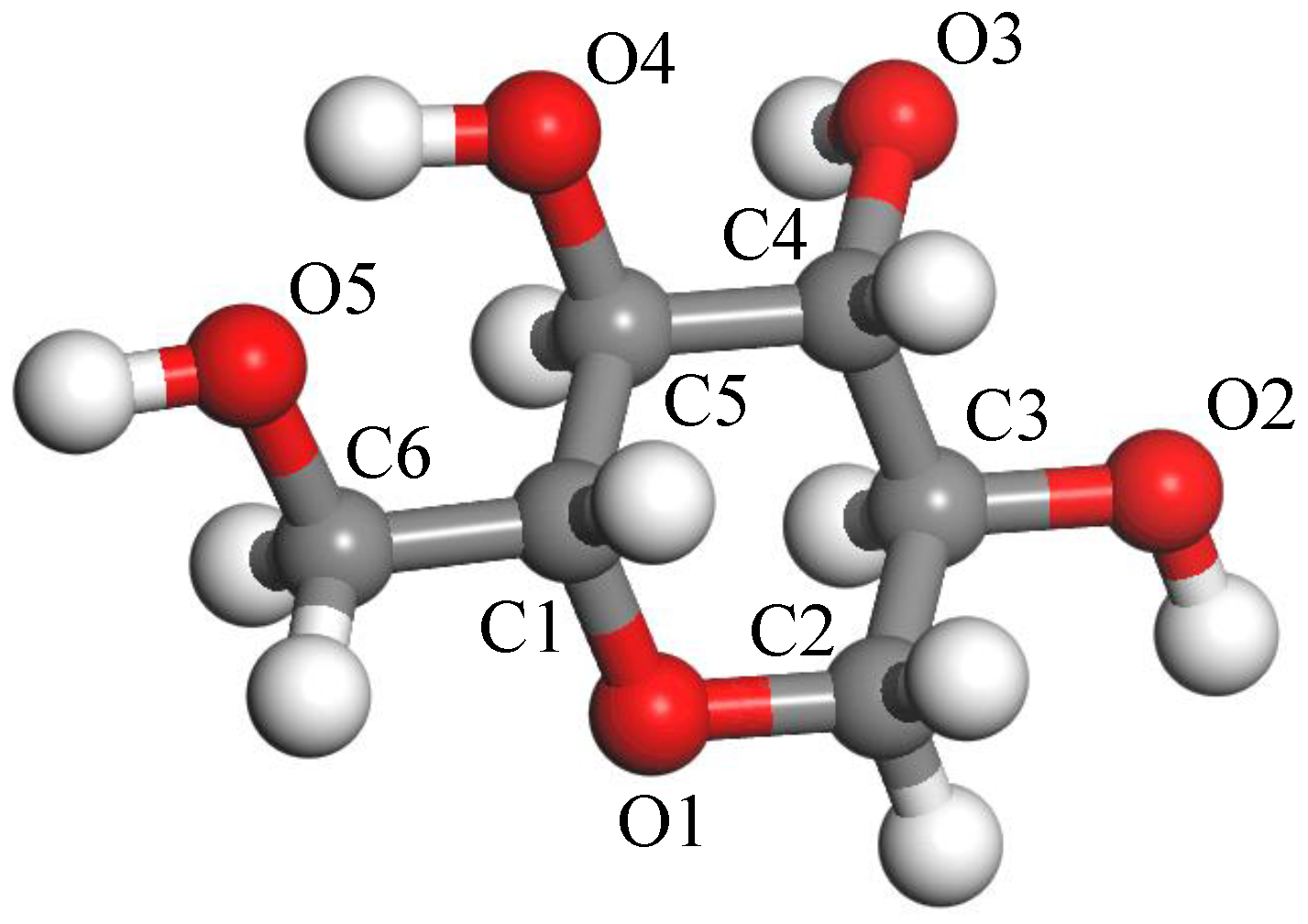
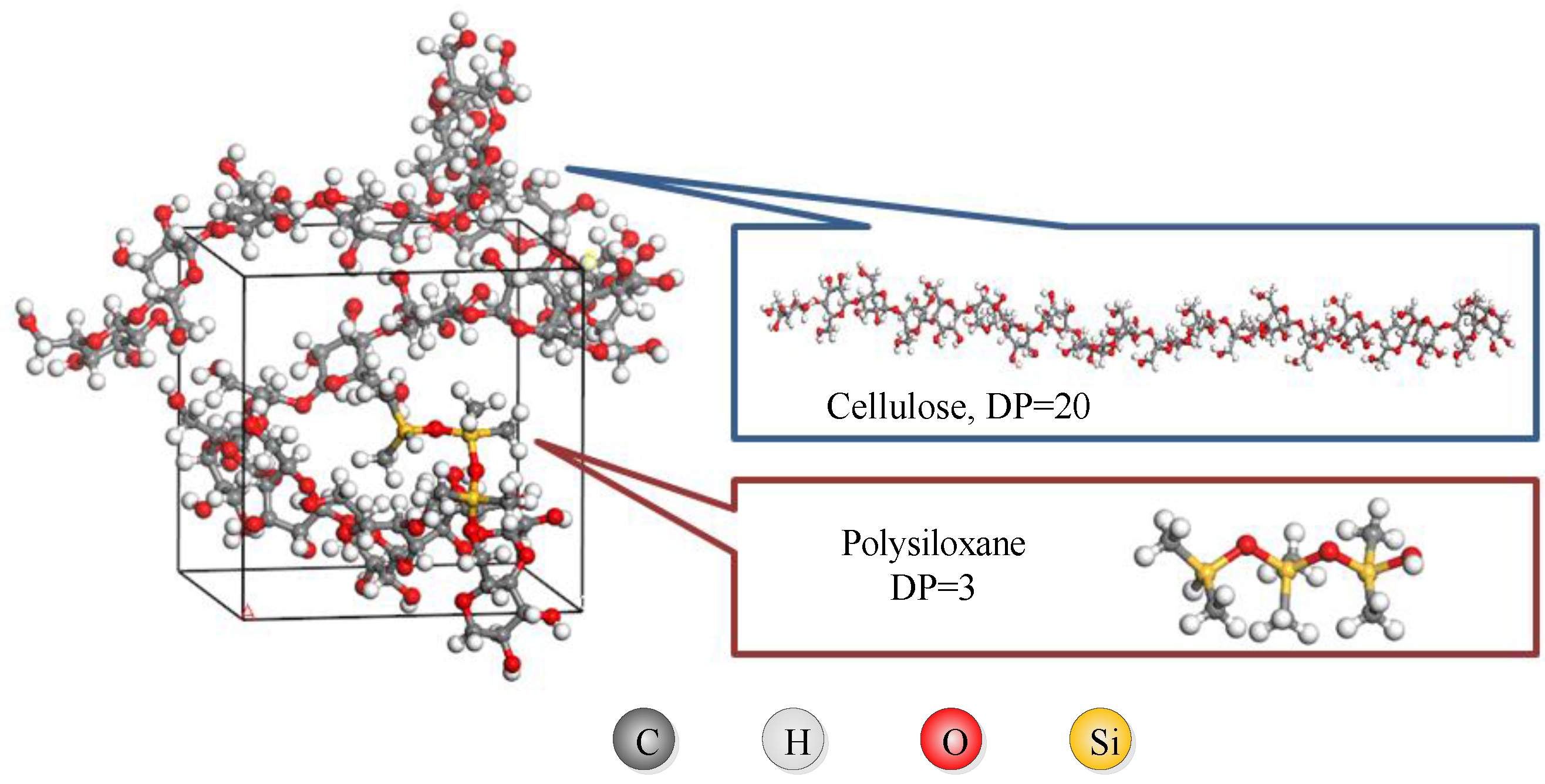
2.1. Model Building
2.2. Parameter Setting
3. Analysis of Simulation Results
3.1. Mechanical Properties
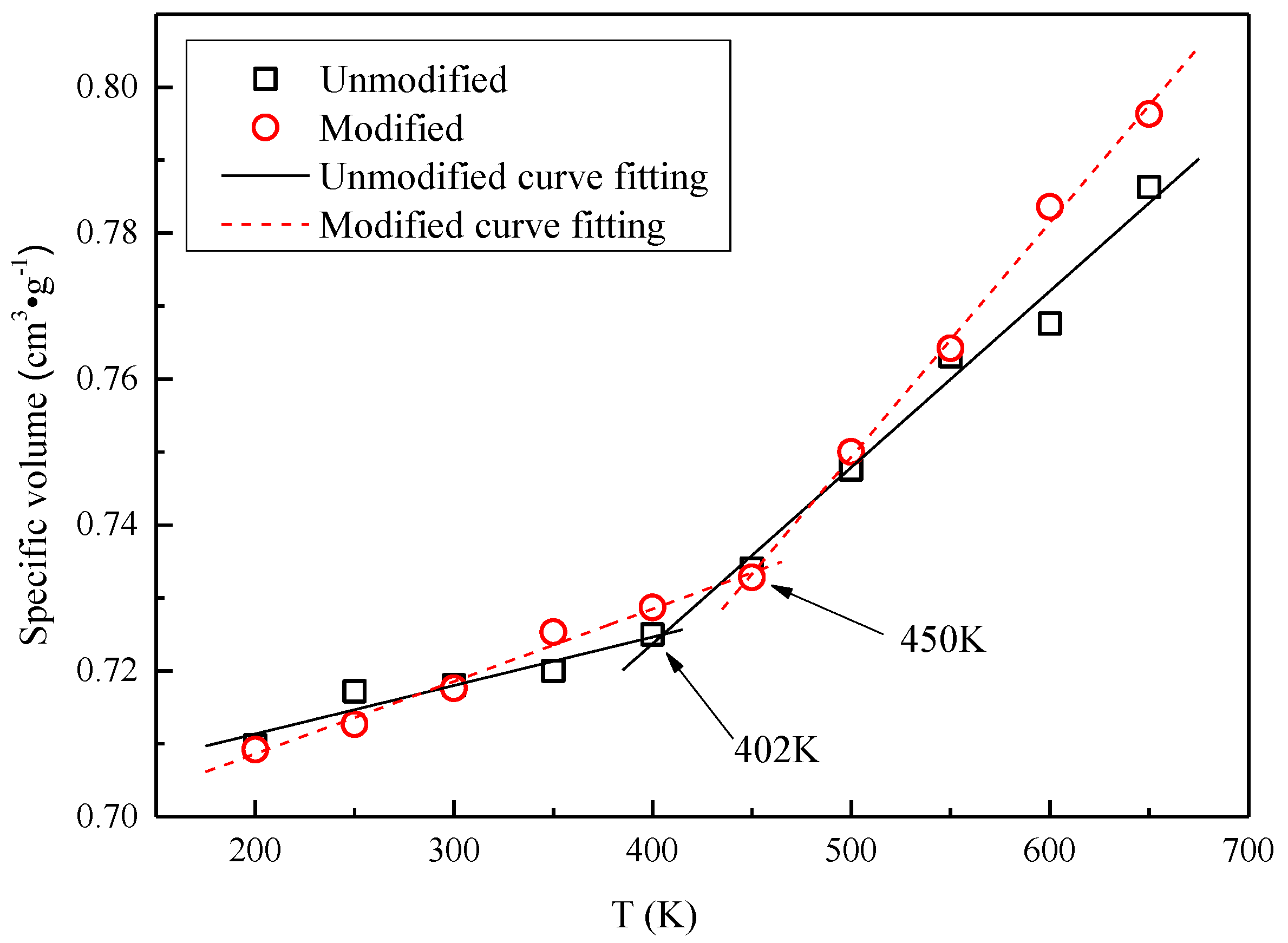
3.2. Glass Transition Temperature
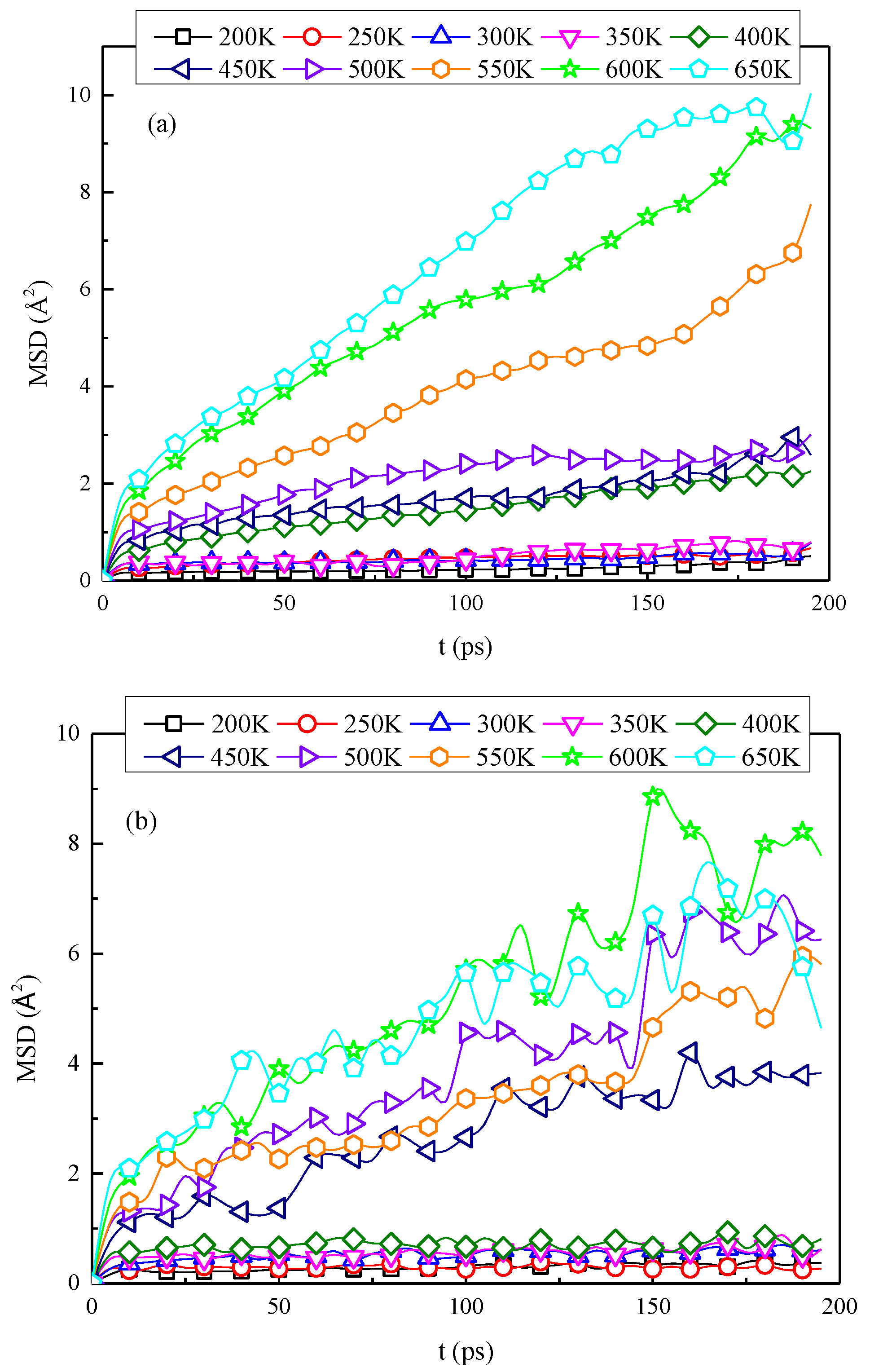
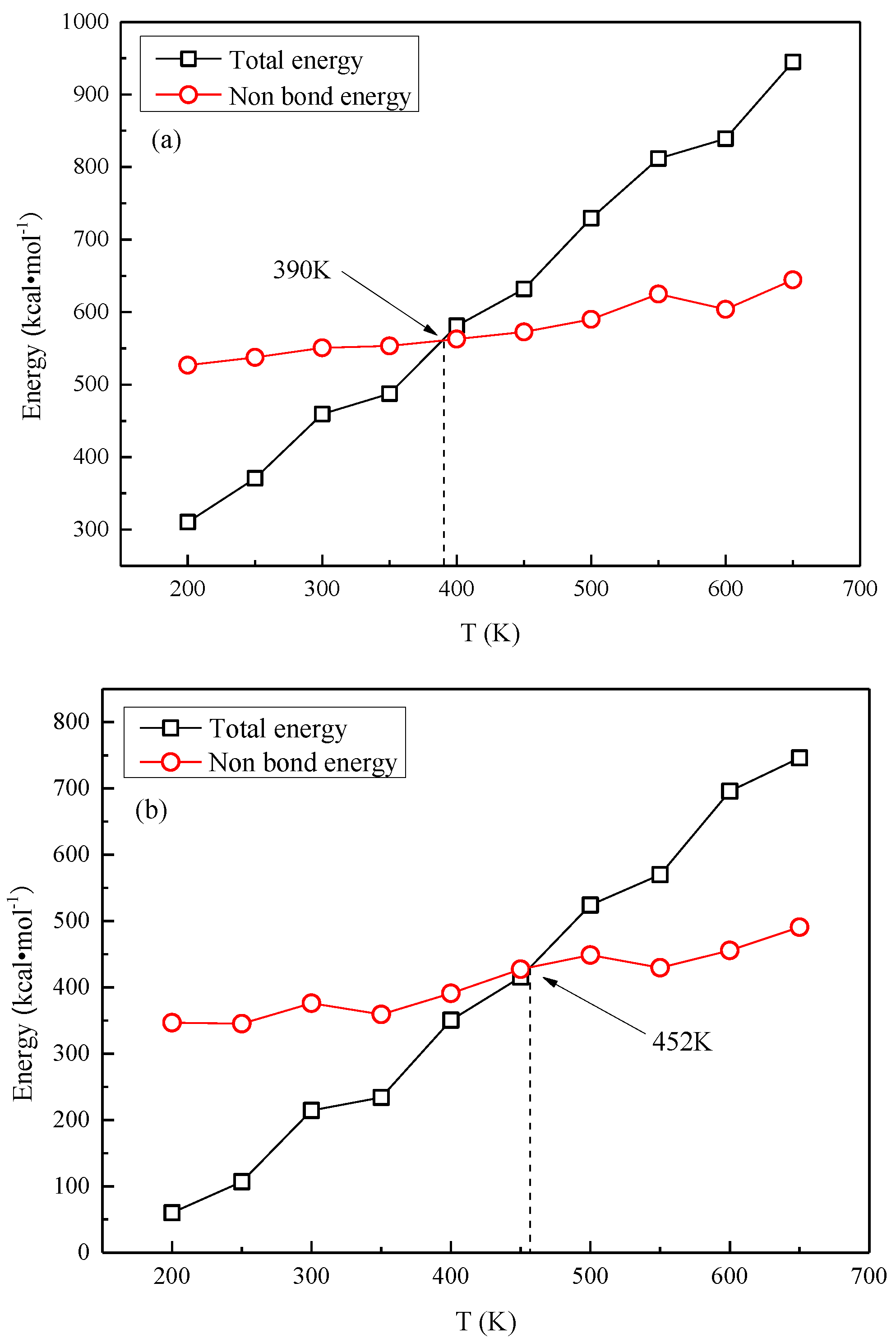
3.3. Glass Transition Temperature Analysis
4. Analysis and Discussion
4.1. Enhancement Mechanism
4.2. Mechanism of Effect of Polysiloxane on Chain Movement Intensity and Glass Transition Temperature of Cellulose
5. Conclusions
- (1)
- For the modified cellulose insulation paper, the anti-deformation energy of the cellulose is increased, and its mechanical properties are improved.
- (2)
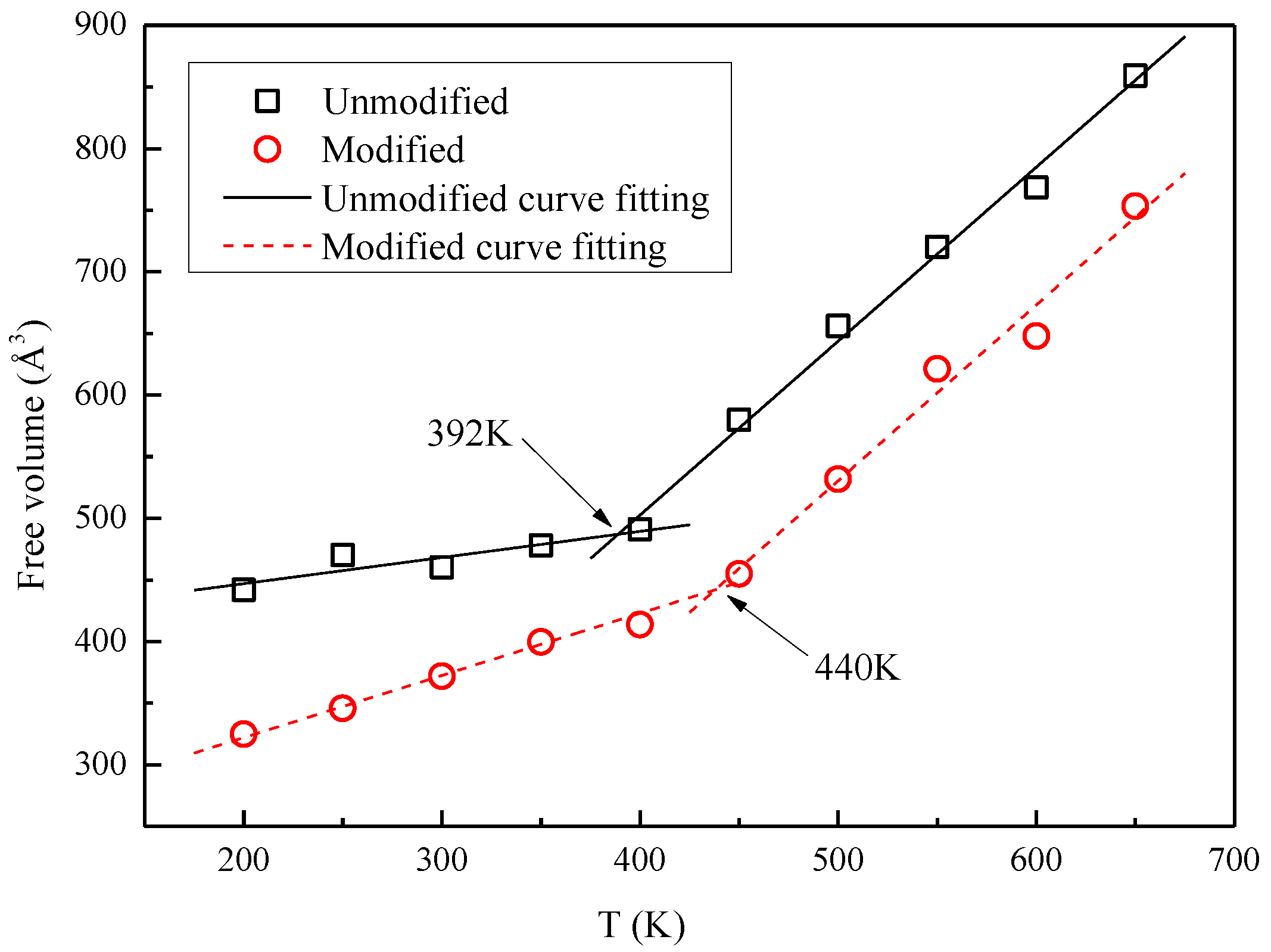
- Based on the specific volume method, the glass transition temperature of the modified cellulose model increased by 48 K than that of the unmodified model. When the temperature increases near the glass transition temperature, the mean square displacement of the two models appears to jump, and the chain movement of the insulation paper cellulose becomes intense.
- (3)
- The mechanism of the change in the mechanical properties of the model is discussed based on energy. The glass transition temperature range obtained from the free volume theory is basically the same as that obtained by the specific volume method. Therefore, the modification of insulation paper cellulose by polysiloxane grafting will greatly enhance the thermal stability of insulation paper.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ke, Y.C.; He, P.S. Macromolecule Physical Tutorial; Chemistry Industry Press: Beijing, China, 2006; pp. 157–161. [Google Scholar]
- Liu, R.Q. Basis of Cellulose Chemistry; Science Press: Beijing, China, 1985; pp. 115–123. [Google Scholar]
- Zhu, M.Z. Molecular Dynamics Study of Thermal Aging of Oil-impregnated Insulation Paper. Ph.D. Thesis, Chongqing Univeristy, Chongqing, China, April 2012. [Google Scholar]
- Yan, J.Y.; Wang, X.L.; Li, Q.M.; Zhou, Y.; Wang, Z.D.; Li, C.R. Molecular dynamics simulation on the pyrolysis of insulation paper. Proc. CSEE 2015, 35, 5941–5949. [Google Scholar]
- Yang, T. Molecular Dynamics Study on the Impact of Temperature and Electric Field on the Microscopic Properties of Oil-Immersed. Ph.D. Thesis, Chongqing Univeristy, Chongqing, China, May 2013. [Google Scholar]
- Wang, Y.Y.; Yang, T.; Liao, R.J.; Zhang, D.W.; Liu, Q.; Tian, M. Glass Transition in amorphous region of transformer insulation paper by molecular dynamics. High Volt. Eng. 2012, 31, 1199–1206. [Google Scholar]
- Li, X.M.; Pan, Q.; Lin, Y.D.; You, B.; Sun, Y.J. Synthesis and Characterization of epoxy/poly siloxane hybrid materials. J. Chongqing Univ. 2011, 34, 112–122. [Google Scholar]
- Zhao, S. Study on Preparation and Properties of Polysiloxane Modified Epoxy Encapsulating Material. Ph.D. Thesis, Harbin University of Science and Technology, Harbin, China, March 2014. [Google Scholar]
- Zhang, H.Z. Study on Polysiloxane Copolymerization Modified Epoxy Resin Capability. Ph.D. Thesis, Chongqing University, Chongqing, China, May 2008. [Google Scholar]
- Li, Y.W.; Shen, M.M.; Huang, H.Y.; Ma, Y.J.; Ha, C.Y. Synthesis and properties of polyphenylsilicone and polymethylphenylsilicone modified epoxy resins. Acta Polym. Sin. 2009, 11, 1086–1090. [Google Scholar] [CrossRef]
- Su, Q.Q.; Liu, W.Q.; Wang, W.R.; Liu, Y.F. Investigation on epoxy resin modified by diethoxydimethylsilane. Chem. Mater. Constr. 2008, 24, 23–25. [Google Scholar]
- Zhang, S.; Xie, J.L.; Deng, L.J. Study on organosilicon modified epoxy resin adhesive for heat-resistance. Mater. Rev. 2006, 20, 23–25. [Google Scholar]
- Guan, D.B.; Cai, Z.Y.; Fang, C.; Qiu, X.M.; Dou, Y.L. Preparation and properties of fluorinated silicone modified epoxy resin composite. Polym. Mater. Sci. Eng. 2015, 31, 120–125. [Google Scholar]
- Zong, J.P.; Zhang, Q.S.; Li, J.S.; Sun, H.F.; Yu, Y.T.; Liu, S.J.; Liu, Y.H. Comparative of polydimethylsiloxane modified graft and block polyurethane dispersions. Polym. Mater. Sci. Eng. 2011, 27, 62–65. [Google Scholar]
- Sun, W.F.; Wang, X. Molecular dynamics simulation study of polyimide/copper-nanoparticle composites. Acta Phys. Sin. 2013, 62, 186202. [Google Scholar]
- Lin, C.P.; Liu, X.J.; Rao, Z.H. Molecular dynamics simulation of the thermophysical properties and phase change behaviors of aluminum nanoparticles. Acta Phys. Sin. 2015, 64, 083601. [Google Scholar]
- Kopper, K.P.; Küpper, D.; Reeve, R.; Mitrelias, T.; Gunn, D.S.D.; Jenkins, S.J. Chemically selective modification of spin polarization in ultrathin ferromagnetic films: Microscopic theory and macroscopic experiment. Phys. Rev. B 2009, 80, 1956–1960. [Google Scholar] [CrossRef]
- Yu, S.; Yang, S.; Cho, M. Molecular dynamics study to identify mold geometry effect on the pattern transfer in thermal nanoimprint lithography. Jpn. J. Appl. Phys. 2009, 48. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Chen, Y.F.; Gu, C.; Liao, R.J.; Zhu, W.B.; Du, X.M. Simulation on thermodynamic properties of amorphous cellulose based on molecular dynamics. High Volt. Eng. 2015, 41, 432–439. [Google Scholar]
- Liao, R.J.; Nie, S.J.; Zhou, X.; Wang, K.; Yuan, L.; Yang, L.J.; Cheng, H.C. Molecular dynamics simulation on the hydrophilicity of physical modification cellulose insulating materials. Proc. CSEE. 2013, 39, 1–7. [Google Scholar]
- Cheng, W.; Wang, K.; Fu, Q. Preparation of inorganic particle grafted cellulose fibre and its thermostability. Plast. Ind. 2014, 42, 113–117. [Google Scholar]
- Mazeau, K.; Heux, L. Molecular dynamics simulations of bulk native crystalline and amorphous structures of cellulose. J. Phys. Chem. B 2003, 107, 2394–2403. [Google Scholar] [CrossRef]
- Chen, W.; Lickfield, C.G.; Yang, Q.C. Molecular modeling of cellulose in amorphous state. Part 1: Model building and plastic deformation study. Polymer 2004, 45, 1063–1071. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, S.; Zhang, F.Z.; Li, X.; Zhou, Q. Simulation and experimental about the thermal aging performance improvement of cellulose insulation paper. Trans. China Electrotech. Soc. 2016, 31, 68–76. [Google Scholar]
- Tang, C.; Zhang, S.; Li, X.; Xiong, B.F.; Xie, J.T. Experimental analyses and molecular simulation of the thermal aging of transformer insulation paper. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 1131–1138. [Google Scholar] [CrossRef]
- Theodorou, D.N.; Wsuter, U. Detailed molecular structure of a vinyl polymer glass. Macromolecules 1985, 18, 1467–1478. [Google Scholar] [CrossRef]
- Sun, H. Ab-initio calculations and force field development for computer simulation of polysilanes. Macromolecules 1995, 28, 701–712. [Google Scholar] [CrossRef]
- Tao, C.G.; Feng, H.J.; Zhou, J.; Lv, L.H.; Lu, X.H. Molecular simulation of oxygen adsorption and diffusion in polypropylene. Acta Phys. Chim. Sin. 2009, 25, 1373–1378. [Google Scholar]
- Watt, J.P.; Davies, G.F.; O’Connell, R.J. The elastic properties of composite materials. Rev. Geophys. Space Phys. 1976, 14, 541–563. [Google Scholar] [CrossRef]
- Tanaka, F.; Iwata, T. Estimation of the elastic modulus of cellulose crystal by molecular mechanics simulation. Cellulose 2006, 13, 509–517. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, C.; Chen, G.; Zhou, Q.; Lv, C.; Li, X. The influence and mechanism of nano Al2O3 to the thermal stability of cellulose insulation paper. Sci. Sin. Techol. 2015, 45, 1167–1179. [Google Scholar]
- He, P.S. The Mechanical Properties of High Polymer; Press of University of Science and Technology of China: Hefei, China, 2008; pp. 185–196. [Google Scholar]
- Fu, Y.Z.; Liu, Y.Q.; Lan, Y.H. Molecular simulation on the glass transition of polypropylene. Polym. Mater. Sci. Eng. 2009, 25, 53–56. [Google Scholar]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. Polymer Handbook; Wiley-Interscience Publication: New York, NY, USA, 1999; pp. 476–479. [Google Scholar]
- Mazur, P.; Maradudin, A.A. Mean-square displacements of atoms in thin crystal films. Phys. Rev. B 1981, 24, 2996–3007. [Google Scholar] [CrossRef]
- Hao, Z.F.; Zhang, J.; Wu, Y.H.; Yu, J.; Yu, L. Synthesis and thermal stability properties of boron-doped silicone resin. J. Appl. Polym. Sci. 2014, 131, 1366–1373. [Google Scholar] [CrossRef]
- Fox, T.G.; Flory, P.J. Second-order transition temperatures and related properties of polystyrene. I. influence of molecular weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Zhang, S.; Wang, Q.; Wang, X.; Hao, J. Thermal Stability of Modified Insulation Paper Cellulose Based on Molecular Dynamics Simulation. Energies 2017, 10, 397. https://doi.org/10.3390/en10030397
Tang C, Zhang S, Wang Q, Wang X, Hao J. Thermal Stability of Modified Insulation Paper Cellulose Based on Molecular Dynamics Simulation. Energies. 2017; 10(3):397. https://doi.org/10.3390/en10030397
Chicago/Turabian StyleTang, Chao, Song Zhang, Qian Wang, Xiaobo Wang, and Jian Hao. 2017. "Thermal Stability of Modified Insulation Paper Cellulose Based on Molecular Dynamics Simulation" Energies 10, no. 3: 397. https://doi.org/10.3390/en10030397





