Methane Adsorption Rate and Diffusion Characteristics in Marine Shale Samples from Yangtze Platform, South China
Abstract
:1. Introduction
2. Diffusion Models
2.1. Unipore Diffusion Model
2.2. Bidisperse Diffusion Model
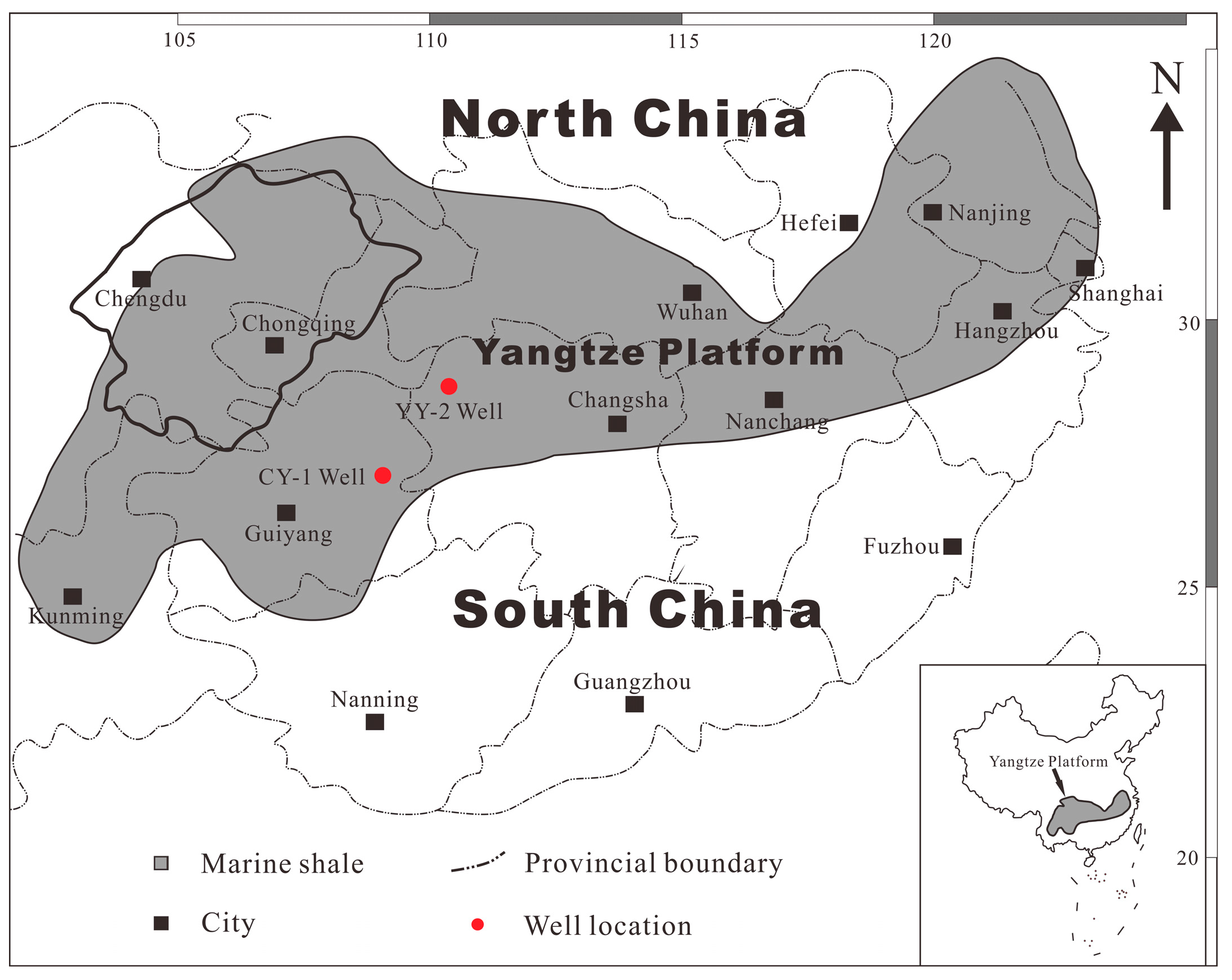
3. Shale Samples and Experimental Methods
3.1. Shale Samples Preparation
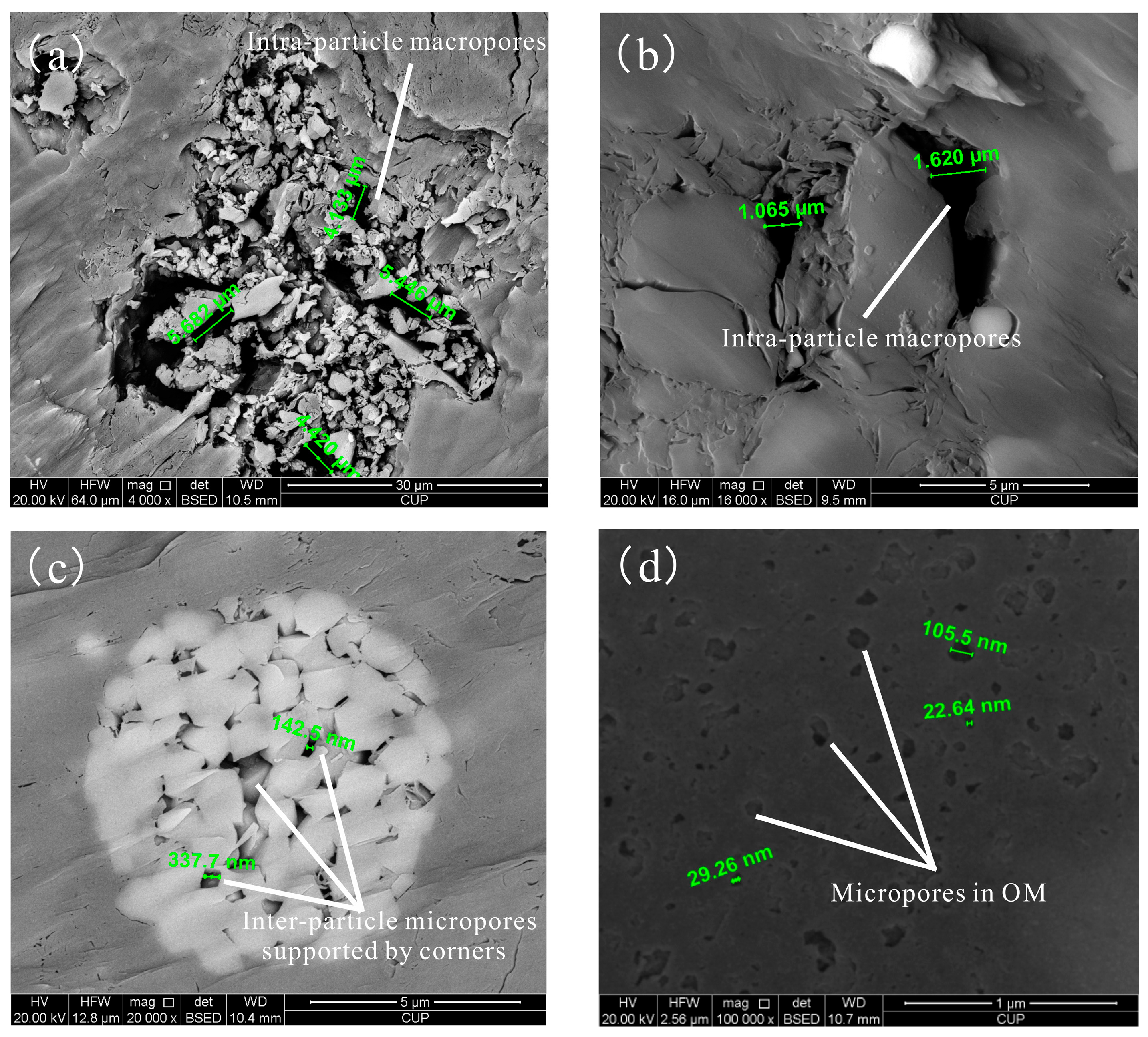
3.2. Scanning Electronic Microscopy (SEM) Observations
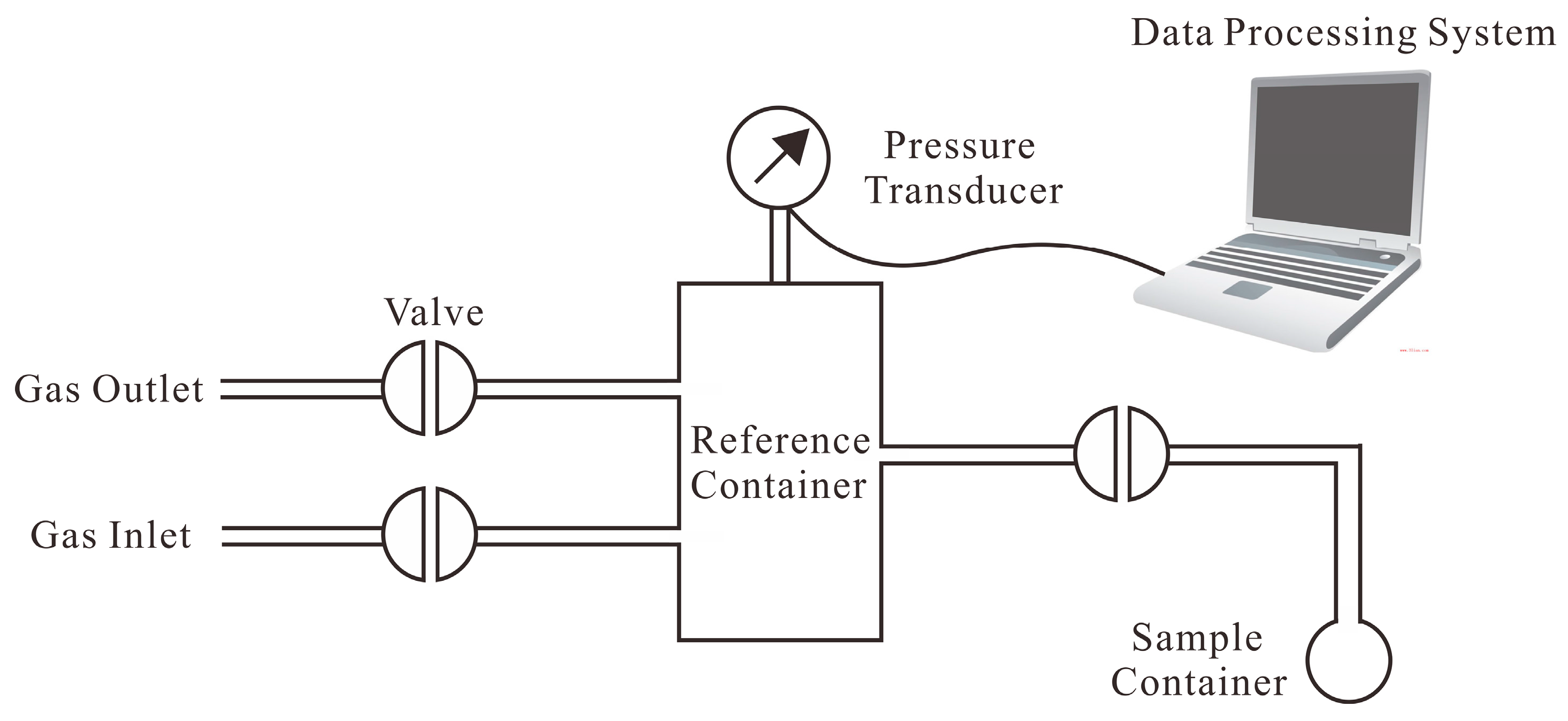
3.3. Methane Adsorption Isotherms and Adsorption Rate Measurements
4. Experimental Results and Discussions
4.1. Pore Characteristics
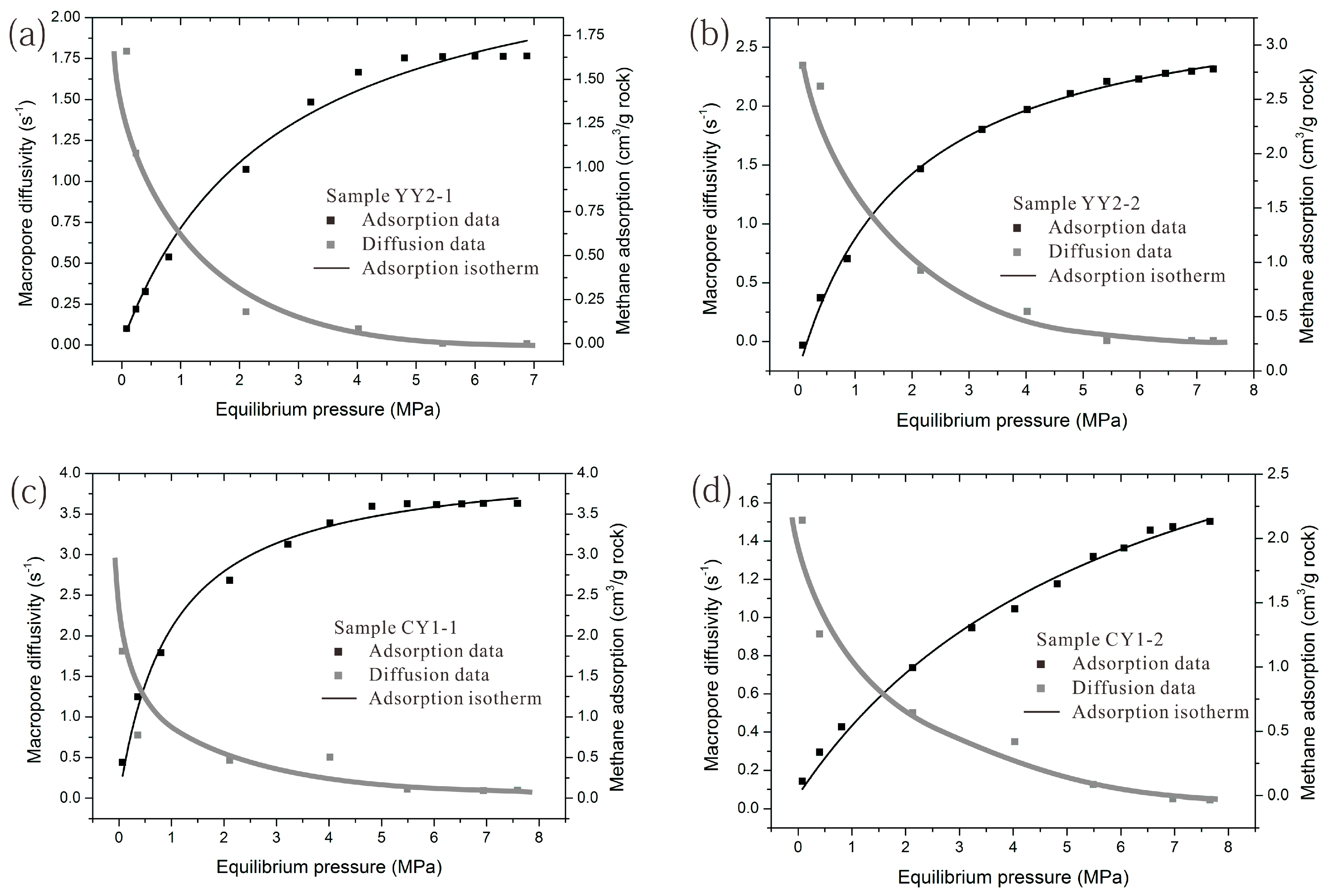
4.2. Methane Adsorption Isotherms
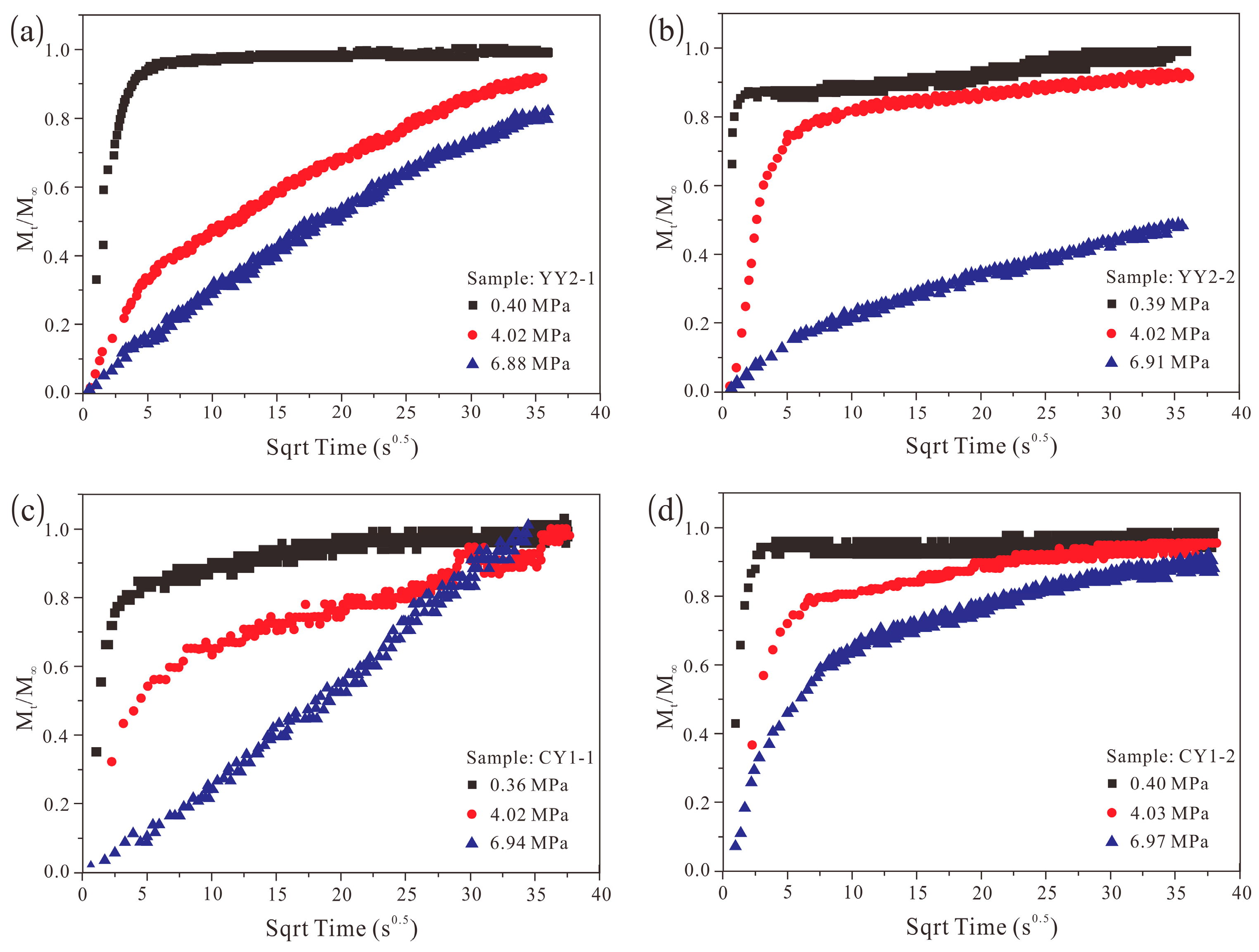
4.3. Methane Adsorption Kinetics
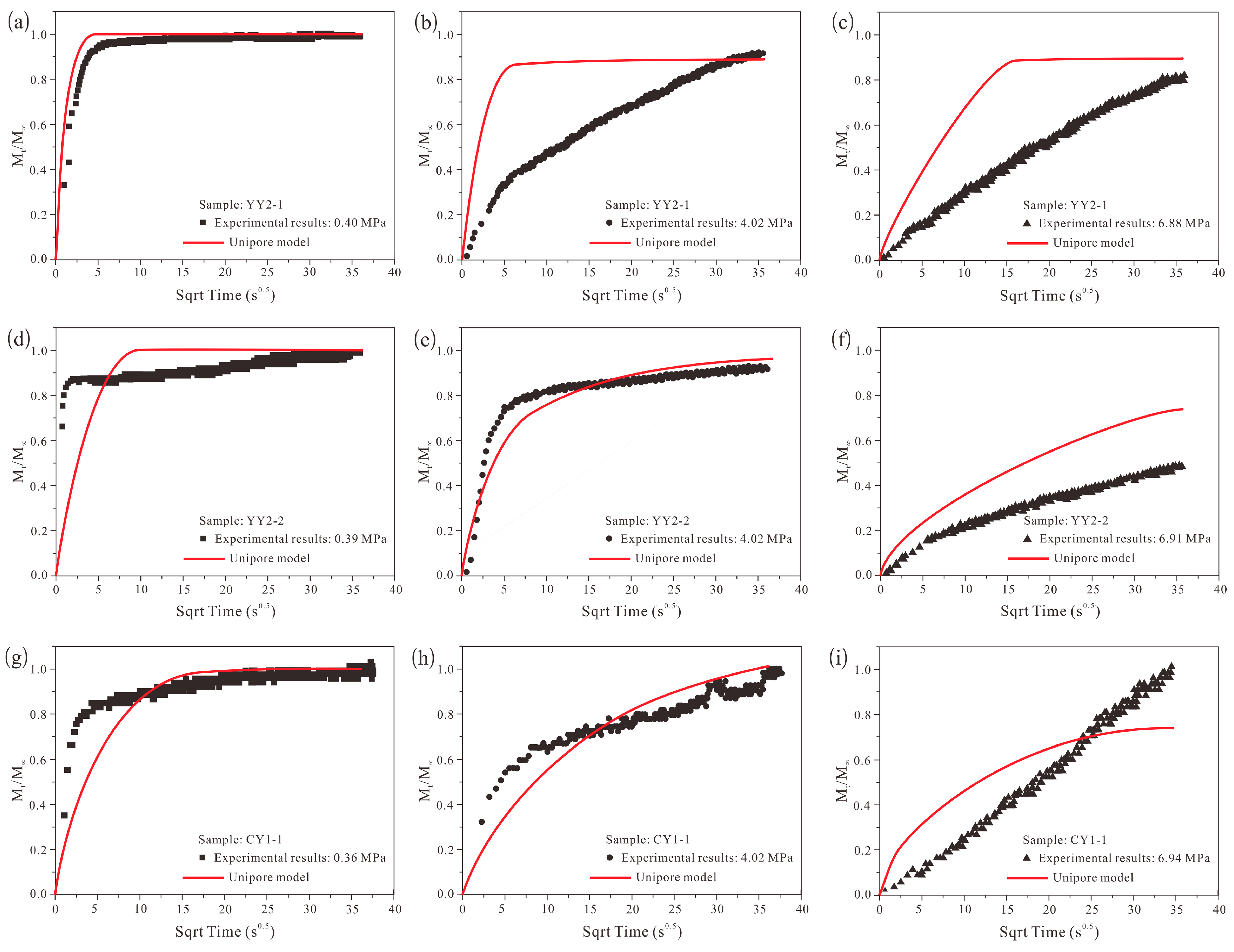
4.4. Methane Adsorption Rate Data: Application of Unipore Model
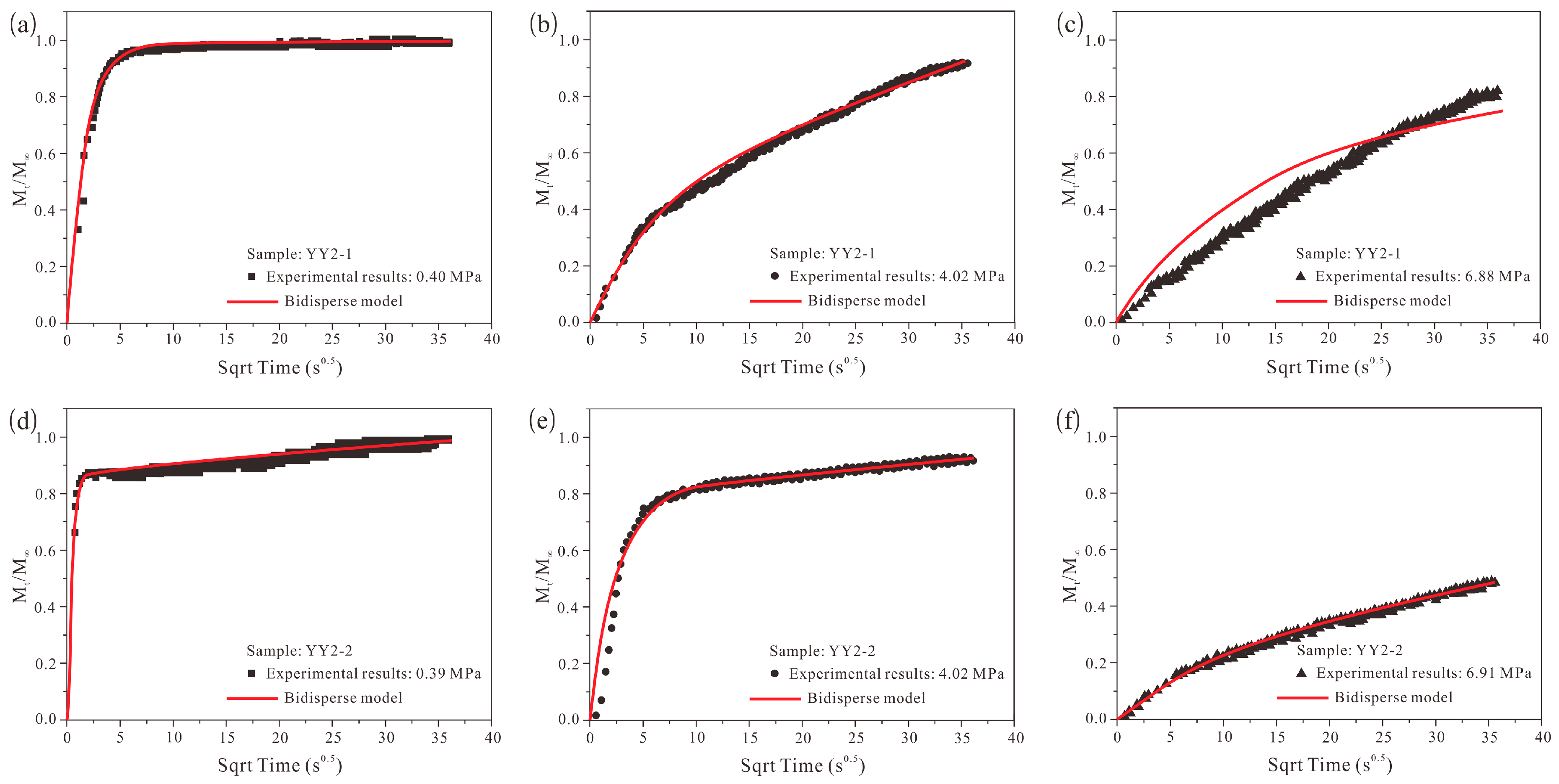
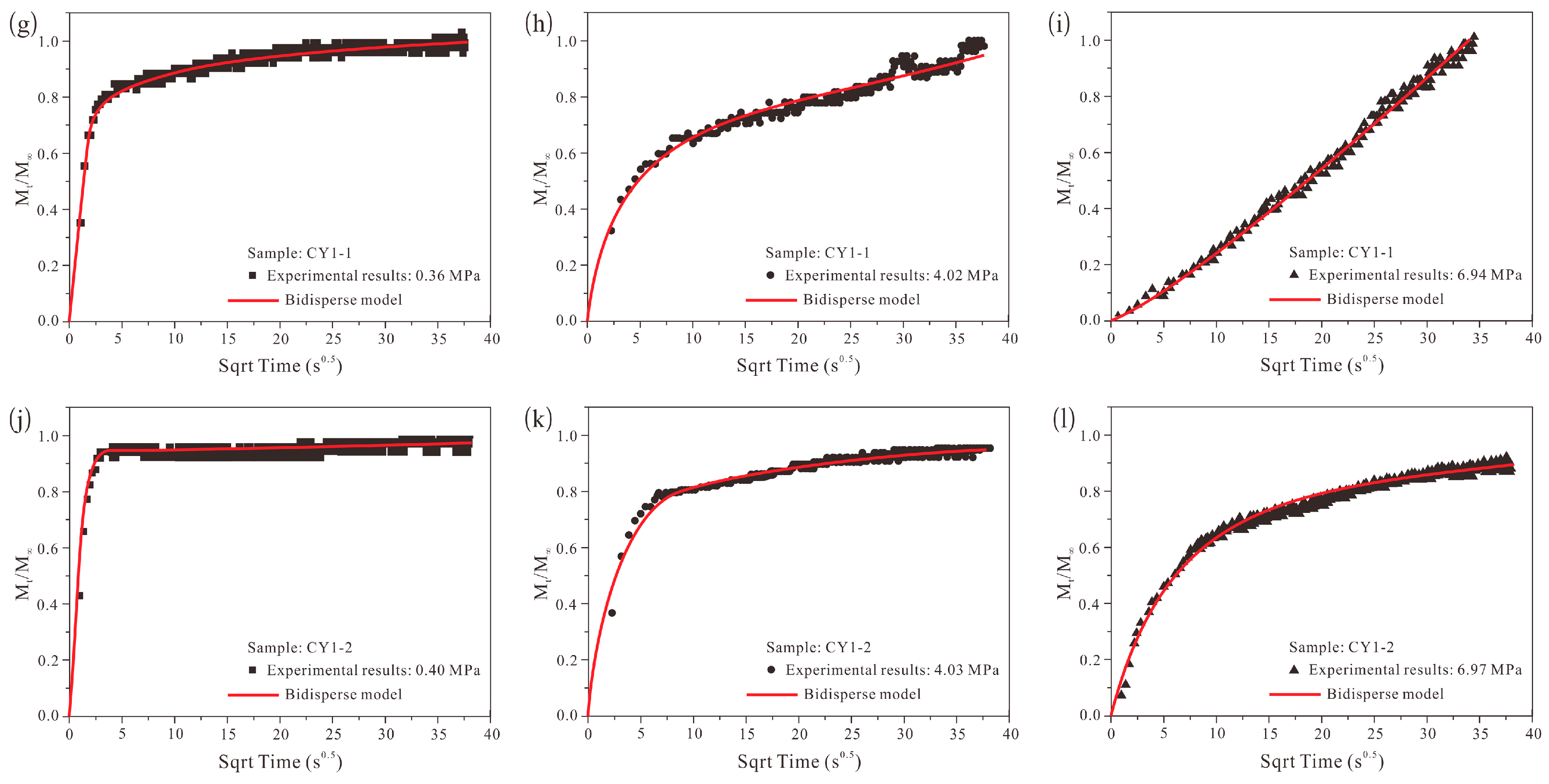
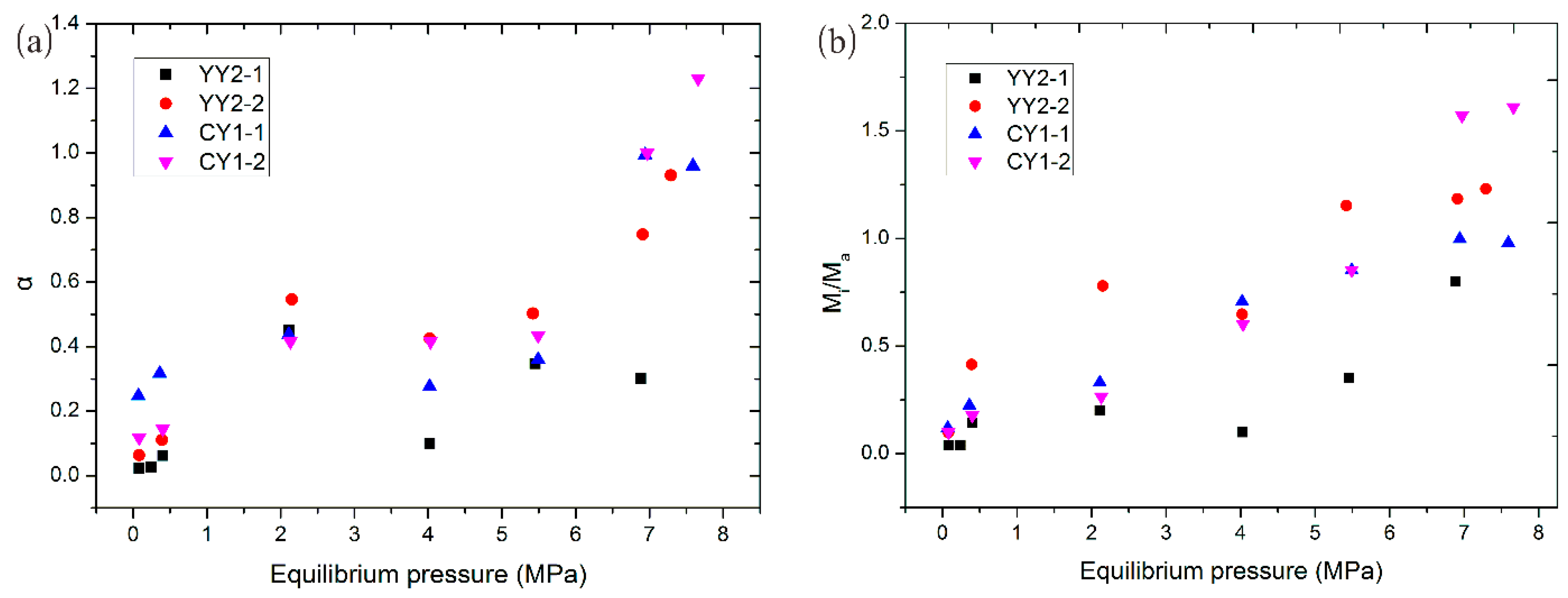
4.5. Methane Adsorption Rate Data: Application of Bidisperse Model
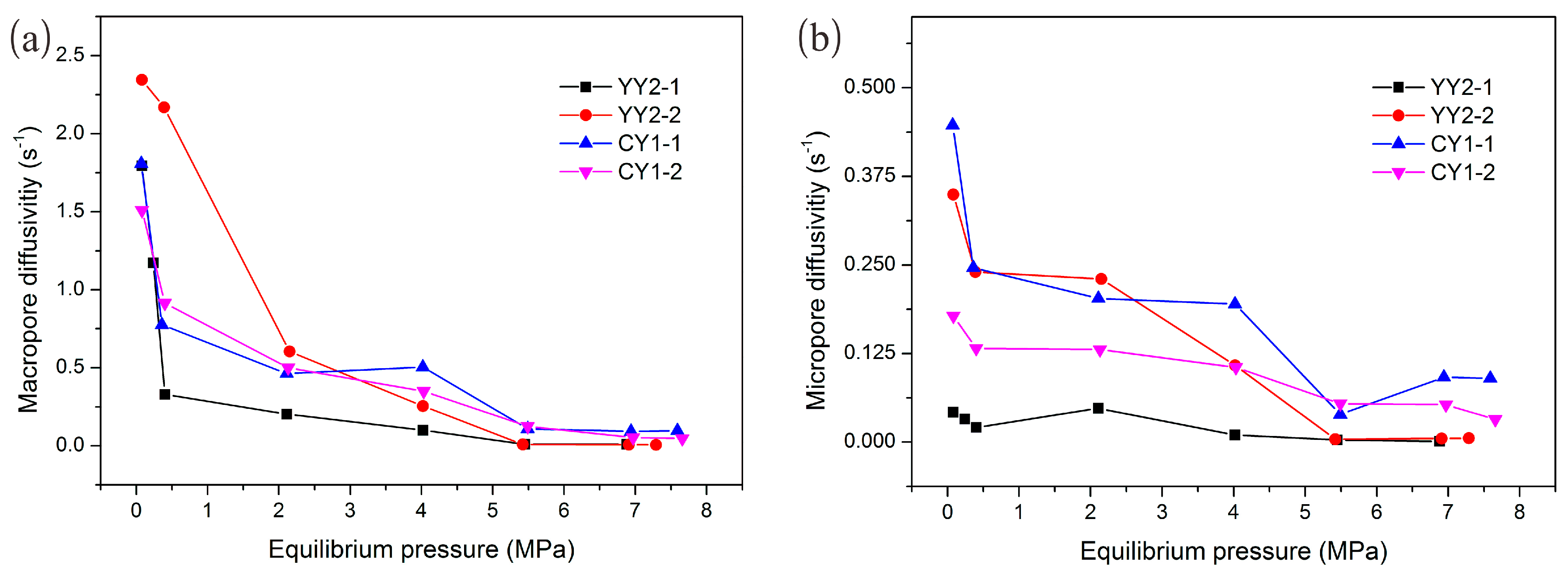
4.6. Effect of Pressure and Surface Coverage on Methane Diffusivity in Shale
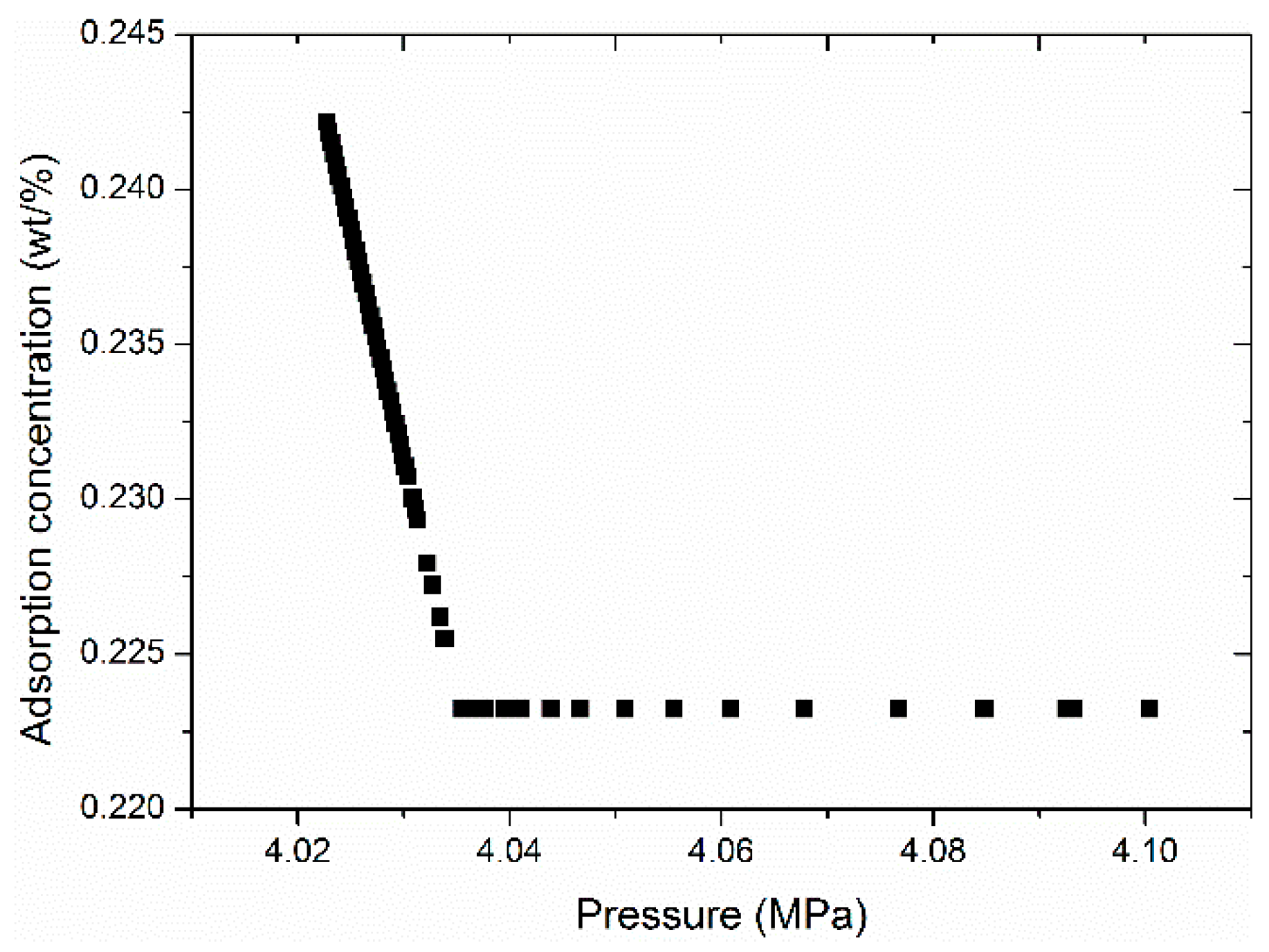
4.7. Analysis of Methane Adsorption Rate Behavior between Different Pressure Steps
4.8. Implications for Gas Shale Reservoir Characterization
5. Conclusions
- (1)
- The unipore model failed to describe the methane adsorption rate data for all pressure steps, and the bidisperse model cannot perfectly model the methane adsorption rate data. However, using the bidisperse model is still justified even if some new diffusion models [19,68] involving a more complicated mathematical process and providing a better description were established.
- (2)
- There is an evident negative correlation between macropore diffusivity and pressure lower than 3–4 MPa, and when the pressure is greater than 5.5 MPa the macropore diffusivity is constant. However, the micropore diffusivity only showed a gentle decreasing trend with pressure. This finding indicated that methane diffusion could play a significant role in later stages of the development of shale gas reservoir. Additionally, the surface coverage or adsorption volume also negatively affected methane diffusivity in shale.
- (3)
- The order of magnitude of macro- and micropore diffusivities in measured marine shale are roughly from 10−3 to 100, and 10−3 to 10−1, respectively. In these shale samples, both macro- and micropores are significant for the methane adsorption rate and total adsorption in measured marine shale samples, and the relative influence and importance of micropore diffusion and adsorption to the adsorption rate and total adsorption increased with increasing pressure. This is because the adsorption sites in macropores become full with increasing pressure steps, so less methane adsorption in macropores can occur at higher pressure steps.
- (4)
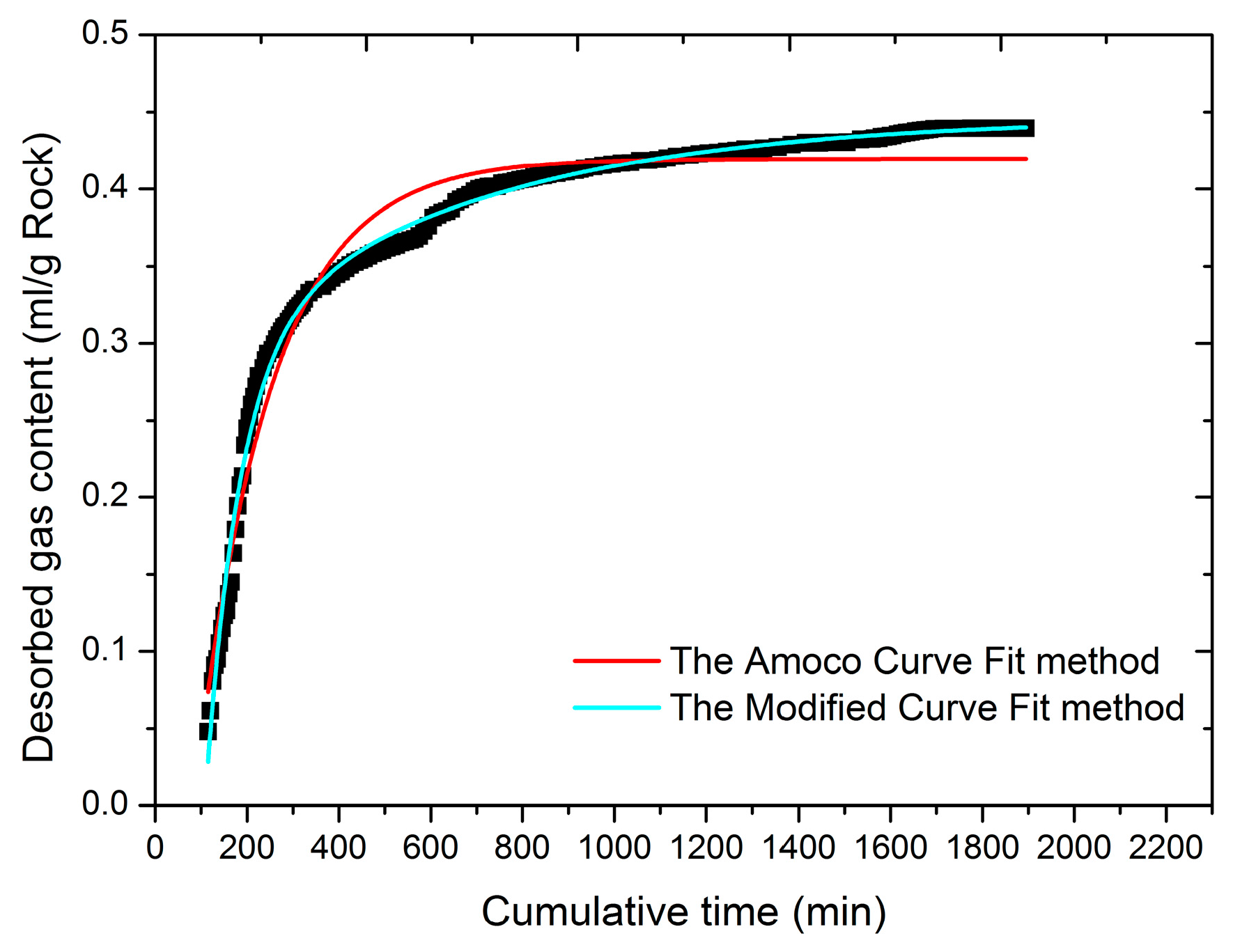
- A new estimation method was proposed here based upon the bidisperse model for lost gas content estimation of shale or coal samples. From a theoretical viewpoint, the method proposed here may give a more accurate prediction of lost gas content than other methods based upon the unipore model, although further study is required.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Curtis, J.B. Fractured shale-gas systems. AAPG Bull. 2002, 86, 1921–1938. [Google Scholar]
- Jarvie, D.M.; Hill, R.J.; Ruble, T.E.; Pollastro, R.M. Unconventional shale-gas systems: The Mississippian Barnett Shale of north-central Texas as one model for thermogenic shale-gas assessment. AAPG Bull. 2007, 91, 475–499. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Bustin, R.M. The effect of pore structure and gas pressure upon the transport properties of coal: A laboratory and modeling study. 2. Adsorption rate modeling. Fuel 1999, 78, 1345–1362. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Bustin, R.M. The effect of pore structure and gas pressure upon the transport properties of coal: A laboratory and modeling study. 1. Isotherms and pore volume distributions. Fuel 1999, 78, 1333–1344. [Google Scholar] [CrossRef]
- Reed, R.M.; Loucks, R.G. Low-thermal-maturity (<0.7% VR) mudrock pore systems: Mississippian Barnett Shale, southern Fort Worth Basin. GCAGS J. 2015, 4, 15–28. [Google Scholar]
- Chen, Q.; Zhang, J.; Tang, X.; Li, W.; Li, Z. Relationship between pore type and pore size of marine shale: An example from the sinian-cambrian formation, upper Yangtze region, South China. Int. J. Coal Geol. 2016, 158, 13–28. [Google Scholar] [CrossRef]
- Wang, F.P.; Reed, R.M. Pore Networks and Fluid Flow in Gas Shales. In Proceedings of the SPE Annual Technical Conference and Exhibition 2009, New Orleans, LA, USA, 4–7 October 2009. [Google Scholar]
- Schieber, J. Common Themes in the Formation and Preservation of Intrinsic Porosity in Shales and Mudstones-Illustrated with Examples across the Phanerozoic. In Proceedings of the SPE Unconventional Gas Conference, Pittsburgh, PA, USA, 23–25 February 2010. [Google Scholar]
- Tan, J.; Weniger, P.; Krooss, B.; Merkel, A.; Horsfield, B.; Zhang, J.; Boreham, C.J.; van Graas, G.; Tocher, B.A. Shale gas potential of the major marine shale formations in the Upper Yangtze Platform, South China, Part II: Methane sorption capacity. Fuel 2014, 129, 204–218. [Google Scholar] [CrossRef]
- Ross, D.J.K.; Bustin, R.M. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Gasparik, M.; Bertier, P.; Gensterblum, Y.; Ghanizadeh, A.; Krooss, B.M.; Littke, R. Geological controls on the methane storage capacity in organic-rich shales. Int. J. Coal Geol. 2014, 123, 34–51. [Google Scholar] [CrossRef]
- Passey, Q.R.; Bohacs, K.; Esch, W.L.; Klimentidis, R.; Sinha, S. From Oil-Prone Source Rock to Gas-Producing Shale Reservoir—Geologic and Petrophysical Characterization of Unconventional Shale Gas Reservoirs. In Proceedings of the International Oil and Gas Conference and Exhibition in China, Beijing, China, 8–10 June 2010; p. 131350. [Google Scholar]
- Chen, C.; Hu, D.; Westacott, D.; Loveless, D. Nanometer-scale characterization of microscopic pores in shale kerogen by image analysis and pore-scale modeling. Geochem. Geophys. Geosyst. 2013, 14, 4066–4075. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, J.; Li, G. Experimental Analysis of Methane Adsorption-diffusion Property in High-maturity Organic-rich Shale and High-Rank Coal. Nat. Gas Geosci. 2015, 26, 1499–1506. [Google Scholar]
- Darabi, H.; Ettehad, A.; Javadpour, F.; Sepehrnoori, K. Gas flow in ultra-tight shale strata. J. Fluid Mech. 2012, 710, 641–658. [Google Scholar] [CrossRef]
- Yuan, W.; Pan, Z.; Xiao, L.; Yang, Y.; Zhao, C.; Connell, L.D.; Li, S.; He, J. Experimental study and modelling of methane adsorption and diffusion in shale. Fuel 2014, 117, 509–519. [Google Scholar] [CrossRef]
- Diamond, W.P.; Schatzel, S.J. Measuring the gas content of coal: A review. Int. J. Coal Geol. 1998, 35, 311–331. [Google Scholar] [CrossRef]
- Bhowmik, S.; Dutta, P. Adsorption rate characteristics of methane and CO2 in coal samples from Raniganj and Jharia coalfields of India. Int. J. Coal Geol. 2013, 113, 50–59. [Google Scholar] [CrossRef]
- Haghshenas, B.; Clarkson, C.R.; Aquino, S.D.; Chen, S. Characterization of Multi-Fractured Horizontal Shale Wells using Drill Cuttings: 2. Permeability/Diffusivity Estimation. J. Nat. Gas Sci. Eng. 2016, 32, 586–596. [Google Scholar] [CrossRef]
- Pan, Z.; Connell, L.D.; Camilleri, M.; Connelly, L. Effects of matrix moisture on gas diffusion and flow in coal. Fuel 2010, 89, 3207–3217. [Google Scholar] [CrossRef]
- Smith, D.M.; Williams, F.L. Diffusional Effects in the Recovery of Methane from Coalbeds. Soc. Pet. Eng. J. 1984, 24, 529–535. [Google Scholar] [CrossRef]
- Busch, A.; Gensterblum, Y.; Krooss, B.M.; Littke, R. Methane and carbon dioxide adsorption–diffusion experiments on coal: Upscaling and modeling. Int. J. Coal Geol. 2004, 60, 151–168. [Google Scholar] [CrossRef]
- Cui, X.; Bustin, R.M.; Dipple, G. Selective transport of CO2, CH4, and N2 in coals: Insights from modeling of experimental gas adsorption data. Fuel 2004, 83, 293–303. [Google Scholar] [CrossRef]
- Nandi, S.P.; Walker, P.L., Jr. Activated diffusion of methane from coals at elevated pressures. Fuel 1975, 54, 81–86. [Google Scholar] [CrossRef]
- Zhao, X. An Experimental Study of Methane Diffusion in Coal Using Transient Approach; University of Arizona: Tucson, AZ, USA, 1991; pp. 85–87. [Google Scholar]
- Smith, D.M.; Williams, F.L. Diffusion models for gas production from coal: Determination of diffusion parameters. Fuel 1984, 63, 256–261. [Google Scholar] [CrossRef]
- Smith, D.M.; Williams, F.L. Diffusion models for gas production from coals: Application to methane content determination. Fuel 1984, 63, 251–255. [Google Scholar] [CrossRef]
- Pillalamarry, M.; Harpalani, S.; Liu, S. Gas diffusion behavior of coal and its impact on production from coalbed methane reservoirs. Int. J. Coal Geol. 2011, 86, 342–348. [Google Scholar] [CrossRef]
- Nikolai, S.; Andreas, B.; Hans, B.; Bernd, K.; Yves, G. Assessing the Kinetics and Capacity of Gas Adsorption in Coals by a Combined Adsorption/Diffusion Method. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 5–8 October 2003. [Google Scholar]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Vaidyanathan, A.S.; Youngquist, G.R. Sorption by solids with bidisperse pore structures. Chem. Eng. Sci. 1971, 26, 1305–1318. [Google Scholar] [CrossRef]
- Wheeler, A. Reaction rates and selectivity in catalyst pores. Adv. Catal. 1951, 3, 250. [Google Scholar]
- Carslaw, H.S.; Jaeger, J.C. Conduction of Heat in Solids, 2nd ed.; Clarendon Press: Oxford, UK, 1959. [Google Scholar]
- Nandi, S.P.; Walker, P.L. Activated diffusion of methane in coal. Fuel 1970, 49, 309–323. [Google Scholar] [CrossRef]
- Crank, J. A diffusion problem in which the amount of diffusing substance is finite. II. Diffusion with nonlinear absorption. Philos. Mag. 1948, 39, 140–149. [Google Scholar] [CrossRef]
- Mianowski, A.; Marecka, A. The isokinetic effect as related to the activation energy for the gases diffusion in coal at ambient temperatures. J. Therm. Anal. Calorim. 2009, 96, 285–292. [Google Scholar] [CrossRef]
- Zhang, J.; He, S.; Yi, J.; Zhang, B.; Zhang, S.; Zheng, L.; Hou, Y.; Wang, Y. Rock thermo-acoustic emission and basin modeling technologies applied to the study of maximum paleotemperatures and thermal maturity histories of Lower Paleozoic marine shales in the western middle Yangtze area. Acta Pet. Sin. 2014, 35, 58–67. [Google Scholar]
- Cao, H.-Y.; Zhu, C.-Q.; Qiu, N.-S. Maximum paleotemperature of main Paleozoic argillutite in the Eastern Sichuan Basin. Chin. J. Geophys. Chin. Ed. 2016, 59, 1017–1029. [Google Scholar]
- MT/T1157-2011, Determination of Equilibrium Moisture in coal Isotherm Adsorption Method. Available online: http://dbpub.cnki.net/grid2008/dbpub/detail.aspx?dbcode=SCSD&dbname=SCSD&filename=SCSDMT/T%201157-2011 (accessed on 5 January 2017).
- Ji, L.M.; Zhang, T.W.; Milliken, K.L.; Qu, J.L.; Zhang, X.L. Experimental investigation of main controls to methane adsorption in clay-rich rocks. Appl. Geochem. 2012, 27, 2533–2545. [Google Scholar] [CrossRef]
- Sudibandriyo, M.; Pan, Z.; Fitzgerald, J.E.; Robinson, R.L.; Gasem, K.A.M. Adsorption of Methane, Nitrogen, Carbon Dioxide, and Their Binary Mixtures on Dry Activated Carbon at 318.2 K and Pressures up to 13.6 MPa. Langmuir 2003, 19, 5323–5331. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Harpalani, S.; And, B.K.P.; Dutta, P. Methane/CO2 Sorption Modeling for Coalbed Methane Production and CO2 Sequestration. Energy Fuels 2006, 20, 1591–1599. [Google Scholar] [CrossRef]
- Bustin, R.; Clarkson, C. Geological controls on coalbed methane reservoir capacity and gas content. Int. J. Coal Geol. 1998, 38, 3–26. [Google Scholar] [CrossRef]
- Chareonsuppanimit, P.; Mohammad, S.A.; Robinson, R.L.; Gasem, K.A.M. High-pressure adsorption of gases on shales: Measurements and modeling. Int. J. Coal Geol. 2012, 95, 34–46. [Google Scholar] [CrossRef]
- Lu, X.C.; Li, F.C.; Watson, A.T. Adsorption Measurements in Devonian Shales. Fuel 1995, 74, 599–603. [Google Scholar] [CrossRef]
- Dutta, P.; Harpalani, S.; Prusty, B. Modeling of CO2 sorption on coal. Fuel 2008, 87, 2023–2036. [Google Scholar] [CrossRef]
- Chalmers, G.R.; Bustin, R.M. Lower Cretaceous gas shales in northeastern British Columbia, Part I: Geological controls on methane sorption capacity. Bull. Can. Pet. Geol. 2008, 56, 1–21. [Google Scholar] [CrossRef]
- Dang, W.; Zhang, J.; Huang, X.; Li, X.; Chen, Q.; Sun, R.; Xue, B.; Han, S. Main-controlling geological factors of gas-bearing property of continental shale gas: A case study of Member 3 of Shahejie Formation in western Liaohe sag. Acta Pet. Sin. 2015, 1516–1530. [Google Scholar]
- Ji, W.; Song, Y.; Jiang, Z.; Wang, X.; Bai, Y.; Xing, J. Geological controls and estimation algorithms of lacustrine shale gas adsorption capacity: A case study of the Triassic strata in the southeastern Ordos Basin, China. BMC Neurosci. 2014, 134, 1–9. [Google Scholar] [CrossRef]
- Rexer, T.F.T.; Benham, M.J.; Aplin, A.C.; Thomas, K.M. Methane Adsorption on Shale under Simulated Geological Temperature and Pressure Conditions. Energy Fuels 2013, 27, 3099–3109. [Google Scholar] [CrossRef]
- Chen, C.; Lin, D. Application of Isothermal Curves in Estimating Minable Resource of Coalbed Methane. J. China Univ. Min. Technol. 2005, 34, 679–682. [Google Scholar]
- Zhang, P.; Hu, L.; Meegoda, J.N.; Gao, S. Micro/Nano-pore Network Analysis of Gas Flow in Shale Matrix. Sci. Rep. 2015, 5, 13501. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Song, Z.; Wang, S.; Cao, X.; Li, Y.; Xia, J. Characterizing the pore structure in the Silurian and Permian shales of the Sichuan Basin, China. Mar. Pet. Geol. 2015, 61, 140–150. [Google Scholar] [CrossRef]
- Ye, Z.; Chen, D.; Pan, Z. A unified method to evaluate shale gas flow behaviours in different flow regions. J. Nat. Gas Sci. Eng. 2015, 26, 205–215. [Google Scholar] [CrossRef]
- Chalmers, G.R.; Bustin, R.M.; Power, I.M. Characterization of gas shale pore systems by porosimetry, pycnometry, surface area, and field emission scanning electron microscopy/transmission electron microscopy image analyses: Examples from the Barnett, Woodford, Haynesville, Marcellus, and Doig unit. AAPG Bull. 2012, 96, 1099–1119. [Google Scholar] [CrossRef]
- Cao, T.; Song, Z.; Luo, H.; Liu, G. The Differences of Microscopic Pore Structure Characteristics of Coal, Oil shale and Shales and Their Storage Mechanisms. Nat. Gas Geosci. 2015, 26, 2208–2218. [Google Scholar]
- Charrière, D.; Pokryszka, Z.; Behra, P. Effect of pressure and temperature on diffusion of CO2 and CH4 into coal from the Lorraine basin (France). Int. J. Coal Geol. 2010, 81, 373–380. [Google Scholar] [CrossRef]
- Chen, Y.D.; Yang, R.T. Concentration dependence of surface diffusion and zeolitic diffusion. AIChE J. 1991, 37, 1579–1582. [Google Scholar] [CrossRef]
- Roberts, P.V.; York, R. Adsorption of Normal Paraffins from Binary Liquid Solutions by 5A Molecular Sieve. Ind. Eng. Chem. Process Des. Dev. 1967, 6, 516–525. [Google Scholar] [CrossRef]
- Kissell, F.N.; Mcculloch, C.M.; Elder, C.H.; Kissell, F.N.; Mcculloch, C.M.; Elder, C.H. The Direct Method of Determining Methane Content of Coal Beds for Ventilation Design; Report of Investigations RI7767; U.S. Bureau of Mines: Washington, DC, USA, 1973. [Google Scholar]
- Yee, D.; Seidle, J.P.; Hanson, W.B. Gas sorption on coal and measurement of gas content. Am. Assoc. Pet. Geol. 1993, 38, 203–218. [Google Scholar]
- Smith, D.M.; Williams, F.L. Direct method of determining the methane content of coal—A modification. Fuel 1984, 63, 425–427. [Google Scholar] [CrossRef]
- Mclennan, J.D.; Schafer, P.S.; Pratt, T.J.; Institute, G. A Guide to Determining Coalbed Gas Content; GRI-94-0396; Gas Research Institute: Chicago, IL, USA, 1995. [Google Scholar]
- Mcculloch, C.M.; Levine, J.R.; Kissell, F.N.; Deul, M.; Mcculloch, C.M.; Levine, J.R.; Kissell, F.N.; Deul, M. Measuring the Methane Content of Bituminous Coalbeds; Report of Investigations 8043; US Bureau of Mines: Washington, DC, USA, 1975; p. 22. [Google Scholar]
- Bertard, C.; Bruyet, B.; Gunther, J. Determination of desorbable gas concentration of coal (direct method). Int. J. Rock Mech. Min. Sci. Geomech. Abstr. 1970, 7, 43–65. [Google Scholar] [CrossRef]
- Chase, R. A Comparison of Methods Used for Determining the Natural Gas Content of Coalbeds from Exploratory Cores; METC/CR-79/18; US Department of Energy: Washington, DC, USA, 1979; p. 25. [Google Scholar]
- Li, Z.; Liu, D.; Cai, Y.; Shi, Y. Investigation of methane diffusion in low-rank coals by a multiporous diffusion model. J. Nat. Gas Sci. Eng. 2016, 33, 97–107. [Google Scholar] [CrossRef]


| Sample | Organic Geochemistry | Mineral Compositions (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TOC (%) | Ro (%) | Quartz | Feldspar | Pyrite | Carbonate | Total Clays | Illite | Chlorite | I/S | |
| YY2-1 | 1.76 | 2.68 | 57 | 5 | 1 | 2 | 35 | 16 | 9 | 10 |
| YY2-2 | 1.61 | 3.04 | 60 | 7 | 2 | 31 | 20 | 4 | 7 | |
| CY1-1 | 2.03 | 3.35 | 51 | 8 | 3 | 1 | 37 | 17 | 7 | 13 |
| CY1-2 | 2.54 | 3.61 | 54 | 5 | 1 | 2 | 38 | 21 | 5 | 12 |
| YY2-1 | YY2-2 | CY1-1 | CY1-2 | ||||
|---|---|---|---|---|---|---|---|
| P (MPa) | V (cm3/g) | P (MPa) | V (cm3/g) | P (MPa) | V (cm3/g) | P (MPa) | V (cm3/g) |
| 0.08 | 0.0858 | 0.08 | 0.238 | 0.07 | 0.2391 | 0.08 | 0.1096 |
| 0.24 | 0.1961 | 0.39 | 0.473 | 0.36 | 0.5080 | 0.40 | 0.3368 |
| 0.40 | 0.2951 | 0.86 | 0.733 | 0.80 | 0.7920 | 0.81 | 0.5333 |
| 0.80 | 0.4921 | 2.15 | 1.359 | 2.11 | 1.3821 | 2.13 | 0.9943 |
| 2.11 | 0.9886 | 3.23 | 1.821 | 3.22 | 1.7253 | 3.23 | 1.3049 |
| 3.21 | 1.3717 | 4.02 | 2.206 | 4.02 | 1.9907 | 4.03 | 1.4517 |
| 4.02 | 1.5401 | 4.78 | 2.553 | 4.82 | 2.3945 | 4.82 | 1.6464 |
| 4.80 | 1.6220 | 5.42 | 2.664 | 5.49 | 2.6267 | 5.49 | 1.8582 |
| 5.45 | 1.6292 | 5.98 | 2.786 | 6.05 | 2.7142 | 6.06 | 1.9253 |
| 6.00 | 1.6311 | 6.45 | 2.838 | 6.53 | 2.8242 | 6.55 | 2.0644 |
| 6.48 | 1.6301 | 6.91 | 2.958 | 6.94 | 2.8501 | 6.97 | 2.0922 |
| 6.88 | 1.6322 | 7.29 | 2.978 | 7.59 | 2.8904 | 7.66 | 2.1312 |
| Sample | VL (MPa) | PL (cm3/g) | R2 |
|---|---|---|---|
| YY2-1 | 2.37 | 2.6105 | 0.98 |
| YY2-2 | 5.55 | 6.0731 | 0.99 |
| CY1-1 | 5.09 | 5.5414 | 0.99 |
| CY1-2 | 3.88 | 6.1741 | 0.99 |
| YY2-1 | YY2-2 | ||
| P (MPa) | D/ (s−1) | P (MPa) | D/ (s−1) |
| 0.08 | 1.5010 | 0.08 | 0.7717 |
| 0.24 | 1.1718 | 0.39 | 0.1801 |
| 0.40 | 0.1934 | 2.15 | 0.2958 |
| 2.11 | 0.2027 | 4.02 | 0.1030 |
| 4.02 | 0.0013 | 5.42 | 0.0122 |
| 5.45 | 0.0009 | 6.91 | 0.0068 |
| 6.88 | 0.00097 | 7.29 | 0.0061 |
| CY1-1 | CY1-2 | ||
| P (MPa) | D/ (s−1) | P (MPa) | D/ (s−1) |
| 0.07 | 0.9091 | 0.08 | 1.3207 |
| 0.36 | 0.1675 | 0.40 | 0.4327 |
| 2.11 | 0.2384 | 2.13 | 0.5001 |
| 4.02 | 0.0549 | 4.03 | 0.0821 |
| 5.49 | 0.0207 | 5.49 | 0.0601 |
| 6.94 | 0.0249 | 6.97 | 0.0129 |
| 7.59 | 0.0012 | 7.66 | 0.0013 |
| YY2-1 | YY2-2 | ||||||||
| P (MPa) | / (s−1) | / (s−1) | α | β/α | P (MPa) | / (s−1) | / (s−1) | α | β/α |
| 0.08 | 1.7948 | 0.0424 | 0.0236 | 0.1149 | 0.08 | 2.3453 | 0.3493 | 0.0636 | 0.2961 |
| 0.24 | 1.1718 | 0.0325 | 0.0277 | 0.1224 | 0.39 | 2.1688 | 0.2402 | 0.1107 | 1.2450 |
| 0.40 | 0.3297 | 0.0207 | 0.0628 | 0.4341 | 2.15 | 0.6042 | 0.2303 | 0.5467 | 2.3397 |
| 2.11 | 0.2027 | 0.0479 | 0.4514 | 0.6033 | 4.02 | 0.2551 | 0.1084 | 0.4251 | 1.9455 |
| 4.02 | 0.0994 | 0.0100 | 0.1003 | 0.3021 | 5.42 | 0.0083 | 0.0042 | 0.5030 | 3.4593 |
| 5.45 | 0.0091 | 0.0032 | 0.3477 | 1.0593 | 6.91 | 0.0068 | 0.0051 | 0.7475 | 3.5553 |
| 6.88 | 0.0097 | 0.0010 | 0.3015 | 2.4030 | 7.29 | 0.0062 | 0.0056 | 0.9309 | 3.6930 |
| CY1-1 | CY1-2 | ||||||||
| P (MPa) | / (s−1) | / (s−1) | α | β/α | P (MPa) | / (s−1) | / (s−1) | α | β/α |
| 0.07 | 1.8084 | 0.4472 | 0.2473 | 0.3609 | 0.08 | 1.5084 | 0.1780 | 0.1180 | 0.3006 |
| 0.36 | 0.7760 | 0.2462 | 0.3172 | 0.6744 | 0.40 | 0.9140 | 0.1325 | 0.1449 | 0.5334 |
| 2.11 | 0.4630 | 0.2028 | 0.4379 | 0.9939 | 2.13 | 0.5012 | 0.1307 | 0.4159 | 0.7941 |
| 4.02 | 0.5047 | 0.1947 | 0.2763 | 2.1202 | 4.03 | 0.3496 | 0.1054 | 0.4160 | 1.8073 |
| 5.49 | 0.1089 | 0.0392 | 0.3598 | 2.5593 | 5.49 | 0.1255 | 0.0545 | 0.4346 | 2.5593 |
| 6.94 | 0.0923 | 0.0917 | 0.9940 | 2.9949 | 6.97 | 0.0529 | 0.0529 | 1.0000 | 4.7118 |
| 7.59 | 0.0967 | 0.0901 | 0.9600 | 2.9400 | 7.66 | 0.0467 | 0.0321 | 1.2310 | 4.8324 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, W.; Zhang, J.; Wei, X.; Tang, X.; Wang, C.; Chen, Q.; Lei, Y. Methane Adsorption Rate and Diffusion Characteristics in Marine Shale Samples from Yangtze Platform, South China. Energies 2017, 10, 626. https://doi.org/10.3390/en10050626
Dang W, Zhang J, Wei X, Tang X, Wang C, Chen Q, Lei Y. Methane Adsorption Rate and Diffusion Characteristics in Marine Shale Samples from Yangtze Platform, South China. Energies. 2017; 10(5):626. https://doi.org/10.3390/en10050626
Chicago/Turabian StyleDang, Wei, Jinchuan Zhang, Xiaoliang Wei, Xuan Tang, Chenghu Wang, Qian Chen, and Yue Lei. 2017. "Methane Adsorption Rate and Diffusion Characteristics in Marine Shale Samples from Yangtze Platform, South China" Energies 10, no. 5: 626. https://doi.org/10.3390/en10050626





