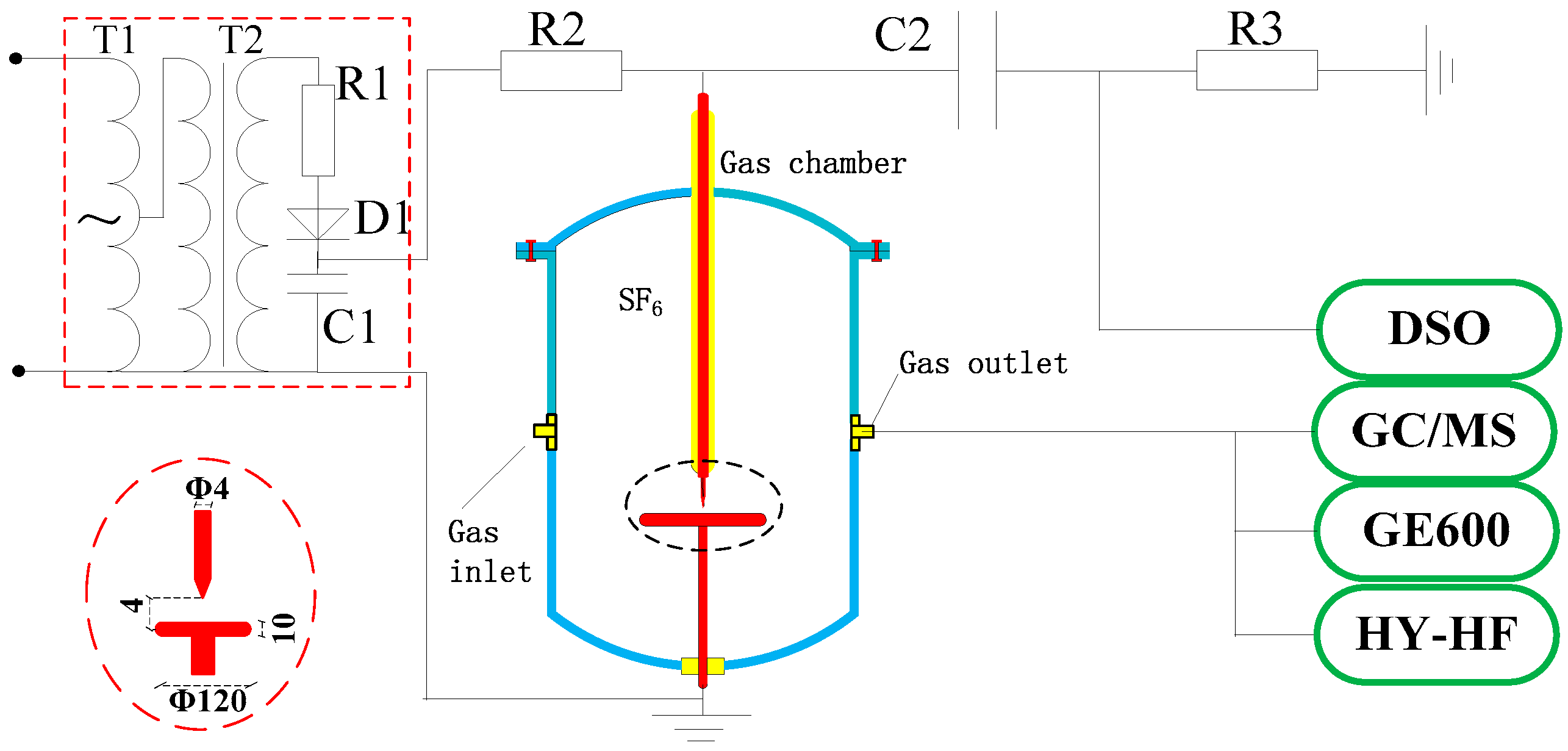
Four voltages (22.75, 29.25, 35.75 and 42.25 kV) were set in the experiments to obtain different PD strengths (PD severity). The PD inception voltage of the needle-plane electrode was 20.5 kV.
3.1. Positive DC Partial Discharge
The generation of SF
6 decomposition components not only relates to the PD pulse’s total discharge quantity (representing PD strength or discharge energy), but is also related to the promotion efficiency that pulses exert on the components. The promotion efficiency would not be same, no matter whether the pulses with different discharge quantities affect the same decomposition components or the pulses with the same discharge quantity affect different decomposition components. Van Burnt [
18] proposed that PD pulse amplitudes are widely distributed, and most of them have a low discharge quantity, whereas few of them have a high discharge quantity.
Then, not only the final effect on the decomposition of pulses have on components could be studied, but also the specific relationship between PD discharge quantity and the generation law of SF
6 decomposition components could be analyzed in depth. Then all pulses could be layered into several classes according to their different discharge quantity value (representing their discharge strength). However, the more classes that are layered, the more specific the relationship that could be obtained will be, but the difficulty of analysis is increased. Therefore, to simplify the amount of analysis, four classes were chosen: lower than 50 pC, 50–100 pC, 100–150 pC and more than 150 pC respectively, and all pulse promotion efficiencies in one class on one kind of decomposition component were assumed the same. In addition, the pulses whose discharge quantity has higher than 150 pC were classified into three classes to obtain more specific pulse distribution information, that is 150–200 pC, 200–400 pC and more than 400 pC. Finally, the pulse number and single pulse discharge quantity in class
i were marked as
Ni and
Qi, respectively, and their average values in six classifications were marked as (
and
. Their average values in different classes were marked as
,
,
,
,
,
,
,
,
,
,
and
, respectively. Furthermore,
was used to present the sum of
to
and
was the sum of
to
, and
was
. The results are listed in
Table 1.
As shown in
Table 1, as the experiment voltage increased from 22.75 kV to 42.25 kV: (1)
,
and
increased sharply,
increased about three-fold.
increased first and then decreased. (2)
,
,
,
,
,
and
increased gradually, and
increased by approximately 32 times. (3)
,
,
,
, and
increased firstly then decreased. (4)
and
under the same experiment voltage conditions increased firstly and then decreased. As also shown in
Table 1, the pulse discharge quantity and pulse number in different classes varied with the experimental voltage, and
, as a key parameter of SF
6 decomposition, was used here to present the PD strength or fault severity, then the four PD strengths were presented as 238,957, 443,476, 641,815 and 718,343 pC, respectively.
Positive DC PD pulses of the needle-plane model are dense and stable and their energy supplied for SF6 decomposition per unit time are similar, although with heterogeneous amplitudes, so per unit time would not change much.
3.2. Generation of SF6 Characteristic Decomposition Components
The decomposition process of SF
6 under positive DC PD is a complex physical and chemical process, which involves collision, ionization, excitation, and composition of different types of particles [
22,
23,
24,
25,
26,
27]. The process affects PD number, PD strength and final concentration of SF
6 decomposition components.
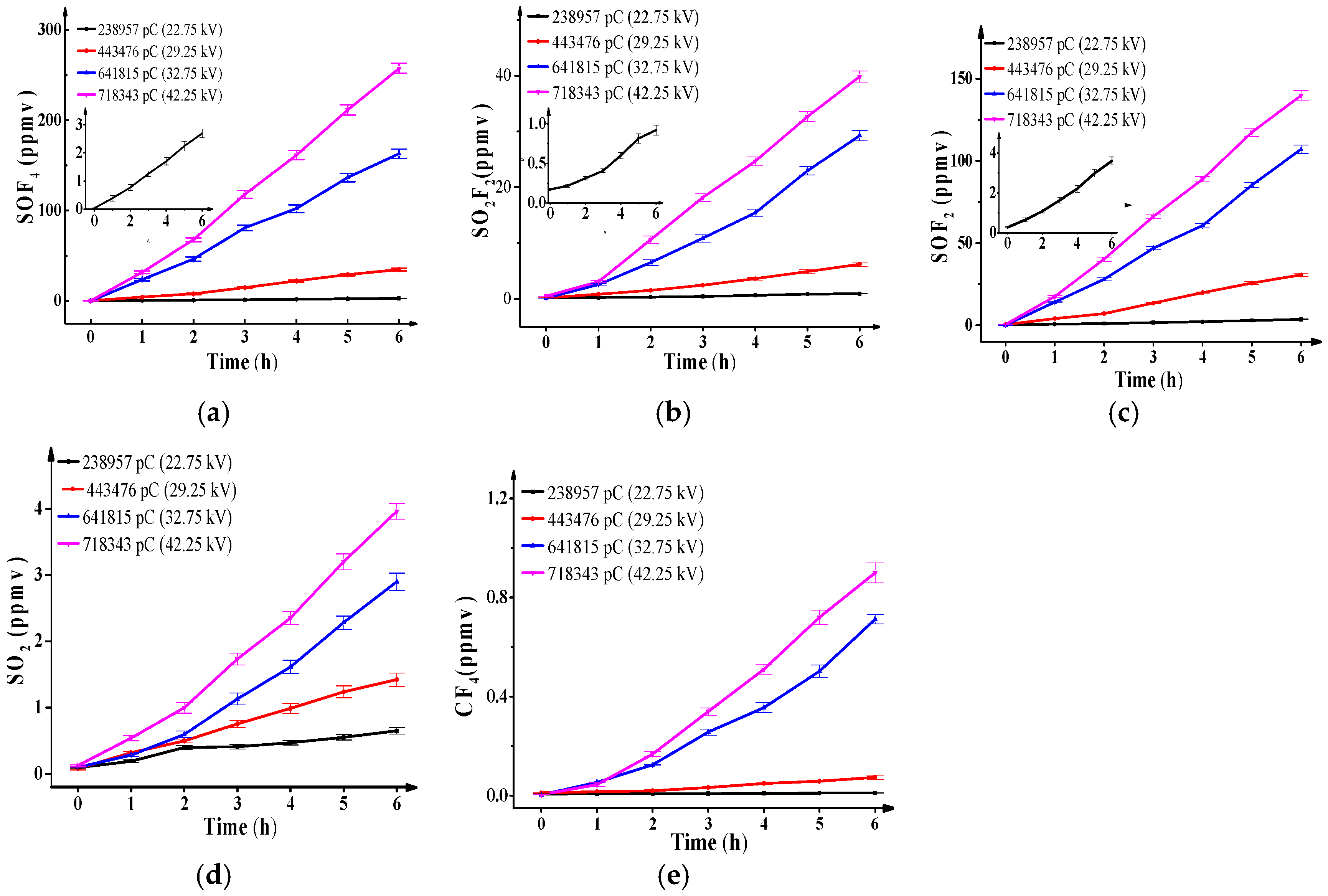
Figure 2 shows the concentration of SO
2F
2, SOF
4, SOF
2, SO
2 and CF
4 against discharge quantities and discharge time. The results are as follows: (1) when the experiment voltage is certain, that is, when the total discharge quantity
is certain, the concentration of all decomposition components increases linearly with the discharge time. (2) The concentration of all decomposition components increases with the discharge quantity level, and the concentration order is: SOF
4 > SOF
2 > SO
2F
2 > SO
2 > CF
4 when the total discharge quantity is higher than 238,957 pC. SOF
4 is the most significant one. Similar results also presented in [
8].
According to the bond dissociation energy [
28], the dissociation energy of the F-SF
X bond is 343.5, 389.1, 286.2, 339.3, 224.7, and 391.6 kJ/mol when the factor x is 5, 4, 3, 2, 1, and 0, respectively, For the SF
x decomposed from SF
6, and the small x corresponds to a greater electron energy. The reactions are given by Equations (1) and (2) [
18]:
As the experimental voltage increases, the ionization intensity of PD gradually strengthens, and pulses with large amplitudes gradually appear (e.g.,
> 150 pC), and the total discharge quantity gradually increases. Furthermore, the number of electrons with high energy gradually increases as well. Therefore, F, SF
5, SF
4, SF
3, SF
2, SF, and S gradually appear, and their generation increases. Similarly, free radicals, such as O and OH, increase as well. Their decomposition reactions are listed in Equations (3)–(5) [
8,
18,
19], the bond energy of O=O is 499 kJ/mol, and H-OH is 498.4 kJ/mol [
28].
The generation of characteristic SF6 decomposition components can be analyzed as follows:
(1) SOF4
Van Brunt proposed that upon discharge most parts of low-fluorine sulfur in the ionization area combined into SF
6 molecules with F, and only a smaller part of them combined into oxyfluorides with O, or OH [
8]. This process is irreversible. SOF
4 is generated from the reactions of SF
5, SF
4, and O, OH as shown in (6)–(8) [
8,
18,
19],
stands for reaction rate coefficient. The
k estimated under negative DC PD or 298 k, respectively, is not a real value of the positive DC discharge, but can provide a reference value due to the similar reaction conditions, such as reaction temperature, reactant, and energy level afford to gas decomposition and no catalyst used in reaction. Then, the rate coefficients in Reactions (9) and (11)–(17) are reference values too for the similar reason. The higher the
is, the easier is it for the reaction to proceed if all other reaction conditions are same, and the higher the concentrations of reactants are:
When the total discharge quantity is 238,957 pC, the maximum discharge quantity of a single pulse is only 176 pC, is approximately 74% of the total discharge quantity , is approximately 91% of the total pulse number . Then, the average energy of electrons is not high, SF5 and SF4 are abundant whereas SF3 and SF2 are scarce. Furthermore, O2 and H2O are scarce in relation to SF6 (approximately 10−6 times), then few O=O, H-OH are broken off and few O, OH generates, so naturally the concentration of SOF4 is extremely low, and less than 3 ppmv at the end of experiment.
On the contrary, when the total discharge quantity increases to 718,343 pC, is 619 pC, is approximately 69% of , and is approximately 53% of . The average energy of electrons increases quickly, and which leads to more broken bonds and greater generation of O and OH as indicated in Reactions (6)–(8). The concentration of SOF4 increases sharply and is up to 257 ppmv at the end of the experiment.
(2) SO2F2
SO
2F
2 is mainly generated from the combination of SOF
4 and H
2O in the main gas chamber [
19] as indicated by Reaction (9):
Given the restrictions of the extremely low hydrolysis rate and water concentration in the reaction, the concentration of SO
2F
2 is lower and less than 1 ppmv at the end of the experiment, it is much lower than the concentration of SOF
4 when
is not high. However, as
increases to 718,343 pC, large amounts of SF
3 and SF
2 are generated under high-energy electrons collisions and combine with O
2 in main gas chamber into SO
2F
2 as Reactions (10) and (11) indicate [
8]. The reaction rate is high, then, the concentration of SO
2F
2 increases rapidly and is close to 40 ppmv at the end of the experiment. The generation depends on the amount of SF
3 and SF
2 generated in the reaction, but it is still far lower than that of SOF
4. The consumptions in Reaction (9) are negligible for the low rate coefficients:
(3) SOF2
SF
5 in the ionization area is not stable and easily combines into SF
6 with F atoms. SF
4 is relatively stable and combines into SOF
2 with OH and H
2O as Reactions (12) and (13) show [
18,
19]:
When
increases, SF
3, SF
2, O, and OH increase and their modes of combination change as indicated in Reactions (14)–(16) [
18,
19]. Strong ionization corresponds to great active particle energy. Then the concentration of SOF
2 can increase sharply up to 140 ppmv at the end of the experiment, which is higher than that of SO
2F
2 but still lower than that of SOF
4.
(4) SO2
Similar to the hydrolysis of SOF
4 shown in Reaction (17) [
19], SOF
2 diffuses to the main gas chamber and combines with gaseous water to form SO
2. Meanwhile, SO
2 continues to react with H
2O in the chamber and generates H
2SO
3 [
19]. Nevertheless, given the extremely low hydrolysis rate of SO
2 and little consumption, the final concentration of SO
2 is still low (less than 4 ppmv) at the end of the experiments:
(5) CF4
CF
4 is formed from C and F atoms [
13], as shown in Reaction (19):
where, F atoms from the decomposition of SF
6 are sufficient, but the amount of C atoms, part of them released from the metal vapor in discharge area on the needle electrode surface or decomposed from impurities in the SF
6 gas (such as trace CO
2 or trace C
2F
6), is extremely limited. Furthermore, the metal vapor is hard to heat with the collision energy of negative ions on the positive electrode. Thus, the concentration of CF
4 is low and near zero. When the discharge quantity
increases from 238,957 pC to 718,343 pC, the collision of higher-energy negative ions crashing onto the metal electrode surface enhances the gasification process of metal and breaks more impurity molecule bonds, then more C atoms are released, and CF
4 generation improves. However, it is still the lowest one among all SF
6 decomposition components. The concentration of CF
4 reflects the PD strength well.
The above analysis show that the increase of the concentrations of SOF4, SO2F2, and SOF2 with PD quantity are more significant than that of SO2 and CF4. The change trend can be used to distinguish the degree of PD strength or PD severity. However, the conclusions are only applicable to a situation where the gas moisture concentration is extremely low and SOF4 cannot easily be hydrolyzed.
3.3. Generation Rates of SF6 Characteristic Decomposition Components
The generation of SF
6 decomposition components is the cumulative result of the decomposition in a certain discharge time under a certain discharge quantity. However, a long time will be needed to distinguish PD strength by observing the change trends of component generation. The observation of the change of generation rate law of components per unit time appears more therefore efficient, to reflect the influence of the discharge quantity on SF
6 decomposition component generation in real time and objectively, the absolute generation rate
and effective generation rate
are proposed for this purpose in this paper. The respective mathematical formulas are written as Equations (20) and (21) [
29]. The effective generation rate has greater statistical significance and contains more information:
where
indicates a sampling interval, which is 1 h,
is the difference between adjacent concentrations, and
is the initial concentration of decomposition components in new SF
6.
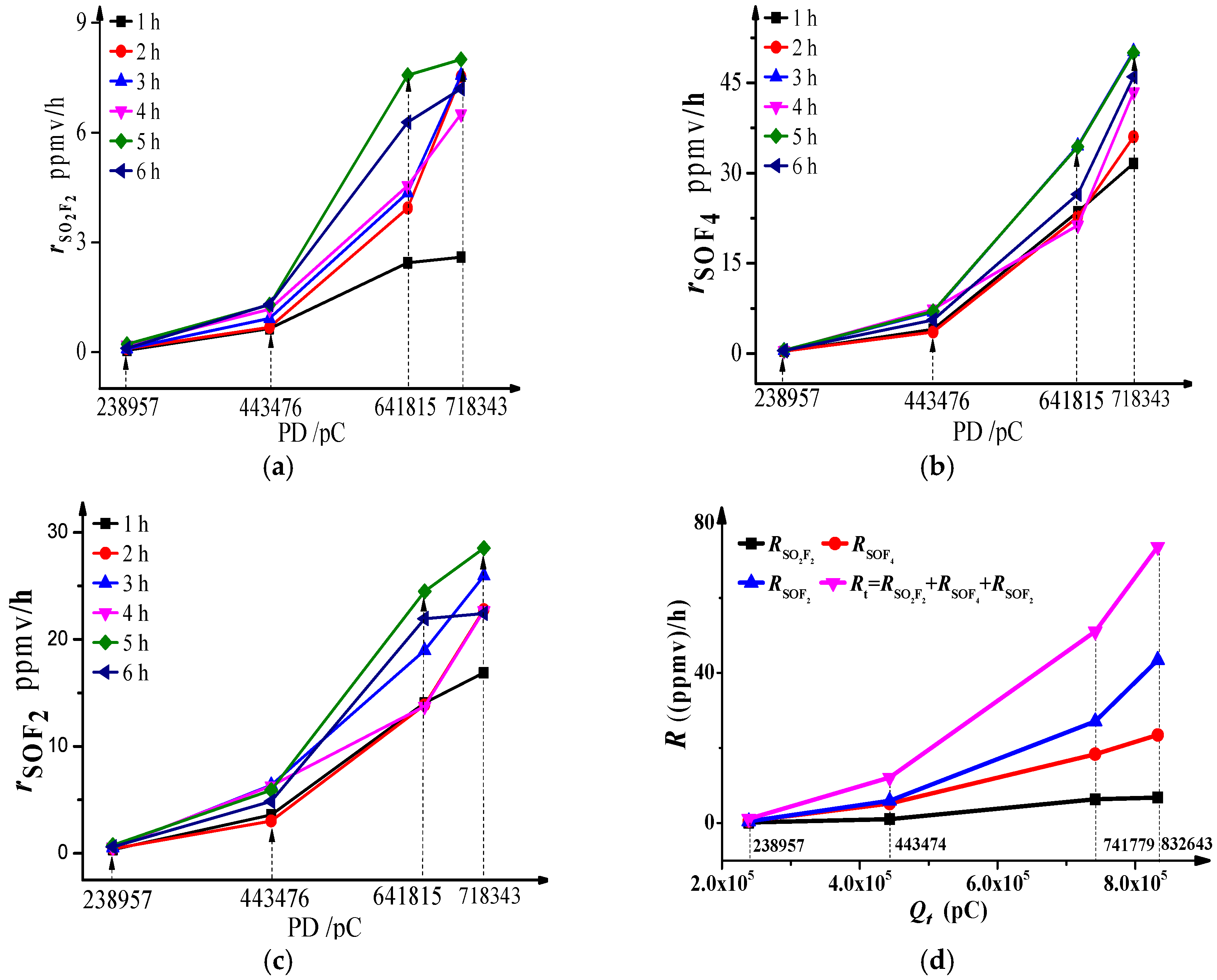
The mathematical relationship between the absolute generation rates and effective generation rates of SOF
4, SOF
2, and SO
2F
2 against discharge quantity are shown in
Figure 3. The results indicate: (1) whatever the total discharge quantity level is, the absolute generation rates of SO
2F
2, SOF
4 and SOF
2 fluctuate around a certain value after 1 h, whereby the values of the three components are 7.36, 45.14 and 24.47 ppmv/h, respectively, when
is 718,343 pC, and the fluctuations (variance) are 0.5, 5.22 and 2.41 ppmv/h. The lower the voltage is, the smaller the sum of the fluctuation of the three components is. (2) As shown in
Figure 3d, the effective generation rates of SOF
4 increase dramatically against total discharge quantity whereas the effective generation rates of SOF
2 and SO
2F
2 do not increase so much. That means the effective generation rate order is:
when
is greater than 238,957 pC. This is similar to the generation order of decomposition components. Van Brunt proposed similar conclusions in [
8].
As mentioned in
Table 1, when the voltage is relatively low, the pulse discharge quantities are small, and most of them are lower than 100 pC, so the total discharge quantity per unit time would not change much. However, as the voltage increases, the pulse amplitudes increase inhomogeneously, and the discharge quantities distribute widely from 50 pC to 619 pC, and the total discharge quantity fluctuates to some degree, so naturally, the generation rates of decomposition components fluctuate too. The smaller the difference of discharge quantity between the discharge pulses, the smaller a fluctuation sum we have. Meanwhile, when the total discharge quantity is low, few active O and OH particles are generated, so SF
4 tends to spread into the ionization area periphery to react with H
2O to generate SOF
2 rather than to generate SOF
4, and the concentration of SOF
2 is slightly higher than that of SOF
4, whereas, when the total discharge quantity grows, SF
4 and SF
5 tend to react with O and OH to form SOF
4, and the reaction amount is more than that of SF
3 and SF
2 reacting with O and OH, because the low-amplitude pulses and low-energy electrons are still a majority, and the concentration of SF
4 is the highest among the low-fluorine sulfur species. Therefore, the generation and generation rate of SOF
4 are higher than that of SOF
2. Furthermore, the consumption of SOF
4 and SOF
2 in hydrolysis reactions is small and has little influence on the final generation. Then, the concentration of their hydrolysis products SO
2F
2 and SO
2 are lower than those of SOF
4 and SOF
2 respectively. When the discharge quantity is high, SF
3 and SF
2 prefer to react with O atoms than O
2 due to their different activities; the generation and generation rate of SOF
2 are higher than that of SO
2F
2.
Actually, the change of main generation way of SOF4, SOF2 and SO2F2 is the main reason for the nonlinear increase of effective generation rates when the discharge quantities increase. The change trend of effective generation rate can be used to distinguish PD strength.
3.4. Concentration Ratios of SF6 Characteristic Decomposition Components
Some studies have focused on the concentration ratios of SF
6 characteristic decomposition components to present PD recognition, such as c(SO
2F
2)/c(SOF
2) in [
14], c(CF
4)/c(CO
2), c(SO
2F
2 + SOF
2)/c(CF
4 + CO
2) in [
13]. Unfortunately, there are no studies on the characteristic concentration ratios of SF
6 decomposition components and their physical significance under positive DC PD conditions. Therefore, the concentration ratio c(SO
2F
2 + SOF
4)/c(SOF
2 + SO
2) was selected as a characteristic to figure out the relationship between the ratios and DC PD quantities. For the ratio, SOF
4 and SO
2F
2, SOF
2 and SO
2 are counted as a whole due to their stable chemical properties in “dry” SF
6 and their role as reactants or as products in hydrolysis reactions. Moreover, the characteristic concentration ratios are independent of the chamber volume comparing generation and effective generation rate. Then the ratios selected to present the discharge faults can improve the PD recognition accuracy.
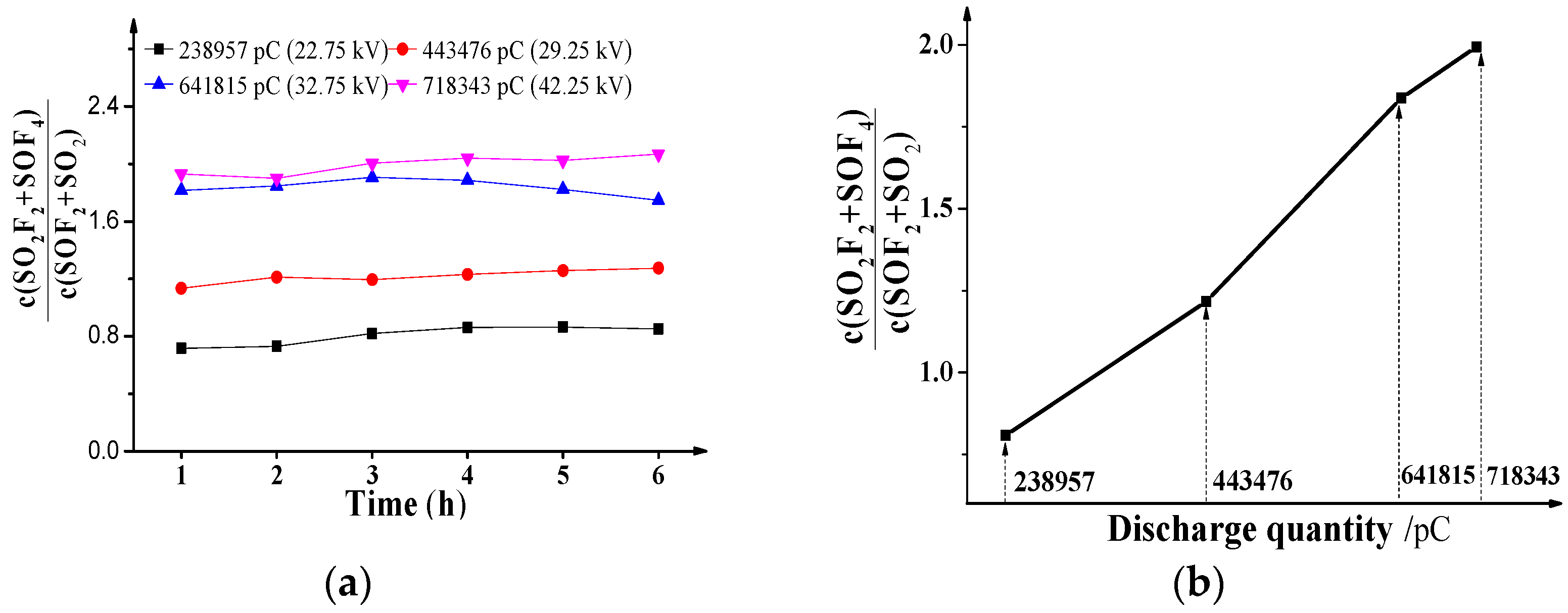
From the concentration data of SO
2F
2, SOF
4, SOF
2 and SO
2 under different voltages in
Figure 2, the effects of positive DC PD discharge quantity on the characteristics concentration ratios c(SO
2F
2 + SOF
4)/c(SOF
2 + SO
2) are shown in
Figure 4a, and the ratio values in
Figure 4b are the means of the values in
Figure 4a.
As shown in
Figure 4a, no matter what the discharge quantity is, the ratio c(SO
2F
2 + SOF
4)/c(SOF
2 + SO
2) does not change much with the extended discharge time, and the values are about 0.8075, 1.2174, 1.8373 and 1.9940, respectively. As show in
Figure 4b, the values of c(SO
2F
2 + SOF
4)/c(SOF
2 + SO
2) increase with the positive DC discharge quantity in an approximately linear manner, which means the discharge quantity has a positive effect on the ratios.
Naturally, the reasons can be attributed to decomposition as shown in
Section 3.3. The effective generation rate order is
when
is greater than 238,957 pC, and the ratios increase with the discharge quantity as seen
Figure 3d, which means the positive effect on SOF
4 and SO
2F
2 is more than that on SOF
2 and SO
2, respectively. Then, the values of c(SO
2F
2 + SOF
4)/c(SOF
2 + SO
2) are greater than 1. Moreover, the greater the positive effect is, the higher the ratios are, and the concentration ratio is closely related to the discharge quantity.
3.5. Quantification of the Promotion Effects of Discharge Quantities on SF6 Decomposition Components
The effective generation rates of decomposition of the components present are the accumulative promotion effects of the pulse discharge quantities in all classes. Then, as mentioned in
Section 3.1, all pulses were divided into four classes, and the promotion efficiency of all pulses in one class on a certain decompose component were assumed to be same. The mathematical relationship of effective generation rate, discharge quantities and promotion coefficients of one kind of decomposition component under a certain experimental voltage is presented as Equation (22), taking SO
2F
2 as an example:
where
is the promotion effect coefficient of pulses in different classes that affect SO
2F
2, and
is the effective generation rate of SO
2F
2. In accordance with that analogy, all coefficient equations of other components can be listed as in Equation (23):
SO
2F
2, SOF
4, SOF
2, and SO
2 are numbered for component
,
as 1, 2, 3, and 4, respectively.
,
,
, and
are numbered for total discharge quantity
,
as 1, 2, 3, 4, respectively, and
is the promotion effect coefficient of the total discharge quantity
of class on the component
. By that analogy, the equation matrix of four experiment voltages could be established and the coefficient matrix be calculated as well (matrix Equation (24)):
Then, as shown in the matrix, the coefficient matrix basically takes the order of
, which indicates that when the total discharge quantity increases from
to
, the pulses whose discharge quantities are higher than 50 pC have promoting effects on the same decomposition component. Higher discharge quantities lead to greater promotion effects on SO
2F
2, SOF
4, SOF
2, and SO
2. When
, which means the pulses whose discharge quantities are lower than 50 pC decrease the positive promotion effects on decomposition components. There are several reasons for this: numerous free low-energy electrons generated in low-amplitude pulses cannot break gas chemical bonds, but they can easily react with active molecules or free radicals (such as SF
4, SF
3, SF
2, O, OH, generated in strong discharges). Then, comparing the high-energy electrons generated in high-amplitude pulses, then they finally form low-energy and inactive negative ions, or react with positive ions to form neutral or stable molecules (e.g., SF
6) [
8]. These reactions inhibit the combination of low fluorine sulfides with O and OH to generate SOF
2, SOF
4, SO
2F
2, etc., but decrease the positive promotion effects that large discharge pulses have on components.
Meanwhile, the coefficient matrix takes the order of
, which indicates that the pulses in the same discharge quantity class have different promoting effects on different decomposition components. The promotion effects take the following order:
, which is consistent with the order of generation and the effective generation rates mentioned in
Section 3.2 and
Section 3.3. Therefore, the results of the coefficient matrix are consistent with the actual situation and reveal the substantial effects of PD severity on SF
6 decomposition components from a mathematical viewpoint.
Therefore, when partial discharges occur in a piece of equipment under a certain voltage, the total discharge quantities of the four classes and their proportion with respect to can be deduced by the effect coefficient matrix and effective generation rate of four types of components in Equation (23). Then, the fault severity can be deduced preliminarily by their discharge quantity. However, the interval discharge quantity of classes in this paper is slightly large, and the assumption that the promotion effect coefficients of pulses in one class on one component are the same seems oversimple and crude, therefore, the diagnosis results may not inaccurate, and more classes must be layered to obtain detailed coefficients. Furthermore, H2O, O2, gas temperature, gas pressure, adsorbents or other influencing factors that could affect the concentration of components should be considered, therefore, more experiments should be performed to obtain more statistical data to get a real and accurate coefficient matrix, which should be helpful for obtaining more accurate and detailed discharge information and reliable judgments on fault severity diagnosis. The coefficient matrix mentioned in this work only offers one method for further research on the use of SF6 decomposition characteristics to diagnose fault severity.