Effect of Carbon Nanoadditives on Lithium Hydroxide Monohydrate-Based Composite Materials for Low Temperature Chemical Heat Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Raw Materials and Synthesis Method of Lithium Hydroxide Monohydrate-Based TCMs
2.2. The Characterization and Heat Storage Performance Test Method of Lithium Hydroxide Monohydrate-Based TCMs
3. Results and Discussion
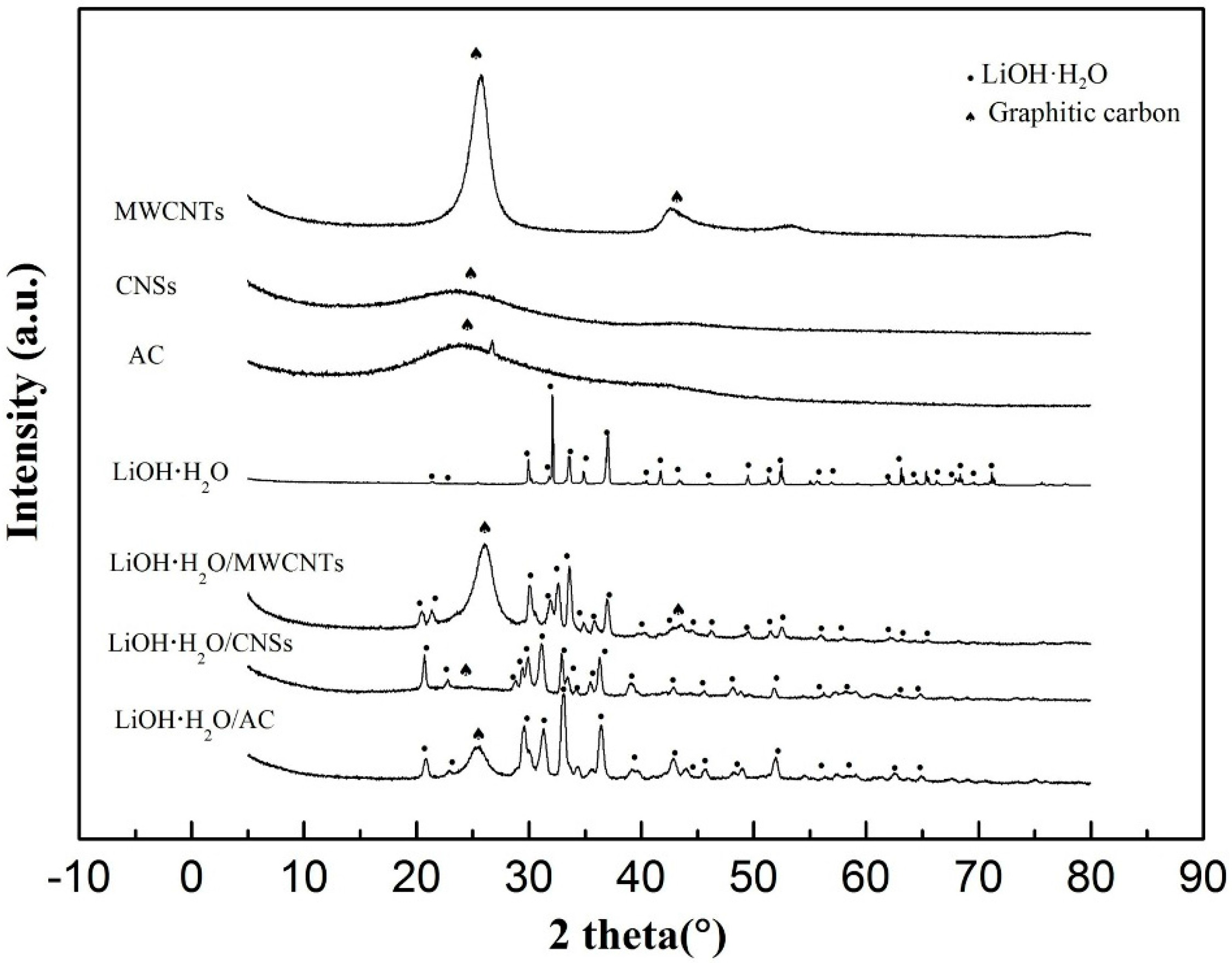
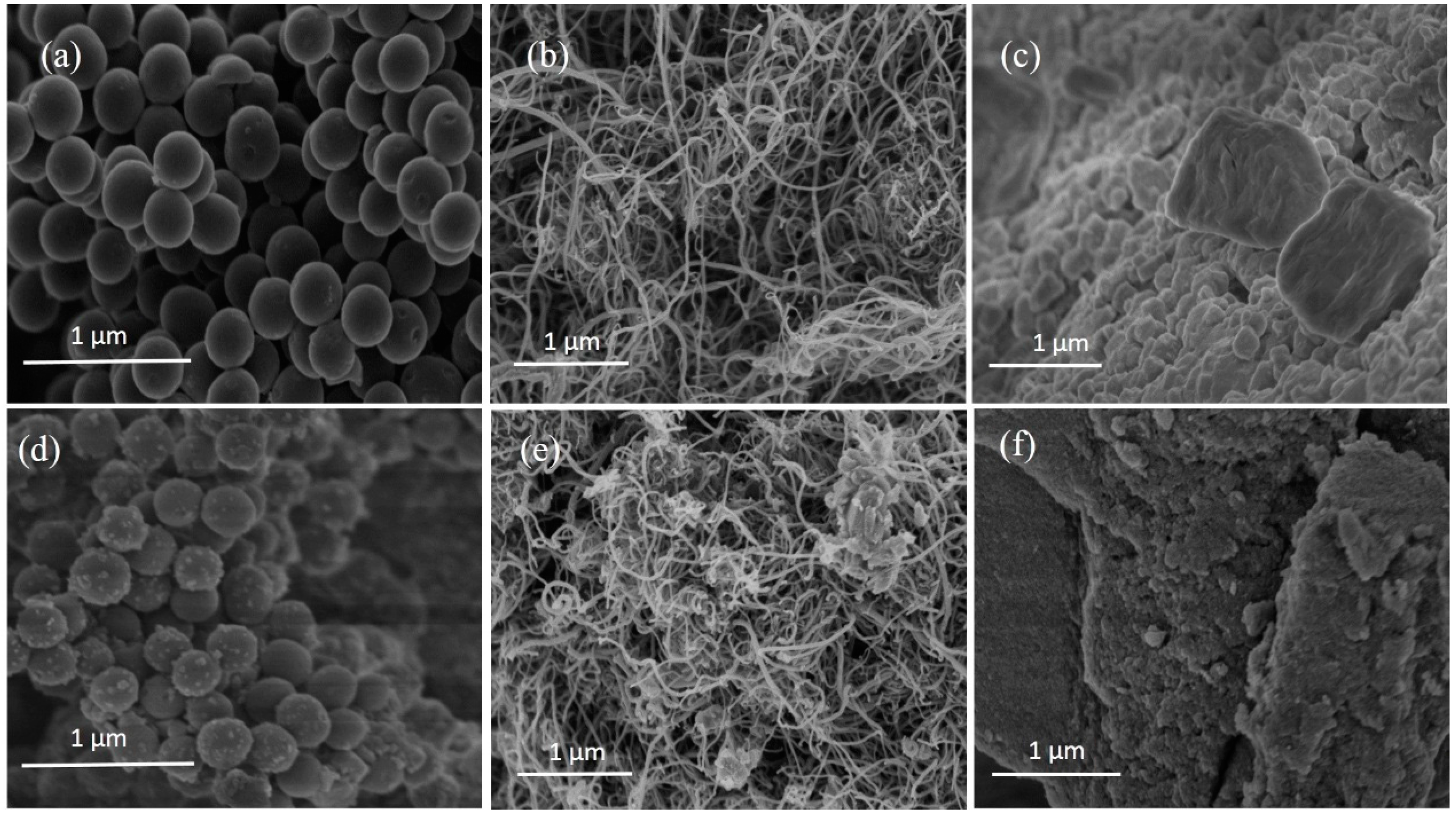
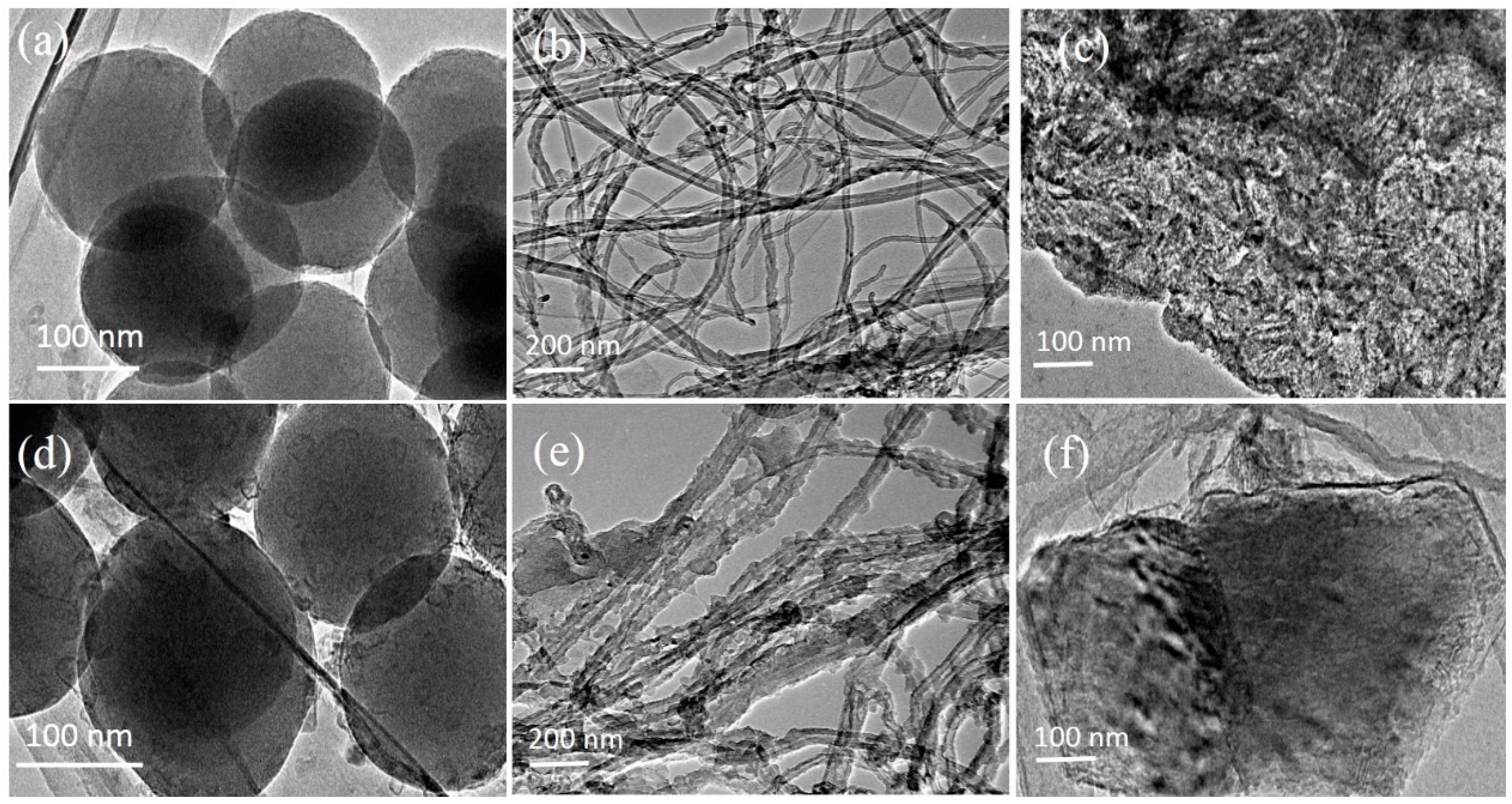
3.1. The Microstructure Characterization of Lithium Hydroxide Monohydrate-Based TCMs
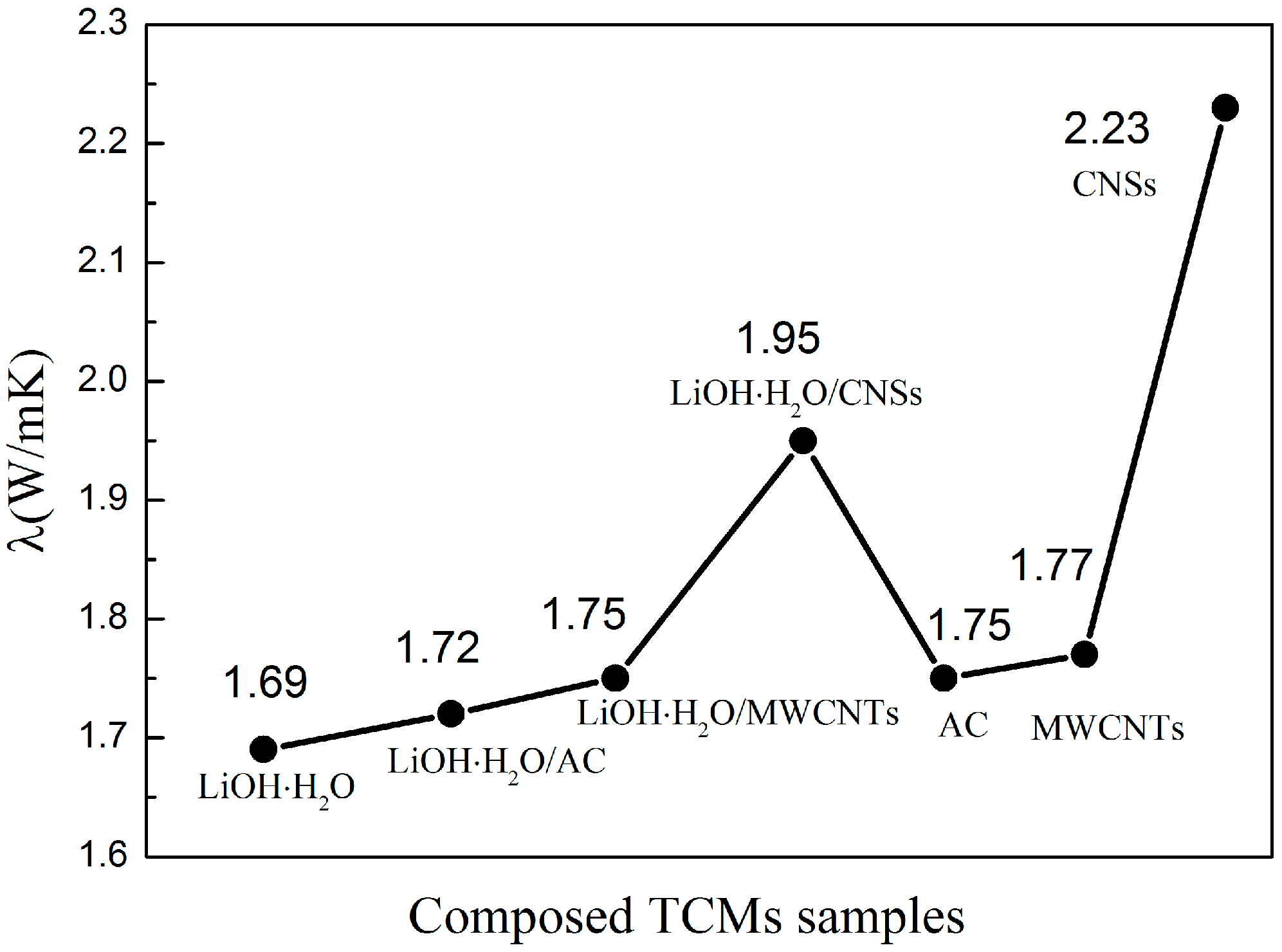
3.2. The Heat Storage Performance Test of Lithium Hydroxide Monohydrate-Based TCMs
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gil, A.; Medrano, M.; Martorell, I.; Lázaro, A.; Dolado, P.; Zalba, B.; Cabeza, L.F. State of the art on high temperature thermal energy storage for power generation. Part 1—Concepts, materials and modellization. Renew. Sustain. Energy Rev. 2010, 14, 31–55. [Google Scholar] [CrossRef]
- Tang, Q.; Sun, J.; Yu, S.; Wang, G. Improving thermal conductivity and decreasing supercooling of paraffin phase change materials by n-octadecylamine-functionalized multi-walled carbon nanotubes. RSC Adv. 2014, 4, 36584–36590. [Google Scholar] [CrossRef]
- Yan, T.; Wang, R.Z.; Li, T.X.; Wang, L.W.; Fred, I.T. A review of promising candidate reactions for chemical heat storage. Renew. Sustain. Energy Rev. 2015, 43, 13–31. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef]
- Ishitobi, H.; Uruma, K.; Takeuchi, M.; Ryu, J.; Kato, Y. Dehydration and hydration behavior of metal-salt-modified materials for chemical heat pumps. Appl. Therm. Eng. 2013, 50, 1639–1644. [Google Scholar] [CrossRef]
- Ogura, H.; Yamamoto, T.; Kage, H. Efficiencies of CaO/H2O/Ca(OH)2 chemical heat pump for heat storing and heating/cooling. Energy 2003, 28, 1479–1493. [Google Scholar] [CrossRef]
- Sheppard, D.A.; Paskevicius, M.; Buckley, C.E. Thermodynamics of hydrogen desorption from NaMgH3 and its application as a solar heat storage medium. Chem. Mater. 2011, 23, 4298–4300. [Google Scholar] [CrossRef]
- Kyaw, K.; Shibata, T.; Watanabe, F.; Matsuda, H.; Hasatani, M. Applicability of zeolite for CO2 storage in a CaO-CO2 high temperature energy storage system. Energy Convers. Manag. 1997, 38, 1025–1033. [Google Scholar] [CrossRef]
- Yang, X.X.; Huang, H.Y.; Wang, Z.H.; Kubota, M.; He, Z.H.; Kobayashi, N. Facile synthesis of graphene oxide-modified lithium hydroxide for low-temperature chemical heat storage. Chem. Phys. Lett. 2016, 644, 31–34. [Google Scholar] [CrossRef]
- Wongsuwan, W.; Kumar, S.; Neveu, P.; Meunier, F. A review of chemical heat pump technology and applications. Appl. Therm. Eng. 2001, 21, 1489–1519. [Google Scholar] [CrossRef]
- Kubota, M.; Horie, N.; Togari, H.; Matsuda, H. Improvement of hydration rate of LiOH/LiOH·H2O reaction for low-temperature thermal energy storage. Procceding of the 2013 Annual Meeting of Japan Society of Refrigerating and Air Conditioning Engineers, Tokyo, Japan, 10–12 September 2013. [Google Scholar]
- Whiting, G.; Grondin, D.; Bennici, S.; Auroux, A. Heats of water sorption studies on zeolite-MgSO4 composites as potential thermochemical heat storage materials. Sol. Energy Mater. Sol. Cells 2013, 112, 112–119. [Google Scholar] [CrossRef]
- Hongois, S.; Kuznik, F.; Stevens, P.; Roux, J.J. Development and characterisation of a new MgSO4-zeolite composite for long-term thermal energy storage. Sol. Energy Mater. Sol. Cells 2011, 95, 1831–1837. [Google Scholar] [CrossRef]
- Mehrali, M.; Tahan Latibari, S.; Mehrali, M.; Mahlia, T.M.I.; Cornelis Metselaar, H.S. Effect of carbon nanospheres on shape stabilization and thermal behavior of phase change materials for thermal energy storage. Energy Convers. Manag. 2014, 88, 206–213. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Candelaria, S.L.; Shao, Y.; Zhou, W.; Li, X.; Xiao, J.; Zhang, J.G.; Wang, Y.; Liu, J.; Li, J.; Cao, G. Nanostructured carbon for energy storage and conversion. Nano Energy 2012, 1, 195–220. [Google Scholar] [CrossRef]
- Cao, Q.; Rogers, J.A. Ultrathin films of single-walled carbon nanotubes for electronics and sensors: A review of fundamental and applied aspects. Adv. Mater. 2010, 21, 29–53. [Google Scholar] [CrossRef]
- Cao, Q.; Han, S.J.; Tulevski, G.S.; Zhu, Y.; Lu, D.D.; Haensch, W. Arrays of single-walled carbon nanotubes with full surface coverage for high-performance electronics. Nat. Nanotechnol. 2013, 8, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Salvo, A.M.P.; La Parola, V.; Liotta, L.F.; Giacalone, F.; Gruttadauria, M. Highly loaded multi-walled carbon nanotubes non-covalently modified with a bis-imidazolium salt and their use as catalyst supports. ChemPlusChem 2016, 81, 471–476. [Google Scholar] [CrossRef]
- Serp, P.; Corrias, M.; Kalck, P. Carbon nanotubes and nanofibers in catalysis. Cheminform 2003, 253, 337–358. [Google Scholar] [CrossRef]
- Tumuluri, K.; Alvarado, J.L.; Taherian, H.; Marsh, C. Thermal performance of a novel heat transfer fluid containing multiwalled carbon nanotubes and microencapsulated phase change materials. Int. J. Heat Mass Transf. 2011, 54, 5554–5567. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z. Paraffin/diatomite/multi-wall carbon nanotubes composite phase change material tailor-made for thermal energy storage cement-based composites. Energy 2014, 72, 371–380. [Google Scholar] [CrossRef]
- Liu, X.C.; Osaka, Y.; Huang, H.Y.; Huhetaoli; Li, J.; Yang, X.X.; Li, S.J.; Kobayashi, N. Development of low-temperature desulfurization performance of a MnO2/AC composite for a combined SO2 trap for diesel exhaust. RSC Adv. 2016, 6, 96367–96375. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Li, Z.; Zheng, H.Y.; Fu, T.J.; Ju, Y.B.; Wang, Y.C. Influence of the surface oxygenated groups of activated carbon on preparation of a nano Cu/AC catalyst and heterogeneous catalysis in the oxidative carbonylation of methanol. Appl. Catal. B Environ. 2015, 179, 95–105. [Google Scholar] [CrossRef]
- Wan, X.; Wang, H.; Yu, H.; Peng, F. Highly uniform and monodisperse carbon nanospheres enriched with cobalt—Nitrogen active sites as a potential oxygen reduction electrocatalyst. J. Power Sources 2017, 346, 80–88. [Google Scholar] [CrossRef]
- Yuan, D.; Chen, J.; Zeng, J.; Tan, S. Preparation of monodisperse carbon nanospheres for electrochemical capacitors. Electrochem. Commun. 2008, 10, 1067–1070. [Google Scholar] [CrossRef]
- Wang, J.; Xie, H.; Xin, Z.; Li, Y.; Chen, L. Enhancing thermal conductivity of palmitic acid based phase change materials with carbon nanotubes as fillers. Sol. Energy 2010, 84, 339–344. [Google Scholar] [CrossRef]
- Wang, B.X.; Zhou, L.P.; Peng, X.F. Surface and size effects on the specific heat capacity of nanoparticles. Int. J. Thermophys. 2005, 27, 139–151. [Google Scholar] [CrossRef]
- Chen, L.J.; Zou, R.Q.; Xia, W.; Liu, Z.P.; Shang, Y.Y.; Zhu, J.L.; Wang, Y.X.; Lin, J.H.; Xia, D.G.; Cao, A.Y. Electro-and photodriven phase change composites based on wax-infiltrated carbon nanotube sponges. ACS Nano 2012, 6, 10884–10892. [Google Scholar] [CrossRef] [PubMed]

| Samples | BET Surface Area (m2/g) | Pore Volume (mL/g) | Average Pore Size (nm) |
|---|---|---|---|
| CNSs | 381 | 0.25 | 4.81 |
| MWCNTs | 343 | 0.61 | 8.95 |
| AC | 324 | 0.75 | 2.71 |
| LiOH·H2O/CNSs | 276 | 0.18 | 2.64 |
| LiOH·H2O/MWCNTs | 140 | 0.31 | 8.81 |
| LiOH·H2O/AC | 84 | 0.03 | 2.54 |
| Pure LiOH·H2O | 15 | 0.06 | 1.75 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Li, S.; Huang, H.; Li, J.; Kobayashi, N.; Kubota, M. Effect of Carbon Nanoadditives on Lithium Hydroxide Monohydrate-Based Composite Materials for Low Temperature Chemical Heat Storage. Energies 2017, 10, 644. https://doi.org/10.3390/en10050644
Yang X, Li S, Huang H, Li J, Kobayashi N, Kubota M. Effect of Carbon Nanoadditives on Lithium Hydroxide Monohydrate-Based Composite Materials for Low Temperature Chemical Heat Storage. Energies. 2017; 10(5):644. https://doi.org/10.3390/en10050644
Chicago/Turabian StyleYang, Xixian, Shijie Li, Hongyu Huang, Jun Li, Noriyuki Kobayashi, and Mitsuhiro Kubota. 2017. "Effect of Carbon Nanoadditives on Lithium Hydroxide Monohydrate-Based Composite Materials for Low Temperature Chemical Heat Storage" Energies 10, no. 5: 644. https://doi.org/10.3390/en10050644





