Natural Gas Hydrate as a Storage Mechanism for Safe, Sustainable and Economical Production from Offshore Petroleum Reserves
Abstract
:1. Introduction
1.1. Safety Concerns
1.2. German SUGAR Project
1.3. Japanese Demonstration Experiment
1.4. Scientific Investigations
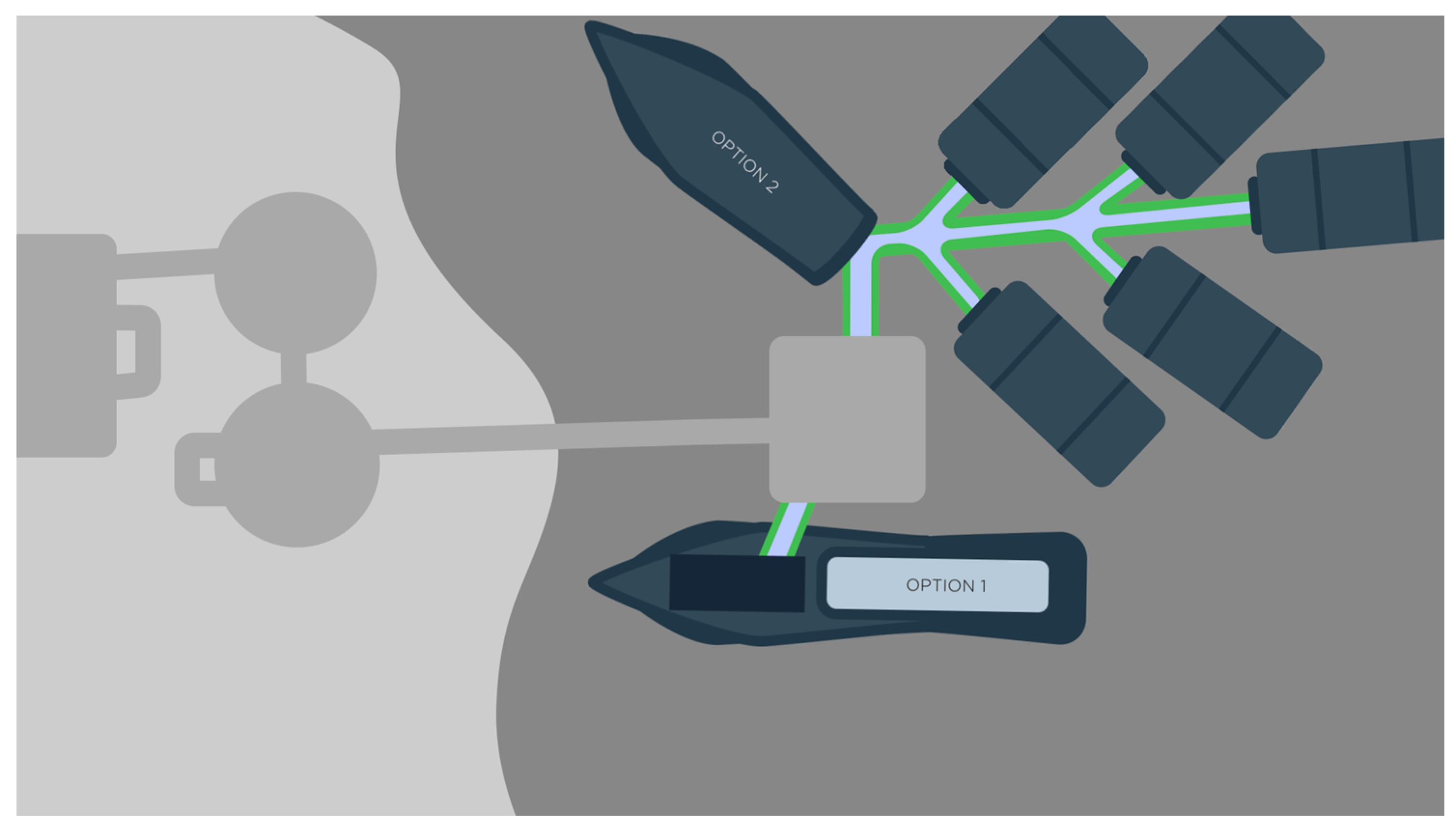
2. Process Constraints Discussion
2.1. Formation of Hydrate in the Deep Ocean
2.2. Shipping Methods
2.3. Re-Gasification of Natural Gas
2.4. Additional Benefits of NGH
3. Discussion
3.1. Formation of NGH—In Situ Conditions
3.2. Self-Preservation Effect
3.3. Containers for Natural Gas Hydrate
3.4. Re-Gasification
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Tusiani, M.D.; Shearer, G. LNG: Fuel for a Changing World—A Nontechnical Guide, 2nd ed.; PennWell: Tulsa, OK, USA, 2016. [Google Scholar]
- Rehder, G.; Eckl, R.; Elfgen, M.; Falenty, A.; Hamann, R.; Kähler, N.; Kuhs, W.F.; Osterkamp, H.; Windmeier, C. Methane Hydrate Pellet Transport Using the Self-Preservation Effect: A Techno-Economic Analysis. Energies 2012, 5, 2499–2523. [Google Scholar] [CrossRef]
- Satoo, N. Development of Natural Gas Hydrate (NGH) Supply Chain. In Proceedings of the 25th World Gas Conference, Kuala Lumpur, Malaysia, 4–8 June 2012. [Google Scholar]
- Sloan, E.D.; Koh, C. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Seo, Y.; Kang, S.P. Inhibition of Methane Hydrate Re-formation in Offshore Pipelines with a Kinetic Hydrate Inhibitor. J. Pet. Sci. Eng. 2012, 88–89, 61–66. [Google Scholar] [CrossRef]
- Brinchi, L.; Castellani, B.; Rossi, F.; Cotana, F.; Morini, E.; Nicolini, A.; Filipponi, M. Experimental Investigations on Scaled-up Methane Hydrate Production with Surfactant Promotion: Energy Considerations. J. Pet. Sci. Eng. 2014, 120, 187–193. [Google Scholar] [CrossRef]
- Ando, N.; Kuwabara, Y.; Mori, Y.H. Surfactant effects on hydrate formation in an unstirred gas/liquid system: An experimental study using methane and micelle-forming surfactants. Chem. Eng. Sci. 2012, 73, 79–85. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.C.; Lee, J.D. Morphology study of methane–propane clathrate hydrates on the bubble surface in the presence of SDS or PVCap. J. Cryst. Growth 2014, 402, 249–259. [Google Scholar] [CrossRef]
- Mori, Y.H. On the Scale-up of Gas-Hydrate-Forming Reactors: The Case of Gas-Dispersion-Type Reactors. Energies 2015, 8, 1317–1335. [Google Scholar] [CrossRef]
- Yapa, P.R.; Chen, F. Behavior of Oil and Gas from Deepwater Blowouts. J. Hydraul. Eng. 2004, 130, 540–553. [Google Scholar] [CrossRef]
- Milkov, A.V. Global estimates of hydrate-bound gas in marine sediments: How much is really out there? Earth Sci. Rev. 2004, 66, 183–197. [Google Scholar] [CrossRef]
- Kvenvolden, K.A.; Ginsburg, G.D.; Soloviev, V.A. Worldwide distribution of subaquatic gas hydrates. Geo-Marine Lett. 1993, 13, 32–40. [Google Scholar] [CrossRef]
- Max, M.D.; Johnson, A.H.; Dillon, W.P. Economic Geology of Natural Gas Hydrate; Springer Science & Business Media: Dordrecht, The Netherlands, 2005; Volume 9. [Google Scholar]
- Bolstad, E.; Clark, L.; Chang, D. Engineers Work to Place Siphon Tube at Oil Spill Site. Available online: http://www.icyte.com/system/snapshots/fs1/7/f/0/c/7f0c761399d7de5ece45aa69ca918ad5f8a1b880/index.html (accessed on 15 May 2010).
- Daniel, C. Giant Dome Fails to Fix Deepwater Horizon Oil Disaster. Available online: http://blogs.nature.com/news/thegreatbeyond/2010/05/_giant_dome_fails_to_fix_deepw.html (accessed on 10 May 2010).
- Stern, L.A.; Circone, S.; Kirby, S.H.; Durham, W.B. Anomalous preservation of pure methane hydrate at 1 atm. J. Phys. B Chem. 2001, 105, 1756–1762. [Google Scholar] [CrossRef]
- Phoenix, S.L.; Kezirian, M.T. Method and System for Extracting Stranded Gas from Underwater Environments, Converting It to Clathrates, and Safely Transporting It for Consumption. International Patent Application No. PCT/US2016/055912, 7 October 2016. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kezirian, M.T.; Phoenix, S.L. Natural Gas Hydrate as a Storage Mechanism for Safe, Sustainable and Economical Production from Offshore Petroleum Reserves. Energies 2017, 10, 828. https://doi.org/10.3390/en10060828
Kezirian MT, Phoenix SL. Natural Gas Hydrate as a Storage Mechanism for Safe, Sustainable and Economical Production from Offshore Petroleum Reserves. Energies. 2017; 10(6):828. https://doi.org/10.3390/en10060828
Chicago/Turabian StyleKezirian, Michael T., and S. Leigh Phoenix. 2017. "Natural Gas Hydrate as a Storage Mechanism for Safe, Sustainable and Economical Production from Offshore Petroleum Reserves" Energies 10, no. 6: 828. https://doi.org/10.3390/en10060828




