Synthesis, Structure, and Sodium Mobility of Sodium Vanadium Nitridophosphate: A Zero-Strain and Safe High Voltage Cathode Material for Sodium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mateirals Synthesis
2.2. Structural Characterization
2.3. Electrochemical Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-ion batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.P.; Chevrier, V.L.; Hautier, G.; Jain, A.; Moore, C.; Kim, S.; Ma, X.; Ceder, G. Voltage, stability and diffusion barrier differences between sodium-ion and lithium-ion intercalation materials. Energy Environ. Sci. 2011, 4, 3680–3688. [Google Scholar] [CrossRef]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-Gonzalez, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Lee, K.T.; Ramesh, T.N.; Nan, F.; Botton, G.; Nazar, L.F. Topochemical synthesis of sodium metal phosphate olivines for sodium-ion batteries. Chem. Mater. 2011, 23, 3593–3600. [Google Scholar] [CrossRef]
- Saravanan, K.; Mason, C.W.; Rudola, A.; Wong, K.H.; Balaya, P. The first report on excellent cycling stability and superior rate capability of Na3V2(PO4)3 for sodium ion batteries. Adv. Energy Mater. 2013, 3, 444–450. [Google Scholar] [CrossRef]
- Kim, H.; Shakoor, R.A.; Park, C.; Lim, S.Y.; Kim, J.-S.; Jo, Y.N.; Cho, W.; Miyasaka, K.; Kahraman, R.; Jung, Y.; et al. Na2FeP2O7 as a promising iron-based pyrophosphate cathode for sodium rechargeable batteries: A combined experimental and theoretical study. Adv. Funct. Mater. 2013, 23, 1147–1155. [Google Scholar] [CrossRef]
- Ellis, B.L.; Makahnouk, W.R.M.; Rowan-Weetaluktuk, W.N.; Ryan, D.H.; Nazar, L.F. Crystal structure and electrochemical properties of A2MPO4F fluorophosphates (A = Na, Li; M = Fe, Mn, Co, Ni). Chem. Mater. 2010, 22, 1059–1070. [Google Scholar] [CrossRef]
- Chihara, K.; Kitajou, A.; Gocheva, I.D.; Okada, S.; Yamaki, J.-I. Cathode properties of Na3M2(PO4)2F3 [M = Ti, Fe, V] for sodium-ion batteries. J. Power Sources 2013, 227, 80–85. [Google Scholar] [CrossRef]
- Singh, P.; Shiva, K.; Celio, H.; Goodenough, J.B. Eldfellite NaFe(SO4)2: An intercalation cathode host for low-cost Na-ion batteries. Energy Environ. Sci. 2015, 8, 3000–3005. [Google Scholar] [CrossRef]
- Barpanda, P.; Chotard, J.-N.; Recham, N.; Delacourt, C.; Ati, M.; Dupont, L.; Armand, M.; Tarascon, J.-M. Structural, transport, and electrochemical investigation of Novel AMSO4F (A = Na, Li; M = Fe, Co, Ni, Mn) metal fluorosulphates prepared using low temperature synthesis routes. Inorg. Chem. 2010, 49, 7401–7413. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, J.; Ye, Z.; Zhao, X.; Wu, S.; Mi, J.-X.; Wang, C.-Z.; Gong, Z.; McDonald, M.J.; Zhu, Z.; et al. A zero-strain Na2FeSiO4 as novel cathode material for sodium ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 17233–17238. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.M.; Eames, C.; Kendrick, E.; Islam, M.S. Sodium ion diffusion and voltage trends in phosphates Na4M3(PO4)2P2O7 (M = Fe, Mn, Co, Ni) for possible high-rate cathodes. J. Phys. Chem. C 2015, 119, 15935–15941. [Google Scholar] [CrossRef]
- Zhang, H.; Hasa, I.; Buchholz, D.; Qin, B.; Geiger, D.; Jeong, S.; Kaiser, U.; Passerini, S. Exploring the Ni redox activity in polyanionic compounds as conceivable high potential cathodes for Na rechargeable batteries. NPG Asia Mater. 2017, 9, e370. [Google Scholar] [CrossRef]
- Barpanda, P.; Ati, M.; Melot, B.C.; Rousse, G.; Chotard, J.N.; Doublet, M.L.; Sougrati, M.T.; Corr, S.A.; Jumas, J.C.; Tarascon, J.M. A 3.90 V iron-based fluorosulphate material for lithium-ion batteries crystallizing in the triplite structure. Nat. Mater. 2011, 10, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Zatovsky, I. NASICON-type Na3V2(PO4)3. Acta Crystallogr. Sect. E 2010, 66, i12. [Google Scholar] [CrossRef] [PubMed]
- Gover, R.K.B.; Bryan, A.; Burns, P.; Barker, J. The electrochemical insertion properties of sodium vanadium fluorophosphate, Na3V2(PO4)2F3. Solid State Ion. 2006, 177, 1495–1500. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Y.-Y.; Dunstan, M.T.; Huo, H.; Hao, X.; Zou, H.; Zhong, G.; Yang, Y.; Grey, C.P. Local structure and dynamics in the Na ion battery positive electrode material Na3V2(PO4)2F3. Chem. Mater. 2014, 26, 2513–2521. [Google Scholar] [CrossRef]
- Liu, J.; Yu, X.; Hu, E.; Nam, K.-W.; Yang, X.-Q.; Khalifah, P.G. Divalent iron nitridophosphates: A new class of cathode materials for Li-ion batteries. Chem. Mater. 2013, 25, 3929–3931. [Google Scholar] [CrossRef]
- Liu, J.; Chang, D.; Whitfield, P.; Janssen, Y.; Yu, X.; Zhou, Y.; Bai, J.; Ko, J.; Nam, K.-W.; Wu, L.; et al. Ionic conduction in cubic Na3TiP3O9N, a secondary Na-ion battery cathode with extremely low volume change. Chem. Mater. 2014, 26, 3295–3305. [Google Scholar] [CrossRef]
- Massiot, D.; Conanec, R.; Feldmann, W.; Marchand, R.; Laurent, Y. NMR characterization of the Na3AlP3O9N and Na2Mg2P3O9N nitridophosphates: Location of the (NaAl)/Mg2 substitution. Inorg. Chem. 1996, 35, 4957–4960. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, S.-J. Synchrotron powder study of Na3V(PO3)3N. Acta Crystallogr. Sect. E 2013, 69, i34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hasa, I.; Qin, B.; Diemant, T.; Buchholz, D.; Behm, J.; Passerini, S. Excellent cycling stability and superior rate capability of Na3V2(PO4)3 cathodes enabled by nitrogen-doped carbon interpenetration for sodium-ion batteries. ChemElectroChem 2017, 4, 1256–1263. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, G. Developments in nanostructured cathode materials for high-performance lithium-ion batteries. Adv. Mater. 2008, 20, 2251–2269. [Google Scholar] [CrossRef]
- Gaberscek, M.; Dominko, R.; Jamnik, J. Is small particle size more important than carbon coating? An example study on LiFePO4 cathodes. Electrochem. Commun. 2007, 9, 2778–2783. [Google Scholar] [CrossRef]
- Wagemaker, M.; Ellis, B.L.; Lützenkirchen-Hecht, D.; Mulder, F.M.; Nazar, L.F. Proof of supervalent doping in olivine LiFePO4. Chem. Mater. 2008, 20, 6313–6315. [Google Scholar] [CrossRef]
- Hung, T.-F.; Cheng, W.-J.; Chang, W.-S.; Yang, C.-C.; Shen, C.-C.; Kuo, Y.-L. Ascorbic acid-assisted synthesis of mesoporous sodium vanadium phosphate nanoparticles with highly sp2-coordinated carbon coatings as efficient cathode materials for rechargeable sodium-ion batteries. Chem. Eur. J. 2016, 22, 10620–10626. [Google Scholar] [CrossRef] [PubMed]
- Recham, N.; Chotard, J.N.; Dupont, L.; Delacourt, C.; Walker, W.; Armand, M.; Tarascon, J.M. A 3.6 V lithium-based fluorosulphate insertion positive electrode for lithium-ion batteries. Nat. Mater. 2010, 9, 68–74. [Google Scholar] [CrossRef] [PubMed]
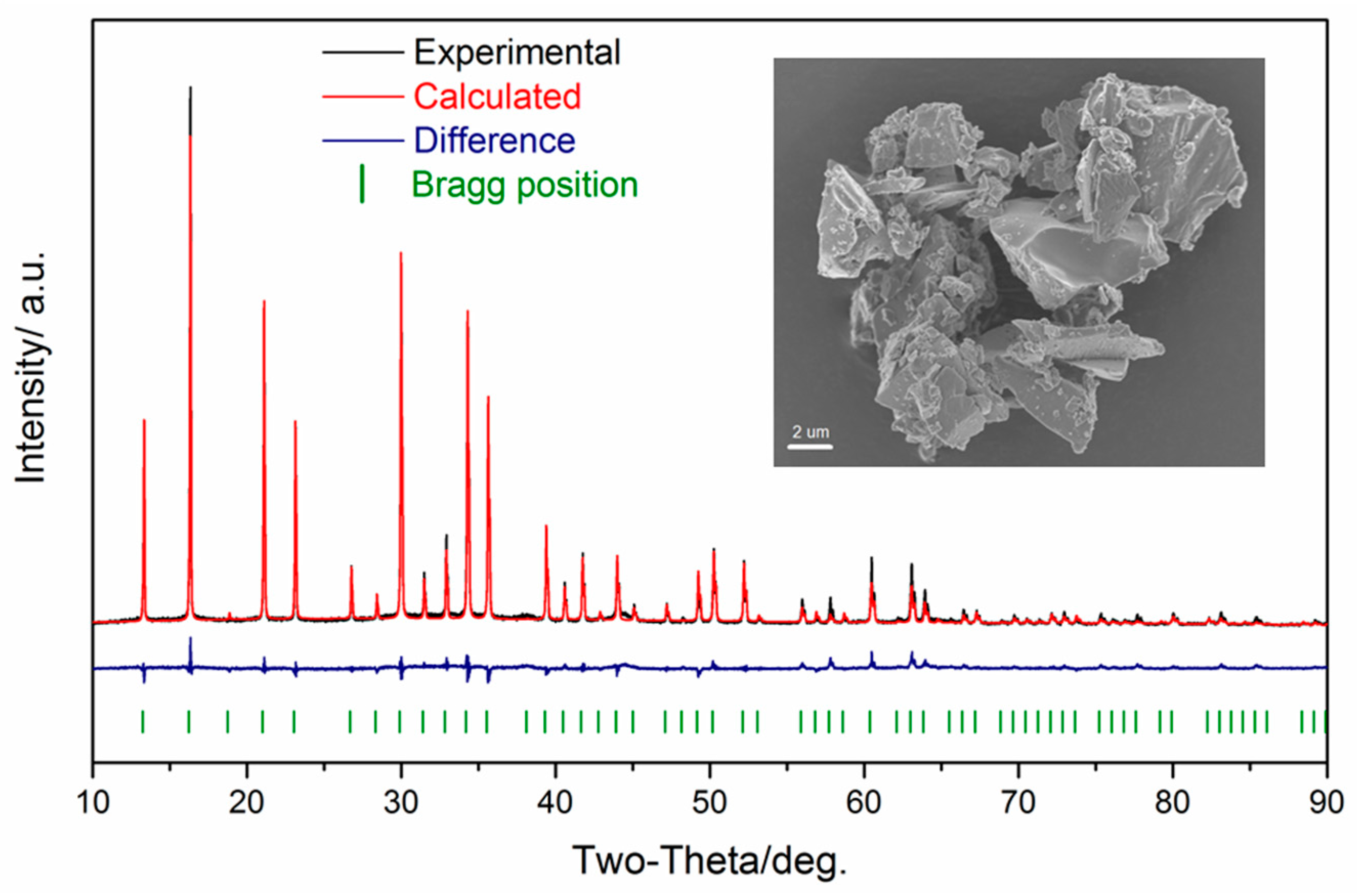
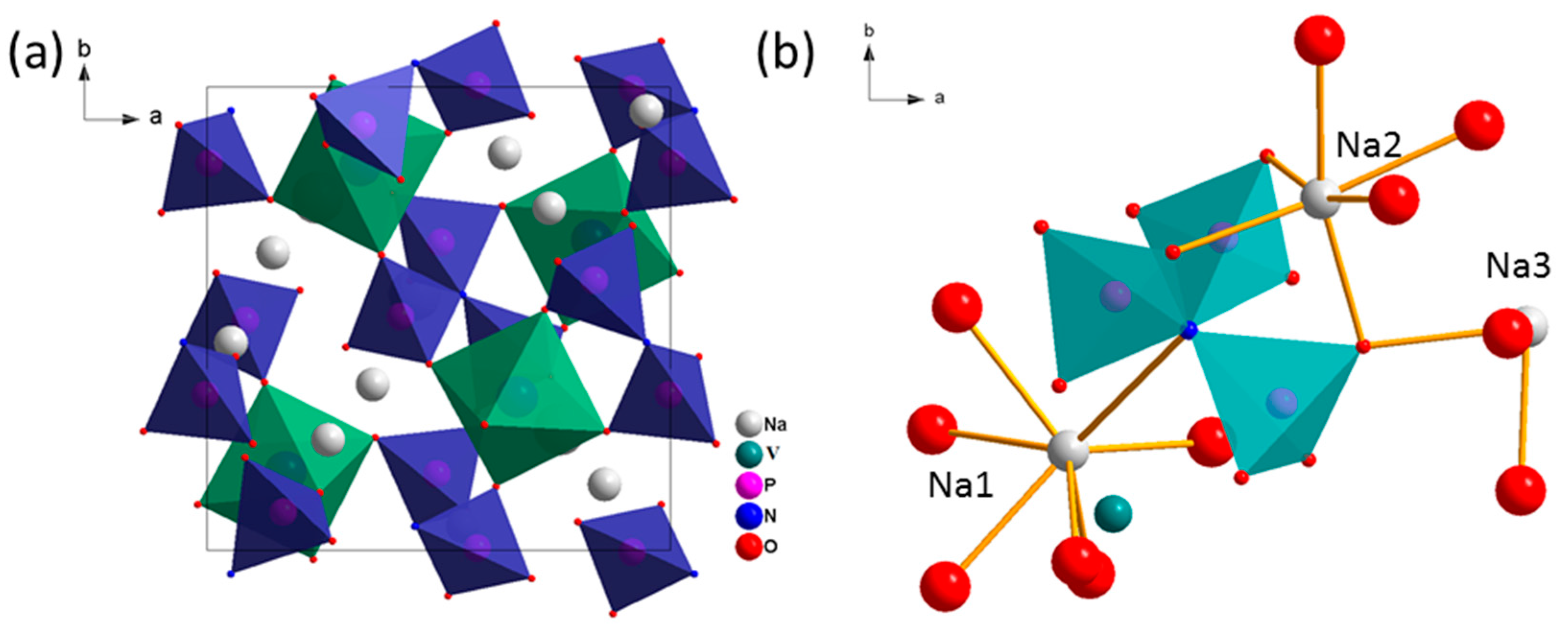
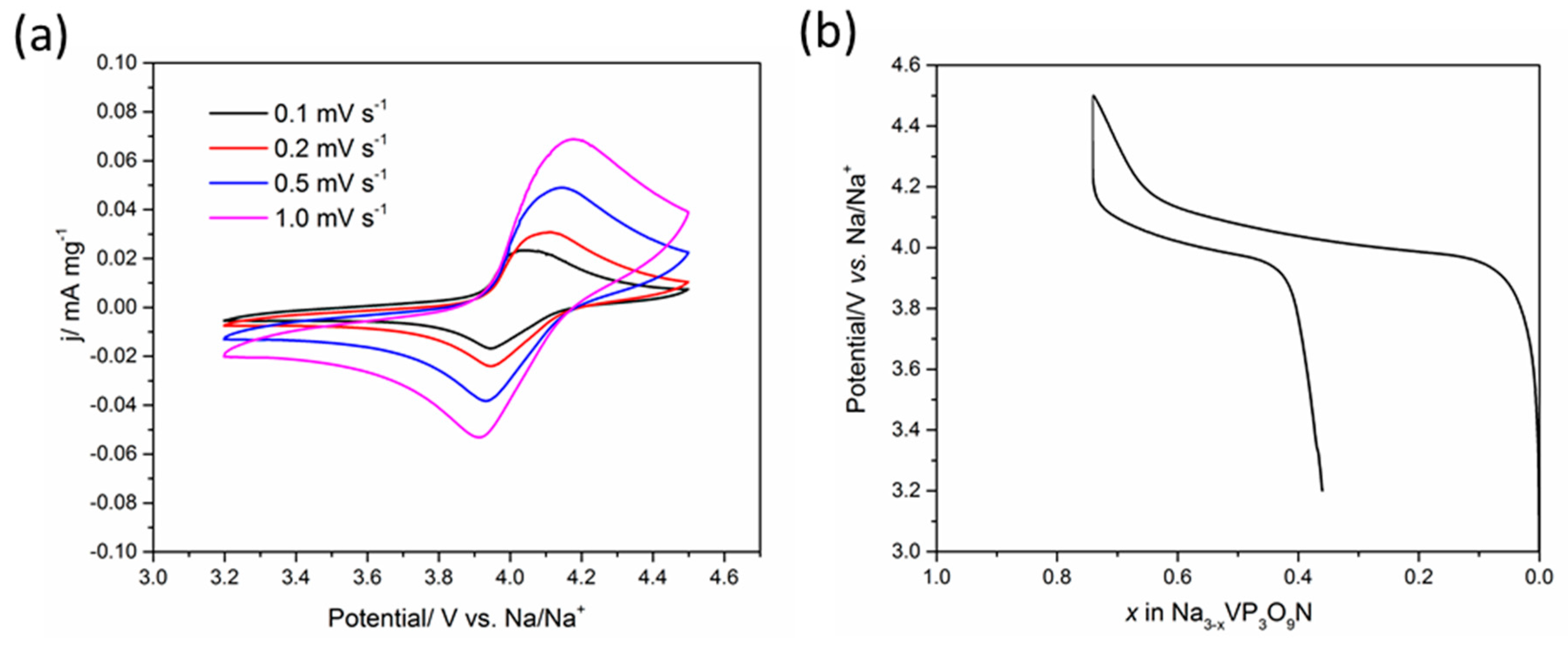

| State | Na1 occ | Na2 occ | Na3 occ | a ( Å) | V ( Å3) | Rwp/% | GOF |
|---|---|---|---|---|---|---|---|
| OCV | 1.05 (3) | 1.00 (2) | 0.91 (3) | 9.445 (1) | 842.7 (2) | 3.38 | 1.14 |
| 1st charge: 4.5 V | 0.58 (4) | 0.97 (2) | 0.76 (3) | 9.438 (1) | 840.8 (3) | 3.43 | 1.16 |
| 1st discharge: 3.2 V | 0.85 (3) | 1.01 (2) | 0.80 (3) | 9.440 (1) | 841.4 (2) | 3.27 | 1.10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Buchholz, D.; Passerini, S. Synthesis, Structure, and Sodium Mobility of Sodium Vanadium Nitridophosphate: A Zero-Strain and Safe High Voltage Cathode Material for Sodium-Ion Batteries. Energies 2017, 10, 889. https://doi.org/10.3390/en10070889
Zhang H, Buchholz D, Passerini S. Synthesis, Structure, and Sodium Mobility of Sodium Vanadium Nitridophosphate: A Zero-Strain and Safe High Voltage Cathode Material for Sodium-Ion Batteries. Energies. 2017; 10(7):889. https://doi.org/10.3390/en10070889
Chicago/Turabian StyleZhang, Huang, Daniel Buchholz, and Stefano Passerini. 2017. "Synthesis, Structure, and Sodium Mobility of Sodium Vanadium Nitridophosphate: A Zero-Strain and Safe High Voltage Cathode Material for Sodium-Ion Batteries" Energies 10, no. 7: 889. https://doi.org/10.3390/en10070889







