On the Response of Nascent Soot Nanostructure and Oxidative Reactivity to Photoflash Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
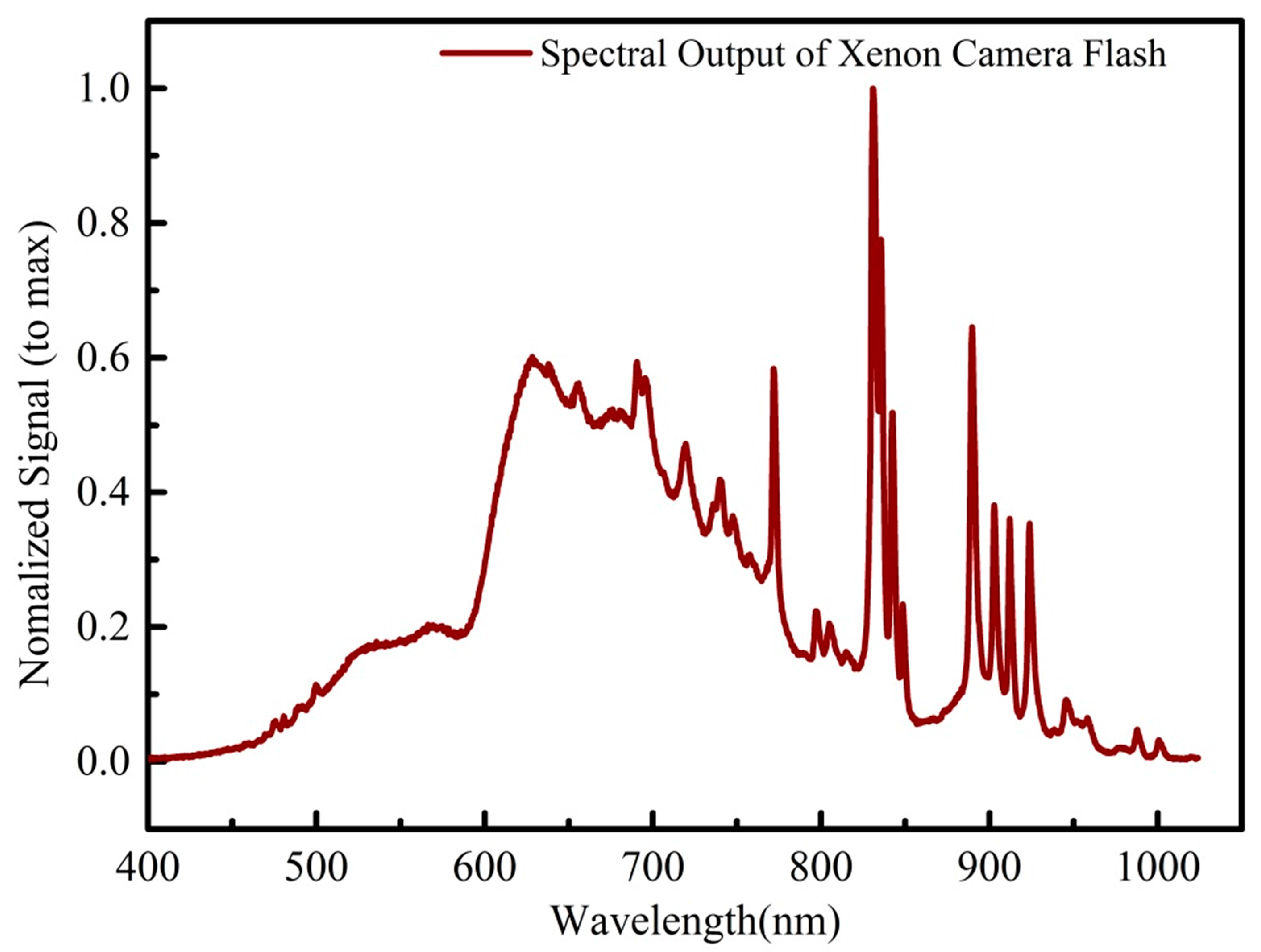
2.2. Flash-Exposure
2.3. Characterization
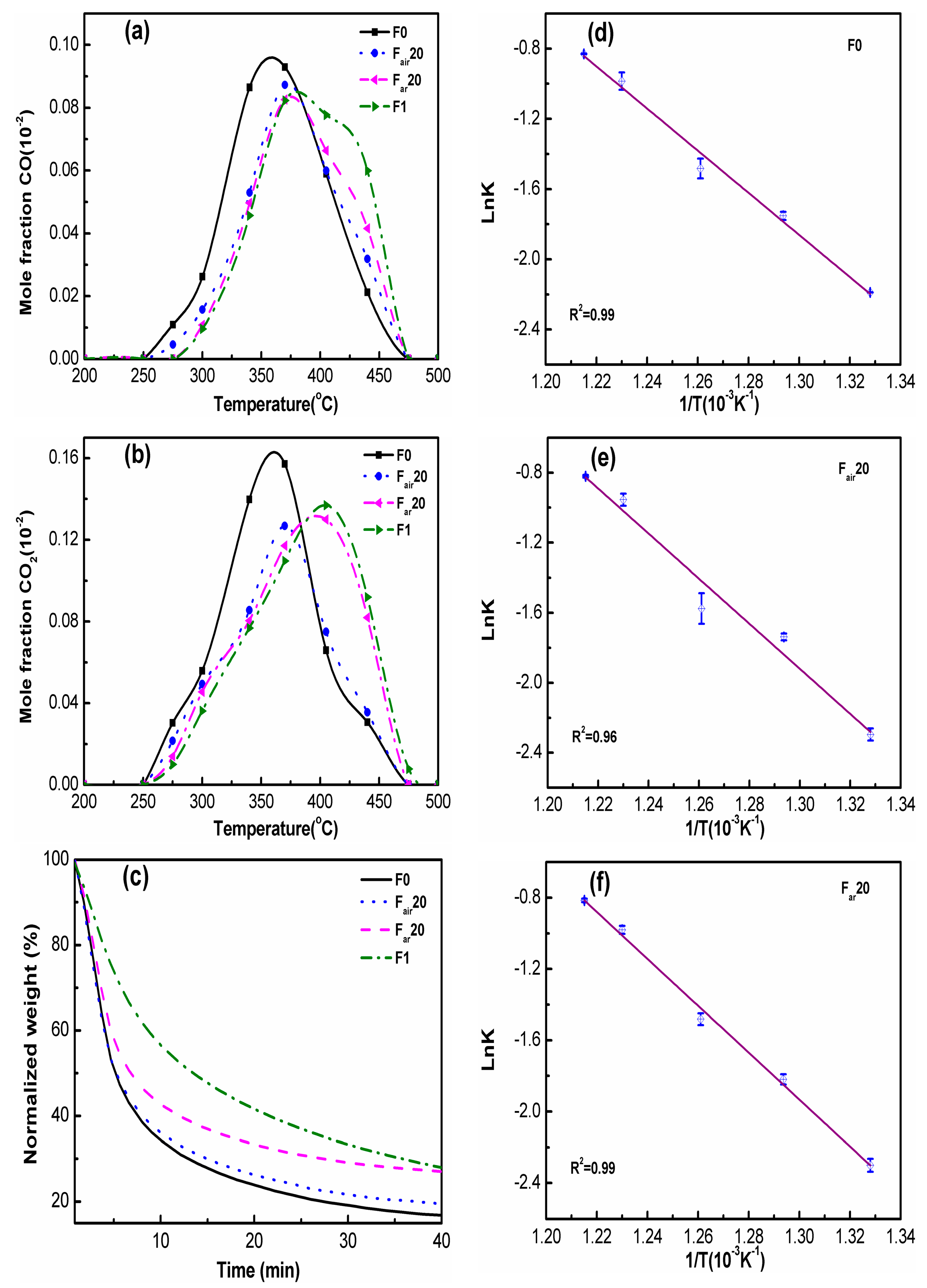
2.4. The Calculation Processes of TGA Experiments
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ramanathan, V.; Carmichael, G. Global and regional climate changes due to black carbon. Nat. Geosci. 2008, 1, 221–227. [Google Scholar] [CrossRef]
- Chameides, W.L.; Bergin, M. Soot takes center stage. Science 2002, 297, 2214–2215. [Google Scholar] [CrossRef] [PubMed]
- Mattarelli, E.; Rinaldini, C.A.; Savioli, T. Combustion Analysis of a Diesel Engine Running on Different Biodiesel Blends. Energies 2015, 8, 3047–3057. [Google Scholar] [CrossRef]
- Giakoumis, E.G.; Zachiotis, A.T. Investigation of a Diesel-Engined Vehicle’s Performance and Emissions during the WLTC Driving Cycle—Comparison with the NEDC. Energies 2017, 10, 240. [Google Scholar] [CrossRef]
- Yoon, S.H.; Han, S.C.; Lee, C.S. Effects of High EGR Rate on Dimethyl Ether (DME) Combustion and Pollutant Emission Characteristics in a Direct Injection Diesel Engine. Energies 2013, 6, 5157–5167. [Google Scholar] [CrossRef]
- Yan, W.; Chen, D.; Yang, Z.; Yan, E.; Zhao, P. Measurement of Soot Volume Fraction and Temperature for Oxygen-Enriched Ethylene Combustion Based on Flame Image Processing. Energies 2017, 10, 750. [Google Scholar] [CrossRef]
- De Falco, G.; Moggia, G.; Sirignano, M.; Commodo, M.; Minutolo, P.; D’Anna, A. Exploring Soot Particle Concentration and Emissivity by Transient Thermocouples Measurements in Laminar Partially Premixed Coflow Flames. Energies 2017, 10, 232. [Google Scholar] [CrossRef]
- Seong, H.J.; Boehman, A.L. Studies of soot oxidative reactivity using a diffusion flame burner. Combust. Flame 2012, 159, 1864–1875. [Google Scholar] [CrossRef]
- Ying, Y.; Xu, C.; Liu, D.; Jiang, B.; Wang, P.; Wang, W. Nanostructure and Oxidation Reactivity of Nascent Soot Particles in Ethylene/Pentanol Flames. Energies 2017, 10, 122. [Google Scholar] [CrossRef]
- Ying, Y.; Liu, D. Effects of butanol isomers additions on soot nanostructure and reactivity in normal and inverse ethylene diffusion flames. Fuel 2017, 205, 109–129. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Yezerets, A.; Currier, N.W.; Kim, D.H.; Wang, C.M. HRTEM Study of diesel soot collected from diesel particulate filters. Carbon 2007, 45, 70–77. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Tomasek, A.J. Soot Nanostructure: Definition, Quantification and Implications. SAE Trans. 2005, 114, 429–436. [Google Scholar]
- Raj, A.; Yang, S.Y.; Cha, D.; Tayouo, R.; Chung, S.H. Structural effects on the oxidation of soot particles by O2: Experimental and theoretical study. Combust. Flame 2013, 160, 1812–1826. [Google Scholar] [CrossRef]
- Choi, M.; Altman, I.S.; Kim, Y.J.; Pikhista, P.V.; Lee, S.T.; Jeong, G.S.; Yoo, J.B. Formation of Shell-Shaped CNPs Above a Critical Laser Power in Irradiated Acetylene. Adv. Mater. 2004, 16, 1721–1725. [Google Scholar] [CrossRef]
- Medwell, P.R.; Nathan, G.J.; Chan, Q.N.; Alwahabi, Z.T.; Dally, B.B. The influence on the soot distribution within a laminar flame of radiation at fluxes of relevance to concentrated solar radiation. Combust. Flame 2011, 158, 1814–1821. [Google Scholar] [CrossRef]
- Wang, C.; Chan, Q.N.; Kook, S.; Hawkes, E.R.; Lee, J.; Medwell, P.R. External irradiation effect on the growth and evolution of in-flame soot species. Carbon 2016, 102, 161–171. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Terrones, M.; de la Guardia, A.; Huc, V.; Grobert, N.; Wei, B.Q.; Lezec, H.; Ramanath, G.; Ebbesen, T.W. Nanotubes in a flash--ignition and reconstruction. Science 2002, 296, 705. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.B.; Choi, M.G.; Kim, D.H.; Kim, K.T.; Lee, H.M.; Lee, H.W.; Kim, J.M.; Kim, S.H. Flash-ignitable nanoenergetic materials with tunable underwater explosion reactivity: The role of sea urchin-like carbon nanotubes. Combust. Flame 2015, 162, 1448–1454. [Google Scholar] [CrossRef]
- Ohkura, Y.; Rao, P.M.; Zheng, X. Flash ignition of Al nanoparticles: Mechanism and applications. Combust. Flame 2011, 158, 2544–2548. [Google Scholar] [CrossRef]
- Cote, L.J.; Cruzsilva, R.; Huang, J. Flash Reduction and Patterning of Graphite Oxide and Its Polymer Composite. J. Am. Chem. Soc. 2009, 131, 11027–11032. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Xiang, S.-B.; Wang, Z.; Wang, X.; Hua, G. Photo-responsive behaviors and structural evolution of carbon-nanotube-supported energetic materials under a photoflash. Mater. Lett. 2012, 88, 27–29. [Google Scholar] [CrossRef]
- Velásquez, M.; Mondragón, F.; Santamaría, A. Chemical characterization of soot precursors and soot particles produced in hexane and diesel surrogated using an inverse diffusion flame burner. Fuel 2013, 104, 684–690. [Google Scholar] [CrossRef]
- Blevins, L.G.; Fletcher, R.A.; Benner, B.A., Jr.; Steel, E.B.; Mulholland, G.W. The existence of young soot in the exhaust of inverse diffusion flames. Proc. Combust. Inst. 2002, 29, 2325–2333. [Google Scholar] [CrossRef]
- Santamaría, A.; Yang, N.; Eddings, E.; Mondragón, F. Chemical and morphological characterization of soot and soot precursors generated in an inverse diffusion flame with aromatic and aliphatic fuels. Combust. Flame 2010, 157, 33–42. [Google Scholar] [CrossRef]
- Yehliu, K.; Vander Wal, R.L.; Boehman, A.L. Development of an HRTEM image analysis method to quantify carbon nanostructure. Combust. Flame 2011, 158, 1837–1851. [Google Scholar] [CrossRef]
- Yehliu, K.; Vander Wal, R.L.; Boehman, A.L. A comparison of soot nanostructure obtained using two high resolution transmission electron microscopy image analysis algorithms. Carbon 2011, 49, 4256–4268. [Google Scholar] [CrossRef]
- Mendiara, T.; Alzueta, M.U.; Millera, A.; Bilbao, R. Oxidation of Acetylene Soot Influence of Oxygen Concentration. Energ. Fuel 2007, 21, 3208–3215. [Google Scholar] [CrossRef]
- Mendiara, T.; Alzueta, M.U.; Millera, A.; Bilbao, R. Acetylene soot reaction with NO in the presence of CO. J. Hazard. Mater. 2009, 166, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Dutcher, B.; Fan, M.; Leonard, B.; Dyar, M.D.; Tang, J.; Speicher, E.A.; Liu, P.; Zhang, Y. Use of Nanoporous FeOOH as a Catalytic Support for NaHCO3 Decomposition Aimed at Reduction of Energy Requirement of Na2CO3/NaHCO3 Based CO2 Separation Technology. J. Phys. Chem. C 2011, 115, 15532–15544. [Google Scholar] [CrossRef]
- Zouaoui, N.; Labaki, M.; Jeguirim, M. Diesel soot oxidation by nitrogen dioxide, oxygen and water under engine exhaust conditions: Kinetics data related to the reaction mechanism”. C. R. Chim. 2014, 17, 672–680. [Google Scholar] [CrossRef]
- Tseng, S.H.; Tai, N.H. Variations in the microstructure and electrical resistance of the SWCNT films under consecutive photoflash exposures. Carbon 2010, 48, 1652–1661. [Google Scholar] [CrossRef]
- Inagaki, M.; Kang, F. Materials Science and Engineering of Carbon: Fundamentals, 2nd ed.; Tsinghua University Press: Beijing, China, 2014; pp. 219–525. [Google Scholar]





| Gas | Gas Flow Rate (L/min) | |
|---|---|---|
| IDF | C2H4 | 0.7 |
| Air | 0.7 | |
| N2 (Carrier) | 0.7 | |
| N2 (Protector) | 13 |
| Model Type (Based on Mechanism) | Symbol | F(α) |
|---|---|---|
| 1D diffusion | D1 | α2 |
| 2D diffusion | D2 | α + (1 − α)ln(1 − α) |
| 3D diffusion | D3 | [1 − (1 − α)1/3]2 |
| Ginstling-Brounshtein | D4 | 1 − (2/3)α − (1 − α)2/3 |
| Prout-Tompkins | Au | ln(αn(α − α)) |
| contracting surface/volume | Rn | 1 − (1 − α)1/n |
| Avrami-Erofeyev | Am | [− ln(1 − α)]1/m |
| Sample | Ea (kJ/mol) | A (min−1) | R2 |
|---|---|---|---|
| F0 | 99.39 ± 1.86 | 8.7 × 105 | 0.99 |
| Fair20 | 106.79 ± 2.14 | 2.6 × 106 | 0.96 |
| Far20 | 108.10 ± 1.25 | 3.2 × 106 | 0.99 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Liu, D.; Ying, Y.; Liu, G.; Wu, Y. On the Response of Nascent Soot Nanostructure and Oxidative Reactivity to Photoflash Exposure. Energies 2017, 10, 961. https://doi.org/10.3390/en10070961
Wang W, Liu D, Ying Y, Liu G, Wu Y. On the Response of Nascent Soot Nanostructure and Oxidative Reactivity to Photoflash Exposure. Energies. 2017; 10(7):961. https://doi.org/10.3390/en10070961
Chicago/Turabian StyleWang, Wei, Dong Liu, Yaoyao Ying, Guannan Liu, and Ye Wu. 2017. "On the Response of Nascent Soot Nanostructure and Oxidative Reactivity to Photoflash Exposure" Energies 10, no. 7: 961. https://doi.org/10.3390/en10070961





