2.1. Samples
In order to demonstrate the applicability of the suggested measurement and simulation procedure, we present data for two completely different types of sorption samples, as shown in
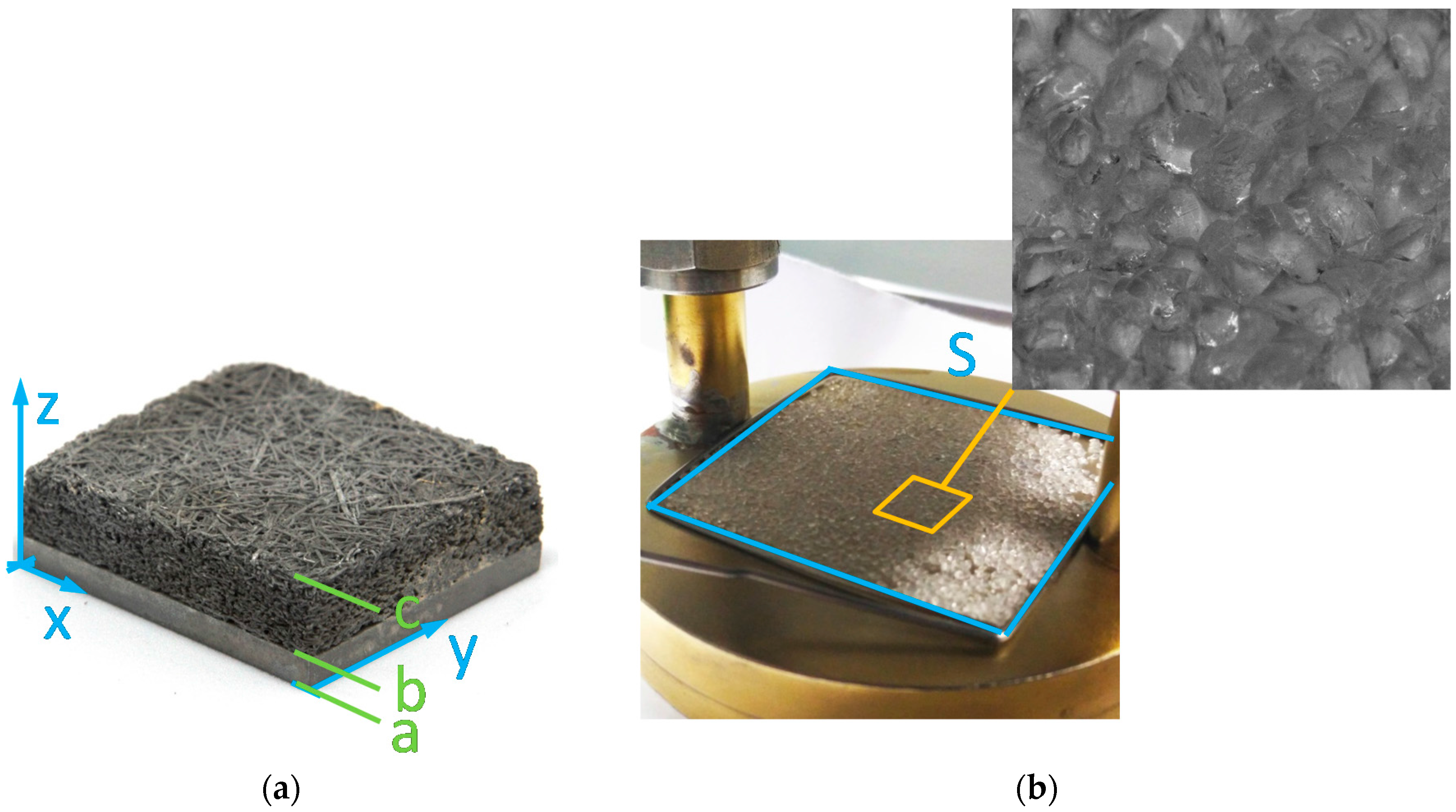
Figure 1 that reflect the wide range of different designs currently discussed in research. Additionally, a sample without adsorbent (flat
plate, called ‘1_Plt’ in the following) was prepared. This sample is a flat aluminum plate, which is anodized. The black surface is best suited for the measurement with the infrared sensor since there are nearly no reflections. The thickness of the plate is 2 mm.
The data for a fibrous structures directly crystallized with SAPO-34 (
fibrous structure called ‘2_Fib’ in the paper) as studied by Füldner [
19], Füldner et al. [
20], Wittstadt et al. [
9] and Velte et al. [
21] can be found in
Table 1. The directly-crystallized SAPO-34 has a chabazite (CHA) structure with a three-dimensional pore structure and a micro pore size of 3.8 Å [
7]. More information about this adsorbent and the partial support transformation synthesis technique was published earlier by Bauer et al. [
7]. The adsorbent mass as listed in
Table 1 was determined by the manufacturer with measurements before and after the calcination. The mean macro pore diameter and the mean adsorbent layer thickness on the fibers are calculated quantities for an ideal cylindrical pore. Their uncertainties depend mainly on the uncertainty of the adsorbent mass. Füldner [
19] also determined macro pore diameters of similar fibrous structures with permeability measurements. The results confirmed the validity of the geometrical model of the ideal cylindrical pores for the fibrous structures.
The data for a loose grains configuration of Siogel as studied by Sapienza et al. [
22] (a silica gel from Oker-Chemie (Goslar, Germany), called ‘3_Sio’ in the paper) can be found in
Table 2. The grains are placed on the carrier plate directly in a monolayer configuration. The mean grain size interval is equivalent to the size of the sieve that was used to separate the grains. The adsorbent mass under atmospheric conditions (room temperature of 20 °C, relative humidity between 40% and 60%) was determined with a microbalance. The adsorbent dry mass was calculated using the equilibrium data of Siogel. The area S is the area on the carrier plate that is covered with the Siogel grains.
2.3. Experimental Setup and Measurement Procedure
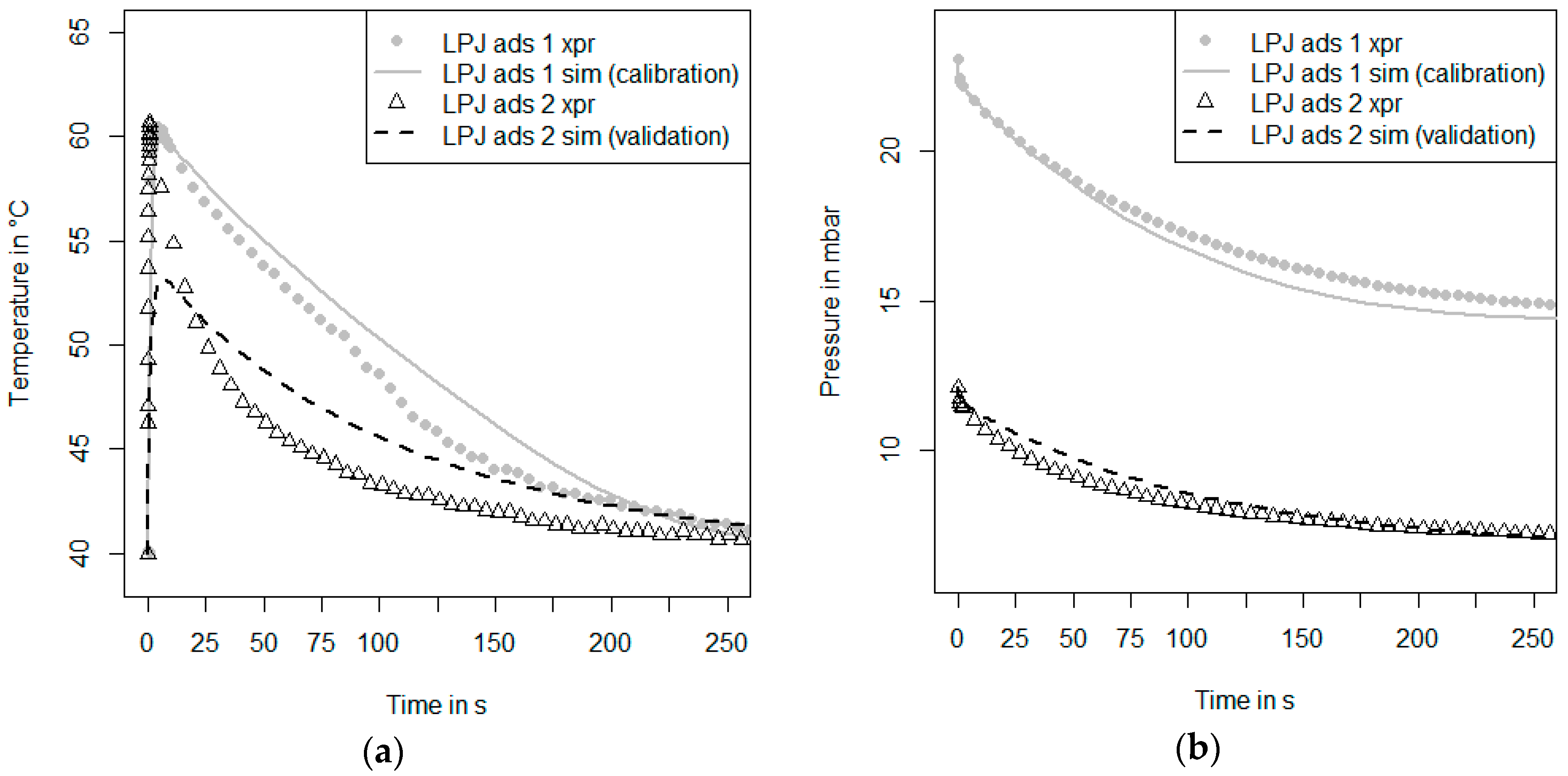
Figure 3 shows a schematic drawing of the recently-modified kinetic setup at Fraunhofer ISE in which both large pressure jump measurements (LPJ) as described by Schnabel et al. [
8] and Füldner et al. [
19], as well as large temperature jump measurements (LTJ) as described by Aristov et al. [
15] can be performed. A brief description of the current setup was given earlier [
22]. The basis for the setup was described first by Schnabel [
18] and Schnabel et al. [
8]. The recent modification is the connection of a nitrogen dosage unit. If both the measurement chamber and the dosing chamber are evacuated, the vacuum pump is switched off. Then, both chambers can be filled with dry nitrogen by opening of the valves V3, V4 and V5. The pressure in the measurement chamber and the dosing chamber is measured with two separate pressure sensors (Baratron 627B (MKS Instruments, Andover, MA, USA)). The surface temperature of the sample placed in the measurement chamber is measured with an infrared sensor (Optris CT Fast (Optris, Berlin, Germany)). In order to indicate the measurement of the surface temperature with the infrared sensor, we call the measurements IR-LPJ and IR-LTJ in the following. The temperature of the thermostats T1 and T2 is measured with Pt-100 sensors. Furthermore, the vapor temperature in the dosing chamber is measured with two Pt-100 sensors. As a prerequisite for each measurement, the measurement chamber with the sample inside is fully evacuated. The carrier plate is heated to a maximum value of 95 °C while the vacuum pump stays connected to the measurement chamber. These conditions are kept over 4 h to make sure that all water vapor or gas is desorbed from the sample.
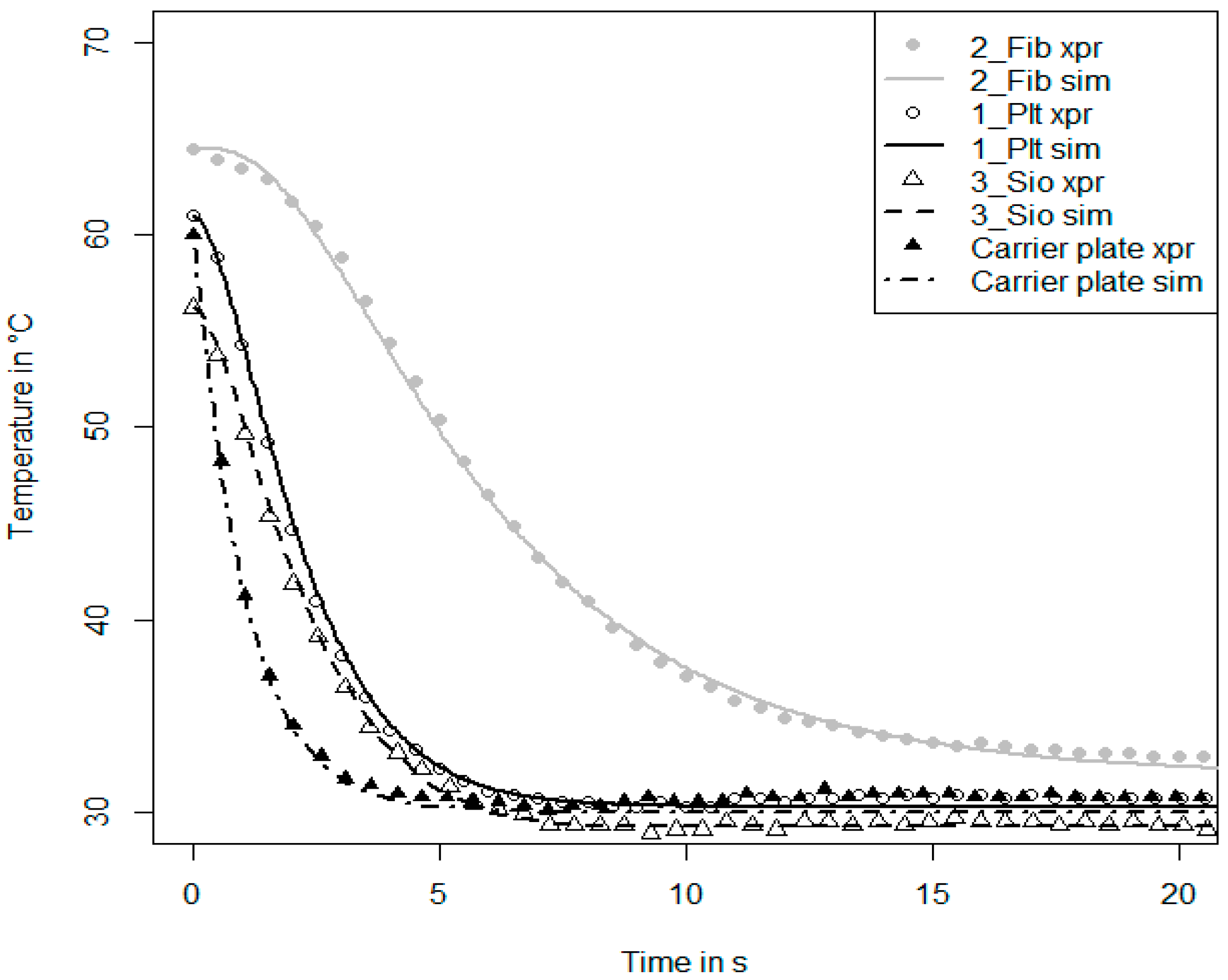
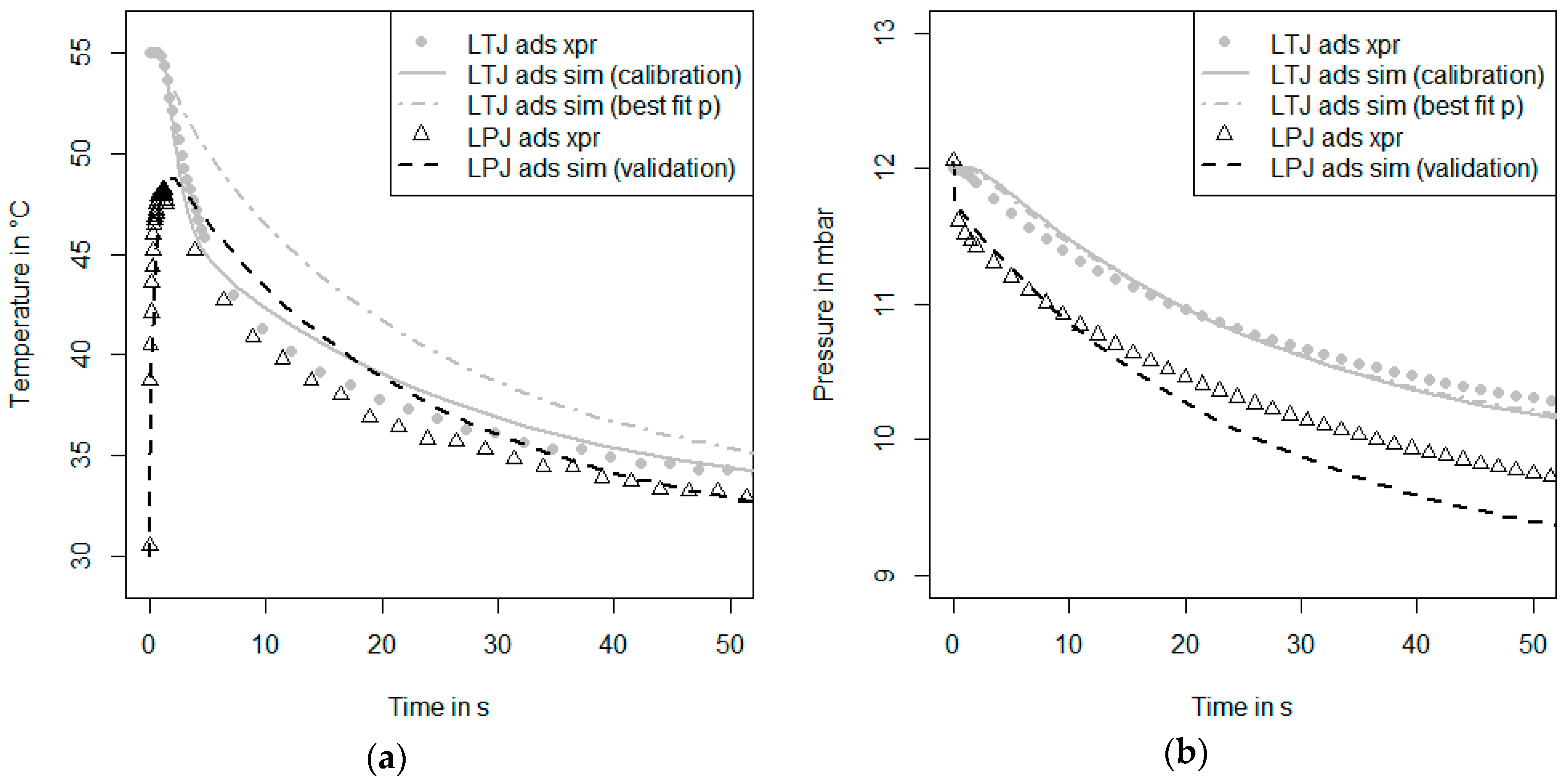
With the nitrogen dosage unit as described above, also another kind of measurement can be performed. Nitrogen can be regarded as an inert species for the adsorbents and the conditions studied here. As soon as a nitrogen pressure in the range of 0.1–100 mbar is set, the adsorbent will neither ad- or de-sorb nitrogen, although the temperature of the sample is changed in the range of 30 °C and 95 °C. Thus, the measurement is isosterical, and hence, there will be no release of the heat of adsorption. The measurement procedure is continued by connecting the carrier plate to the thermostat T1 (low temperature). Then, the hydraulic valves are switched and the carrier plate is connected to the thermostat T2 (high temperature). In such an inert large temperature jump (inert-LTJ) measurement, the nitrogen pressure is set to the value of the water vapor pressure of the LTJ experiment. In such measurements, only the heat capacity of the sample, the thermal conductivity of the sample and the thermal coupling between the sample and the carrier plate influence the temperature change of the sample. The time-dependent surface temperature change of the sample can be measured with the infrared sensor and can be used as a signal for parameter identification with an appropriate numerical heat transfer model. Especially in the case of a loose grains configuration, it can be expected that the heat transfer processes between the grain and the carrier plate strongly depend on the presence of a vapor or a gas phase, since the direct contact area is very small compared to the maximum cross-sectional area of a grain [
33]. If the chambers are evacuated to a pressure below 0.01 mbar, the only heat transfer process in the absence of the vapor or gas phase is the radiative heat transfer between the grain and carrier plate. However, nitrogen and water vapor do have different thermo-physical properties. It has to be carefully discussed to what extent the identified heat transfer parameters of an inert-LTJ measurement can be directly related to the parameters obtained from LTJ or LPJ experiments.
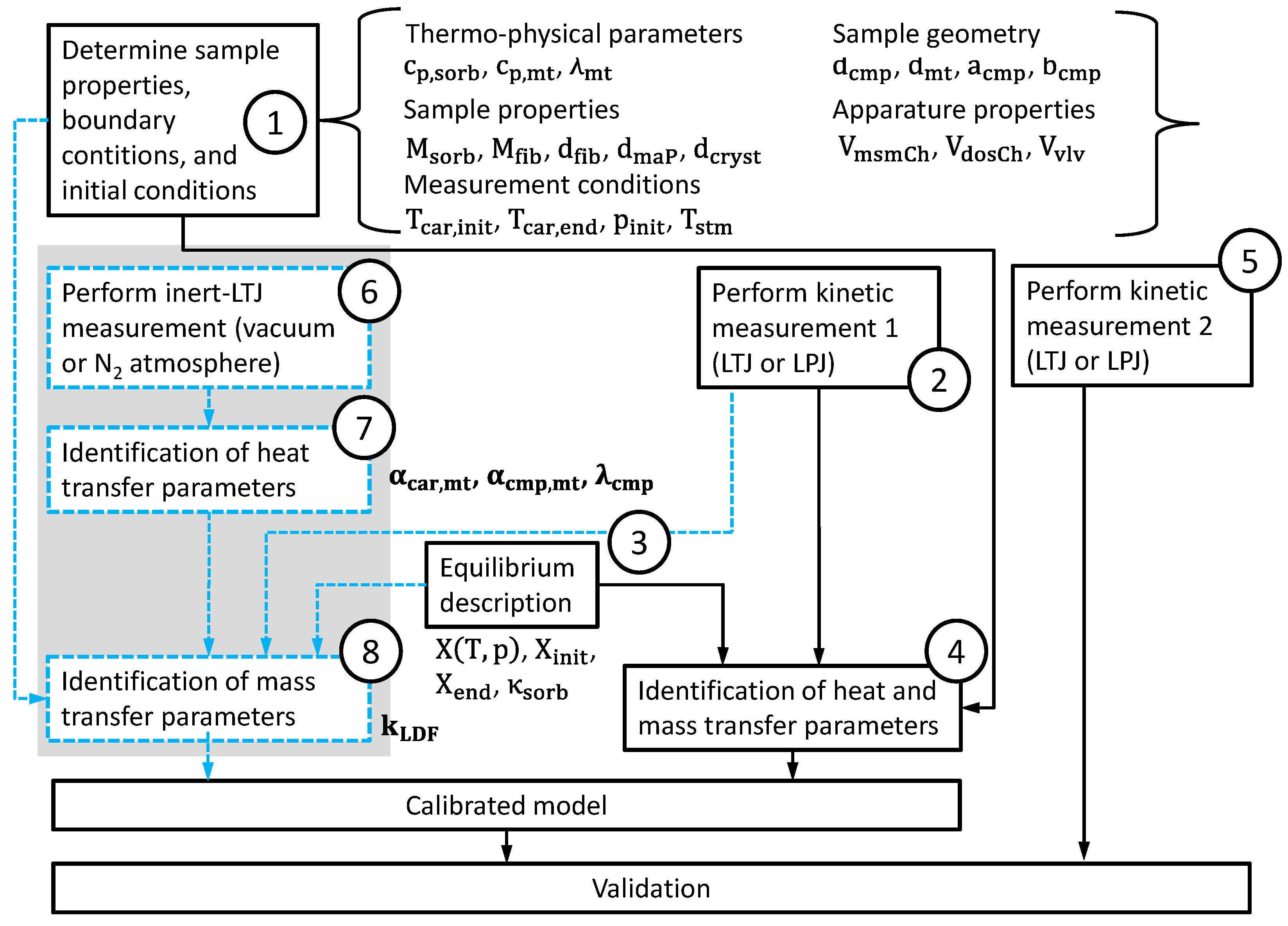
Figure 4 shows the suggested measurement and simulation procedure with the additional inert-LTJ measurements. In the first step (1), all known sample parameters and aperture parameters, as well as the boundary conditions and the initial conditions are collected or defined. Then, the measurement procedure starts. The well-known part of the procedure is the kinetic experiment and the identification of heat and mass transfer parameters (Steps 2, 3 and 4). As soon as the equilibrium description is defined (Step 3), the equilibrium loading at the beginning
and
can be calculated with the initial and end values for pressure and sample temperature. Due to the measurement errors in pressure and temperature, the uncertainty in the determination of the mass of the adsorbent and differences between the equilibrium description and the actual behavior of the adsorbent, the measured uptake
might differ from the calculated uptake
. A measure for this difference is the ratio
as defined in Equation (18). This ratio is used in the simulation to calibrate the simulated uptake in order to achieve the experimental end pressure without adapting any other parameters.
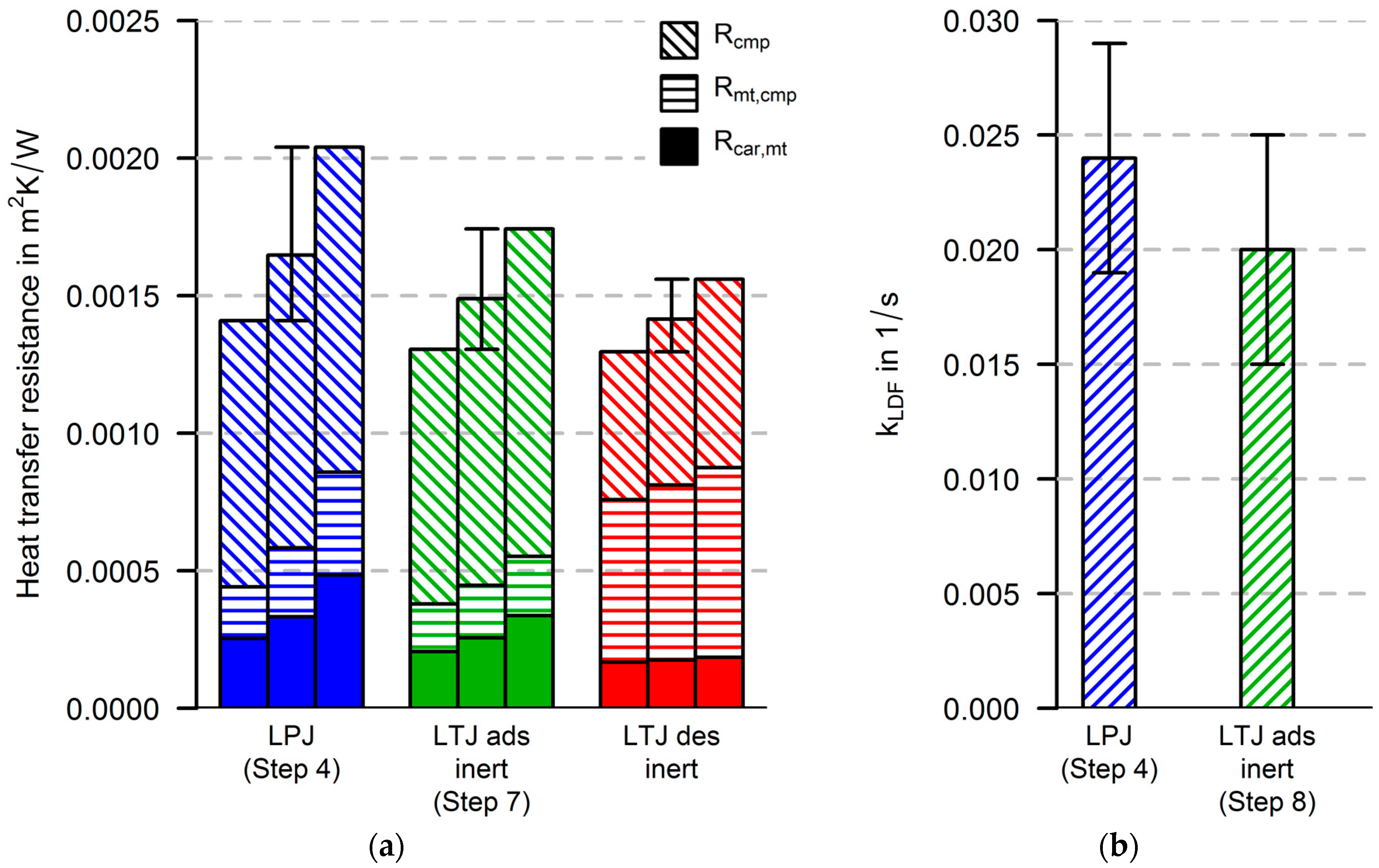
For the new measurement and simulation procedure, three more steps (6, 7 and 8) are suggested in order to separate the identification of heat transfer parameters from the identification of mass transfer parameters. The heat transfer parameters are identified by fitting simulation data to the experimental data of an inert-LTJ experiment (Step 6). This kind of experiment can be compared to flash analysis as first presented by Parker et al. [
34] or other methods that are suited to determine heat transfer parameters. It is worth noting that also in the field of air-to-air energy wheels that are used for air dehumidification, kinetic measurements (temperature jumps or humidity jumps) are performed in order to determine model parameters [
30,
31]. Here, it could also be an option to perform inert temperature jumps in order to study only the heat transfer characteristics without the influence of the adsorption material.
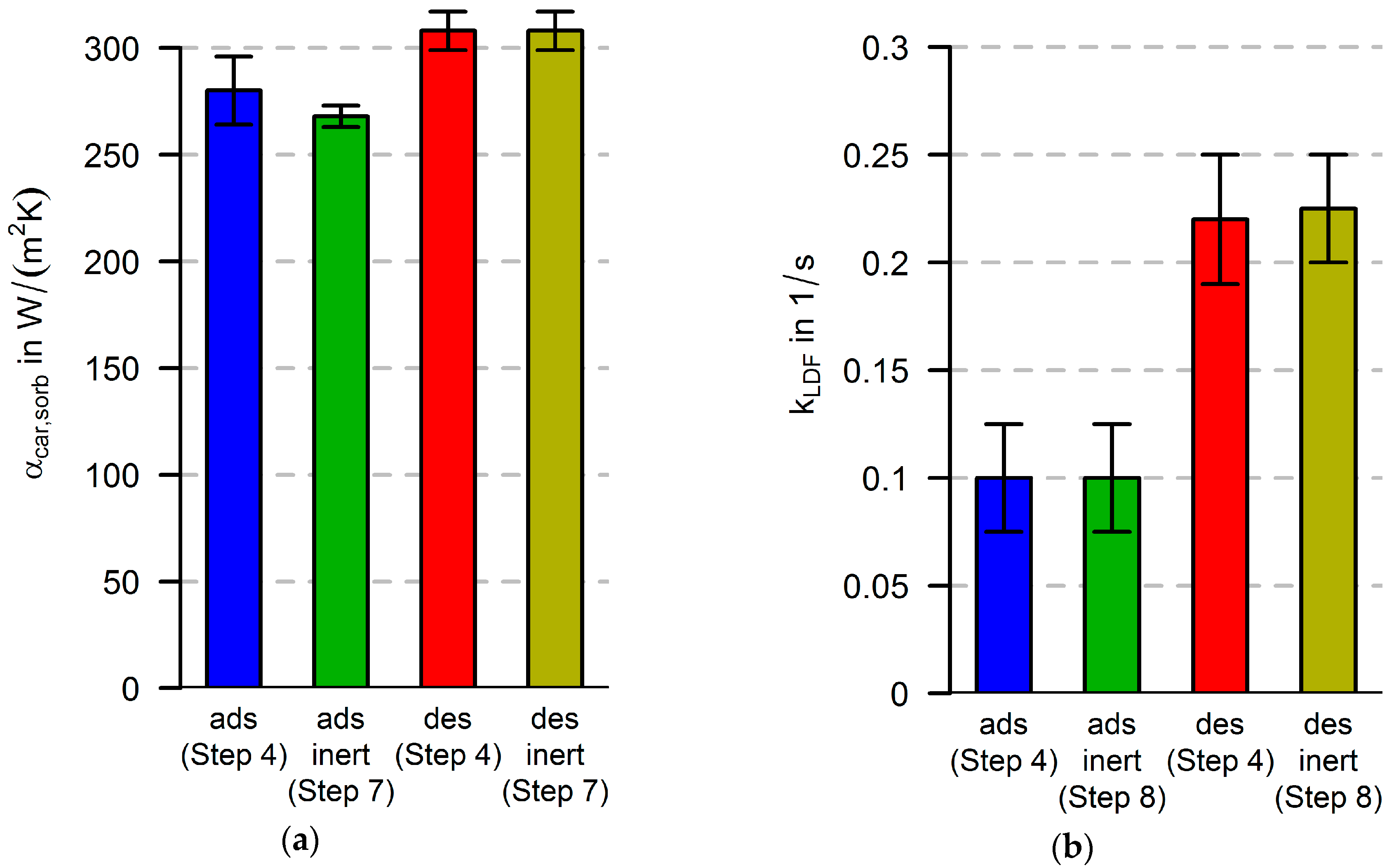
In this paper, both procedures are performed, and the parameters from both procedures are evaluated. Thus, two parameter sets for heat and mass transfer parameters are obtained. If there are strong differences in the parameter sets, they will have to be discussed. This can be an indicator that improvements have to be made in at least one of the following model parts: the heat transfer part (e.g., heat capacities, spatial resolution), the equilibrium description of the adsorbent and the model for the calculation of the adsorption enthalpy.
As soon as we have a well-calibrated model that is able to describe both the inert LTJ and the first kinetic experiment, a second kinetic measurement under different initial conditions can be evaluated. This is the validation Step 5 in
Figure 4.
The initial conditions are listed in
Table 3 for all measurements. For IR-LPJ measurements, the carrier plate temperature is not varied during the measurement. In the case of IR-LTJ measurements, the initial pressure in the measurement chamber is the same as in the dosage chamber, since both chambers are connected throughout the measurement. The initial and end temperatures of the IR-LTJ measurements and the inert-LTJ measurements are chosen according to the typical temperature conditions of a heat pump or a chiller that can be addressed with the material. Since SAPO-34 and Siogel have different adsorption equilibria, the temperatures differ.
2.4. Model Calibration and Validation
Depending on sample and type of the measurement, different models that reflect the different boundary conditions and physics behind the measurement were developed. In
Table 4, an overview is given.
In the case of the inert-LTJ measurements, we use the surface temperature signal for the calibration procedure. For the IR-LPJ and IR-LTJ measurements, we use both the surface temperature signal and the pressure signal. For each signal, we evaluate the root mean square deviation (RMSD) according to Equation (19) and the coefficient of variance (CV) according to Equation (20) as defined by Lanzerath [
35].
The model calibration is done by the variation of unknown parameters. For each calibration measurement, the set of unknown parameters is varied, and the RMSD and the CV are evaluated. The objective function for the calibration is shown in Equation (21). The weighting factors are chosen
and
in the case of the IR-LPJ and IR-LTJ measurements. For the inert-LTJ measurements, the objective function is calculated with
and
, since there is no pressure signal in these measurements.
After finding an optimum (minimum value of the objective function in Equation (21)) for a certain parameter combination, we define the trusted region as the parameter range, in which the CV is at a maximum 5% larger than the optimum. None of the parameter sets within the trusted region are allowed to lie on the boundary of the parameter range. If a combination is found on the boundary of the specified parameter range, the parameter range is extended. An optimum is regarded as a distinct optimum if there is only one parameter combination that fulfills this criterion. As an indication, a mean value and a standard deviation are given for each parameter in the trusted region. This is a multi-variable and multi-objective optimization problem. In order to avoid local minima and the dependence of the optimization result on the initial values, we performed a complete parameter variation for all combinations in the parameter range. The inverse numerical method as described by Özışık [
36] provides a sound theoretical background for the method of parameter variation to identify unknown quantities. An advantage of the inverse numerical method is the possibility of evaluating sensitivities as carried out for example by Naghash et al. [
37]. However, the numerical implementation of our method and the inverse numerical method differs. Since we worked with a specific implementation of solvers for coupled PDEs (COMSOL Multiphysics
®, Stockholm, Sweden), we implemented the direct model without having the option to perform the calculation of the inverse problem.