Pyrolysis Characteristics and Kinetics of Food Wastes
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
2.1.1. Cereals
2.1.2. Meat
2.1.3. Fruits and Vegetables
2.1.4. Mixed Food
2.2. Characterization Analysis of Model Compounds
2.3. Thermogravimetic Analysis
2.4. Pyrolysis Activation Energy Calculation
3. Result and Discussion
3.1. Characterization of Model Compounds
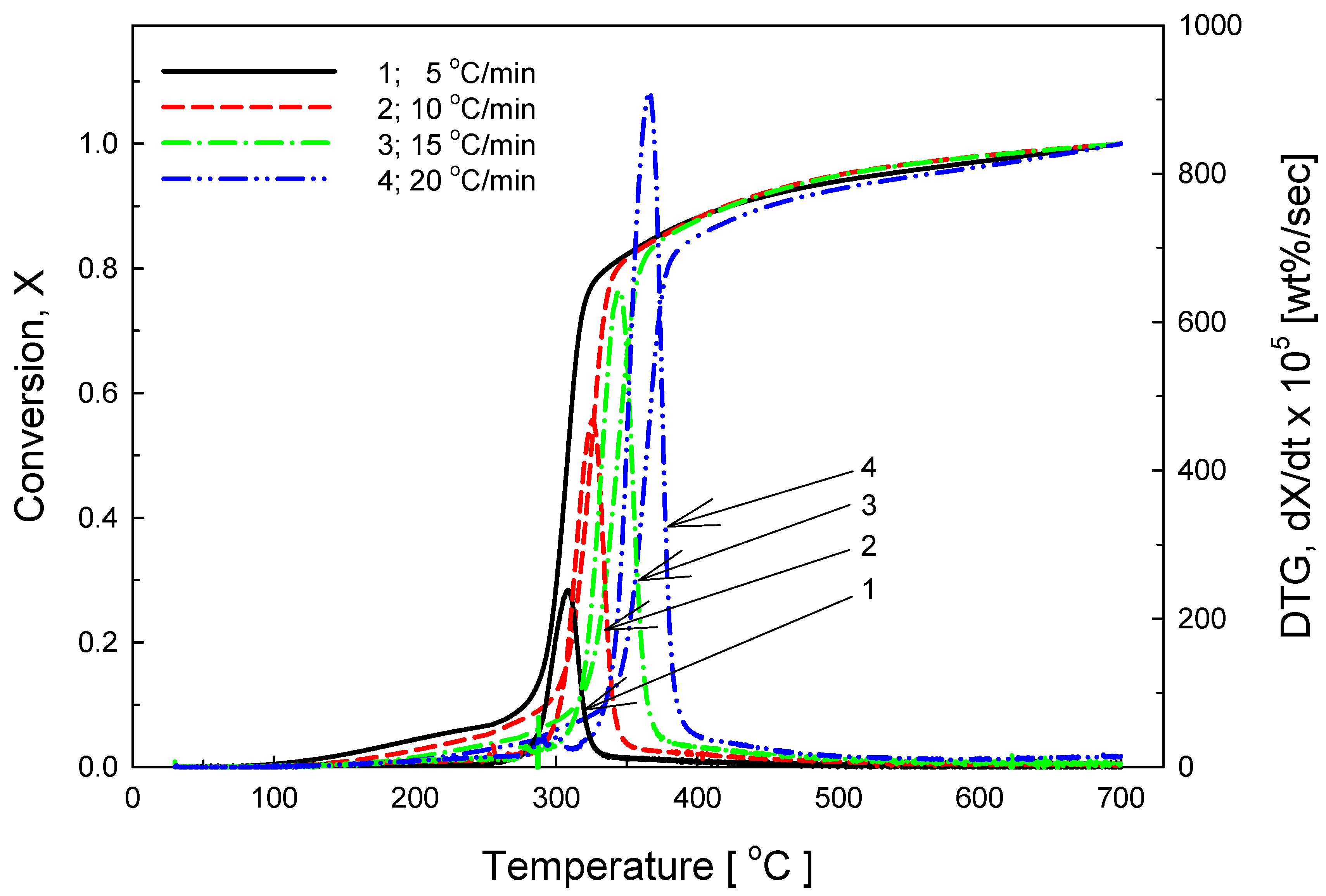
3.2. Thermal Decomposition Characteristics
3.3. Kinetics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gustavsson, J.; Cederberg, C.; Sonesson, U. Global Food Losses and Food Waste; Food and Agriculture Organization of the United Nations: Rome, Italy, 2001; ISBN 978-92-5-107205-9. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Food Wastage Footprint Impacts on Natural Resources, Summary Report; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 978-92-5-107752-8. [Google Scholar]
- Kim, D.W. A study on the Odor Reduction of Food Waste and by Microorganisms. Master’s Thesis, Hoseo University, Chungnam, Korea, 2005. [Google Scholar]
- Kwon, B.; Na, S.; Lim, H.; Lim, C.; Chung, S. Slurry phace decomposition of food waste by using various microorganisms. J. Korean Soc. Environ. Eng. 2014, 36, 303–310. [Google Scholar] [CrossRef]
- Hong, K.H.; Cha, J.D.; Ko, Y.H.; Lee, J.H.; Lim, E.J.; Kim, K.S. Evaluation of odor concentration for food waste compost facility. Korean J. Odor Res. Eng. 2006, 5, 151–155. [Google Scholar]
- Chiang, L.; Chang, J.; Wen, T. Indirect oxidation effect in electrochemical oxidation treatment of landfill leachate. Water Res. 1995, 29, 671–678. [Google Scholar] [CrossRef]
- Jakob, A.; Stucki, S.; Kuhn, P. Evaporation of Heavy Metals during the Heat Treatment of Municipal Solid Waste Incinerator Fly Ash. Environ. Sci. Technol. 1995, 29, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Sakuragi, K.; Li, P.; Otaka, M.; Makino, H. Recovery of Bio-Oil from Industrial Food Waste by Liquefied Dimethyl Ether for Biodiesel Production. Energies 2016, 9, 106. [Google Scholar] [CrossRef]
- Hao, H.-T.N.; Karthikeyan, O.P.; Heimann, K. Bio-Refining of Carbohydrate-Rich Food Waste for Biofuels. Energies 2015, 8, 6350–6364. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, S.; Liao, Y.; Zhou, J.; Gu, Y.; Cen, K. Research on biomass fast pyrolysis for liquid fuel. Biomass Bioenergy 2004, 29, 455–462. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Va´zquez, A. Thermal degradation of cellulose derivatives/starch blends and sisal fibre biocomposites. Polym. Degrad. Stab. 2004, 84, 13–21. [Google Scholar] [CrossRef]
- Guizani, C.; Jeguirim, M.; Valin, S.; Limousy, L.; Salvador, S. Biomass Chars: The Effects of Pyrolysis Conditions on Their Morphology, Structure, Chemical Properties and Reactivity. Energies 2017, 10, 796. [Google Scholar] [CrossRef]
- Kabir, M.J.; Chowdhury, A.A.; Rasul, M.G. Pyrolysis of Municipal Green Waste: A Modelling, Simulation and Experimental Analysis. Energies 2015, 8, 7522–7541. [Google Scholar] [CrossRef]
- Ninan, K.N.; Krishnan, K.; Krishnamurthy, V.N. Kinetics and mechanism of thermal decomposition of insitu generated calcium carbonate. J. Therm. Anal. Calorim. 1991, 37, 1533–1543. [Google Scholar] [CrossRef]
- Campanella, L.; Tomassetti, M.; Tomellini, R. Thermoanalysis of ancient, fresh and waterlogged woods. J. Therm. Anal. Calorim. 1991, 37, 923–932. [Google Scholar] [CrossRef]
- Jaber, J.O.; Probert, S.D. Pyrolysis and gasification kinetics of Jordanian oil-shales. Appl. Energy 1999, 63, 269–286. [Google Scholar] [CrossRef]
- Vamvuka, D.; Kakaras, E.; Kastanakia, E.; Grammelis, P. Pyrolysis characteristics and kinetics of biomass residuals mixtures with lignite. Fuel 2003, 82, 1949–1960. [Google Scholar] [CrossRef]
- Zambon, I.; Colosimo, F.; Monarca, D.; Cecchini, M.; Gallucci, F.; Proto, A.R.; Lord, R.; Colantoni, A. An Innovative Agro-Forestry Supply Chain for Residual Biomass: Physicochemical Characterisation of Biochar from Olive and Hazelnut Pellets. Energies 2016, 9, 526. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Agblevor, F.A. Pyrolysis characteristics and kinetics of chicken litter. Waste Manag. 2007, 27, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Sorum, L.; Grønli, M.G.; Hustad, J.E. Pyrolysis characteristics and kinetics of municipal solid wastes. Fuel 2001, 80, 1217–1227. [Google Scholar] [CrossRef]
- Conesa, J.A.; Marcilla, A.; Prats, D.; Rodriguez, M. Kinetic study of the pyrolysis of sewage sludge. Waste Manag. Res. 1997, 15, 293–305. [Google Scholar] [CrossRef]
- Othman, M.R.; Park, Y.H. Thermogravimetric characteristics and pyrolysis kinetics of Giheung Respia sewage sludge. Korean J. Chem. Eng. 2010, 27, 163–167. [Google Scholar] [CrossRef]
- Nowicki, L.; Antecka, A.; Bedyk, T. The kinetics of gasification of char derived from sewage sludge. J. Therm. Anal. Calorim. 2010, 12, 1032–1041. [Google Scholar] [CrossRef]
- He, P.; Shao, L.; Gu, G.; Li, G. Mechanism and kinetics of low temperature thermo-chemical conversion process of sewage sludge. Water Sci. Technol. 2001, 44, 341–347. [Google Scholar] [PubMed]
- Calvo, L.F.; Otero, M.; Jenkins, B.M. Heating process characteristics and kinetics of sewage sludge in different atmospheres. Thermochim. Acta 2004, 409, 127–135. [Google Scholar] [CrossRef]
- Shao, J.G.; Yan, R.; Chen, H.P. Pyrolysis characteristics and kinetics of sewage sludge by thermogravimetry Fourier transform infrared analysis. Energy Fuels 2008, 22, 38–45. [Google Scholar] [CrossRef]
- Lee, K.I.; Choi, J.W.; Heo, S.Y.; Ban, H.J.; Lim, S.G.; Park, I.H.; Kim, T.H. The Consumption Behaviour Survey for Food 2015; Korea Rural Economic Institute: JeolLaNam-Do, Korea, 2015; Volume 1, ISBN 978-89-6013-880-393520. [Google Scholar]
- Ministry of Environment (MOE). Product Category Rules for Carbon Labeling; Ministry of Environment: Sejong City, Korea, 2009.
- Granada, E.; Eguía, P.; Comesaña, J.A.; Patiño, D.; Porteiro, J.; Miguez, J.L. Devolatilization behaviour and pyrolysis kinetic modelling of Spanish biomass fuels. J. Therm. Anal. Calorim. 2013, 113, 569–578. [Google Scholar] [CrossRef]
- Heikkinen, J.M.; Hordijk, J.C.; de Jong, W.; Spliethoff, H. Thermogravimetry as a tool to classify waste components to be used for energy generation. J. Anal. Appl. Pyrolysis 2004, 71, 883–900. [Google Scholar] [CrossRef]
- Jeguirim, M.; Elmay, Y.; Limousy, L.; Lajili, M.; Said, R. Devolatilization behavior and pyrolysis kinetics of potential Tunisian biomass fuels. Environ. Prog. Sustain. Energy 2014, 33, 1452–1458. [Google Scholar] [CrossRef]
- Guizani, C.; Haddad, K.; Jeguirim, M.; Colin, B.; Limousy, L. Combustion characteristics and kinetics of torrefied olive pomace. Energy 2016, 107, 453–463. [Google Scholar] [CrossRef]
- Weber, R. Extracting mathematically exact kinetic parameters from experimental data on combustion and pyrolysis of solid fuels. J. Energy Inst. 2008, 81, 226–233. [Google Scholar] [CrossRef]
- Anastasakis, K.; Ross, A.B.; Jones, J.M. Pyrolysis behaviour of the main carbohydrates of brown macro-algae. Fuel 2011, 90, 598–607. [Google Scholar] [CrossRef]







| Contents | Materials | Composition Ratio (%) |
|---|---|---|
| Cereals (16) | Rice/Ramen | 16 |
| Fish & meat (19) | Meat | 4 |
| Fish | 12 | |
| Egg | 3 | |
| Vegetables (51) | Napa cabbage | 9 |
| Potato | 20 | |
| Onion | 20 | |
| Daikon | 2 | |
| Fruits (14) | Apple | 7 |
| Orange/Mandarin | 7 |
| Sample | Moisture (%) | (Dry Basis) | ||||
| Volatile Matter (%) | Fixed Carbon (%) | Ash (%) | ||||
| Cereals | 65.74 ± 2.17 | 88.95 ± 0.03 | 10.82 | 0.23 ± 0.03 | ||
| Meat | 71.27 ± 4.83 | 86.67 ± 3.54 | 8.84 | 4.49 ± 1.50 | ||
| Vegetables | 95.28 ± 3.52 | 72.84 ± 2.10 | 9.40 | 17.76 ± 0.42 | ||
| Mixed food | 85.71 ± 2.94 | 79.00 ± 2.74 | 17.25 | 3.75 ± 0.07 | ||
| Sample | Element (%) | HHV (kcal/kg) | ||||
| C | H | N | O | |||
| Cereals | 41.4 | 8.3 | 1.1 | 47.6 | 3930.6 | |
| Meat | 54.5 | 11.3 | 10.5 | 22.4 | 6019.9 | |
| Vegetables | 38.4 | 6.8 | 4.9 | 36.3 | 3565.9 | |
| Mixed food | 47.5 | 12.2 | 2.9 | 29.7 | 4657.6 | |
| T peak (°C) | DTG peak dX/dt × 102 (wt %/min) | |
|---|---|---|
| Cereal | 308 | 14.32 |
| Meat | 378 | 3.63 |
| Vegetable | 314 | 3.86 |
| Mixed food | 307 | 5.43 |
| Wood pellet1 a | 334 | 5.61 |
| Wood pellet2 a | 328 | 4.61 |
| Brassica pellet a | 296 | 4.25 |
| Poplar pellet a | 322 | 4.46 |
| RDF pellet a | 445 | 3.79 |
| Olive stone a | 315 | 4.96 |
| Almond shell a | 323 | 2.91 |
| Pine shavings a | 305 | 5.43 |
| Starch b | 313 | - |
| Banana(dry) b | 312 | - |
| Bread(dry) b | 292 | - |
| Cacao residue b | 209; 280; 328 | - |
| Lemon pellet b | 216; 261; 346 | - |
| Palm pit b | 296 | - |
| Reaction Order | Conversion (wt %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 85 | 90 | ||
| rice | 0th | 7.33 × 10−2 | 3.52 × 10−3 | 1.10 × 10−1 | 1.20 × 10−1 | 1.91 × 10−2 | 8.30 × 101 | 7.27 × 101 | 5.99 × 101 | 1.81 × 101 | 3.41 × 10−4 |
| 1st | 7.33 × 10−1 | 1.76 × 10−2 | 3.67 × 10−1 | 3.00 × 10−1 | 3.82 × 10−2 | 1.38 × 102 | 1.04 × 102 | 7.49 × 101 | 2.13 × 101 | 3.79 × 10−4 | |
| 2nd | 7.33 × 10 | 8.80 × 10−2 | 1.23 × 100 | 7.49 × 10−1 | 7.64 × 10−2 | 2.31 × 102 | 1.48 × 102 | 9.36 × 101 | 2.51 × 101 | 4.21 × 10−4 | |
| meat | 0th | 8.76 × 10−4 | 1.65 × 10−1 | 7.10 × 10−2 | 2.69 × 10−3 | 1.84 × 10−1 | 5.78 × 101 | 1.03 × 102 | 7.77 × 101 | 2.84 × 101 | 2.72 × 103 |
| 1st | 8.76 × 10−3 | 8.27 × 10−1 | 2.37 × 10−1 | 6.71 × 10−3 | 3.68 × 10−1 | 9.63 × 101 | 1.48 × 102 | 9.71 × 101 | 3.34 × 101 | 3.02 × 103 | |
| 2nd | 8.76 × 10−2 | 4.14 × 100 | 7.89 × 10−1 | 1.68 × 10−2 | 7.36 × 10−1 | 1.60 × 102 | 2.11 × 102 | 1.21 × 101 | 3.93 × 101 | 3.36 × 103 | |
| vegetable | 0th | 9.64 × 10−1 | 3.24 × 101 | 7.15 × 10−1 | 4.16 × 10−1 | 2.10 × 10−3 | 3.55 × 101 | 5.01 × 101 | 4.18 × 101 | 2.39 × 101 | 2.42 × 10−4 |
| 1st | 9.64 × 100 | 1.62 × 102 | 2.38 × 100 | 1.04 × 100 | 4.21 × 10−3 | 5.91 × 101 | 7.16 × 101 | 5.23 × 101 | 2.81 × 101 | 2.68 × 10−4 | |
| 2nd | 9.64 × 101 | 8.09 × 102 | 7.94 × 100 | 2.60 × 100 | 8.41 × 10−3 | 9.86 × 101 | 1.02 × 102 | 6.54 × 101 | 3.31 × 101 | 2.98 × 10−4 | |
| mixed | 0th | 1.76 × 101 | 4.39 × 10−3 | 1.01 × 104 | 8.74 × 10−1 | 1.25 × 100 | 8.62 × 101 | 9.28 × 101 | 5.71 × 101 | 2.27 × 101 | 3.50 × 10−4 |
| 1st | 1.76 × 102 | 2.20 × 10−2 | 3.36 × 104 | 2.18 × 100 | 2.51 × 100 | 1.44 × 102 | 1.33 × 102 | 7.14 × 101 | 2.68 × 101 | 3.89 × 10−4 | |
| 2nd | 1.76 × 103 | 1.10 × 10−1 | 1.12 × 105 | 5.46 × 100 | 5.02 × 100 | 2.39 × 102 | 1.89 × 102 | 8.93 × 101 | 3.15 × 101 | 4.32 × 10−4 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, J.-H.; Kim, S.-S.; Shim, J.-W.; Lee, Y.-E.; Yoo, Y.-S. Pyrolysis Characteristics and Kinetics of Food Wastes. Energies 2017, 10, 1191. https://doi.org/10.3390/en10081191
Jo J-H, Kim S-S, Shim J-W, Lee Y-E, Yoo Y-S. Pyrolysis Characteristics and Kinetics of Food Wastes. Energies. 2017; 10(8):1191. https://doi.org/10.3390/en10081191
Chicago/Turabian StyleJo, Jun-Ho, Seung-Soo Kim, Jae-Wook Shim, Ye-Eun Lee, and Yeong-Seok Yoo. 2017. "Pyrolysis Characteristics and Kinetics of Food Wastes" Energies 10, no. 8: 1191. https://doi.org/10.3390/en10081191






