Combustion and Heat Release Characteristics of Biogas under Hydrogen- and Oxygen-Enriched Condition
Abstract
:1. Introduction
2. Numerical Simulations
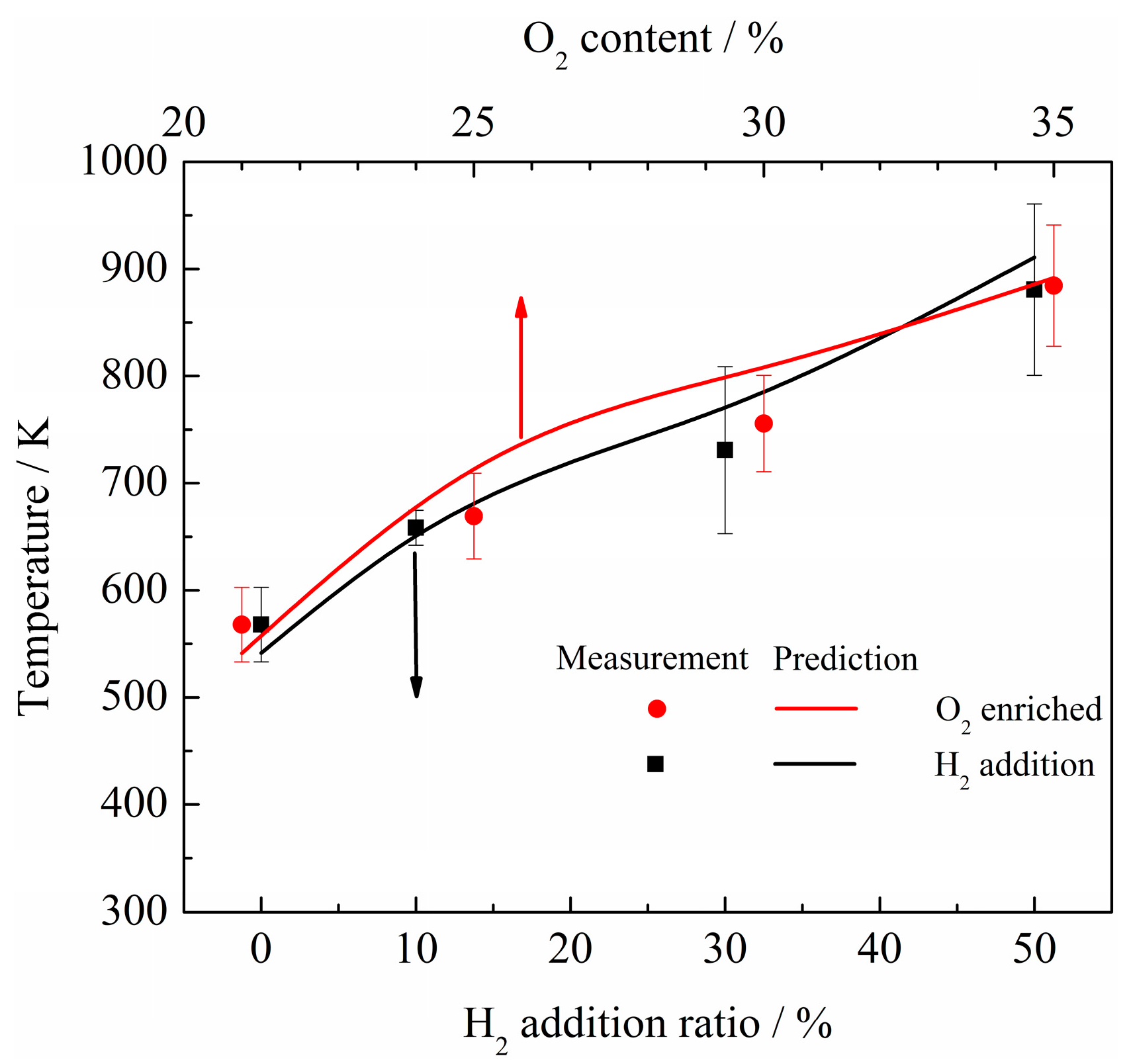
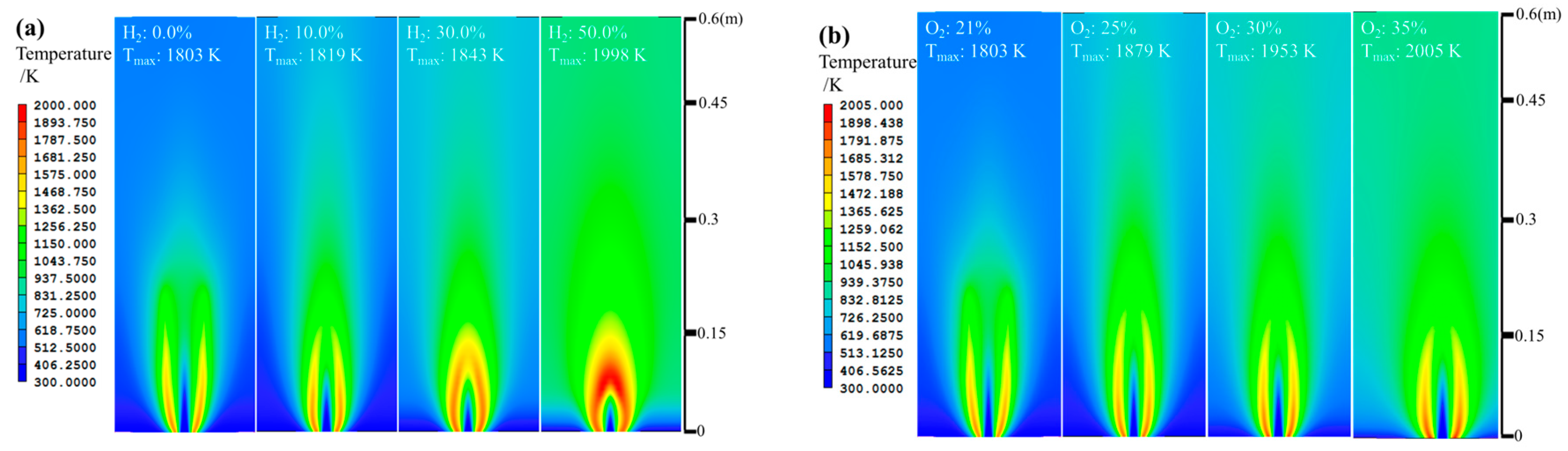
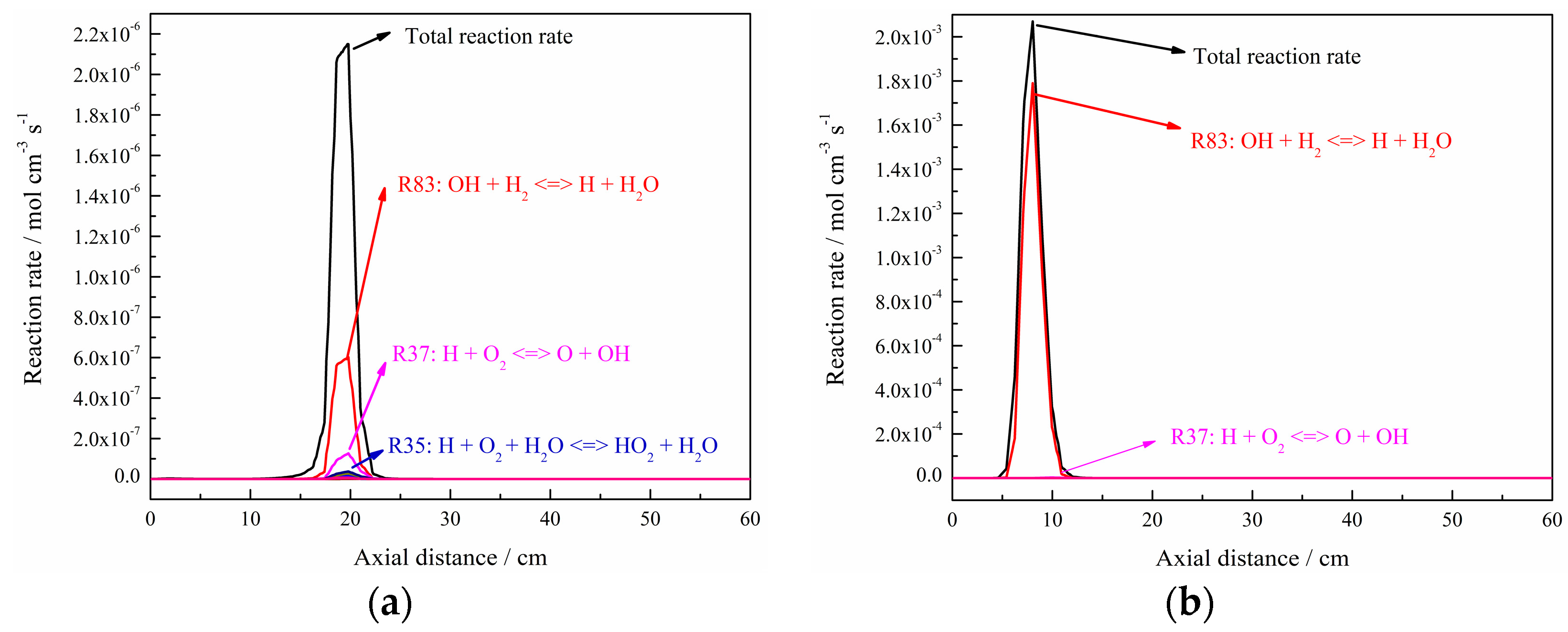
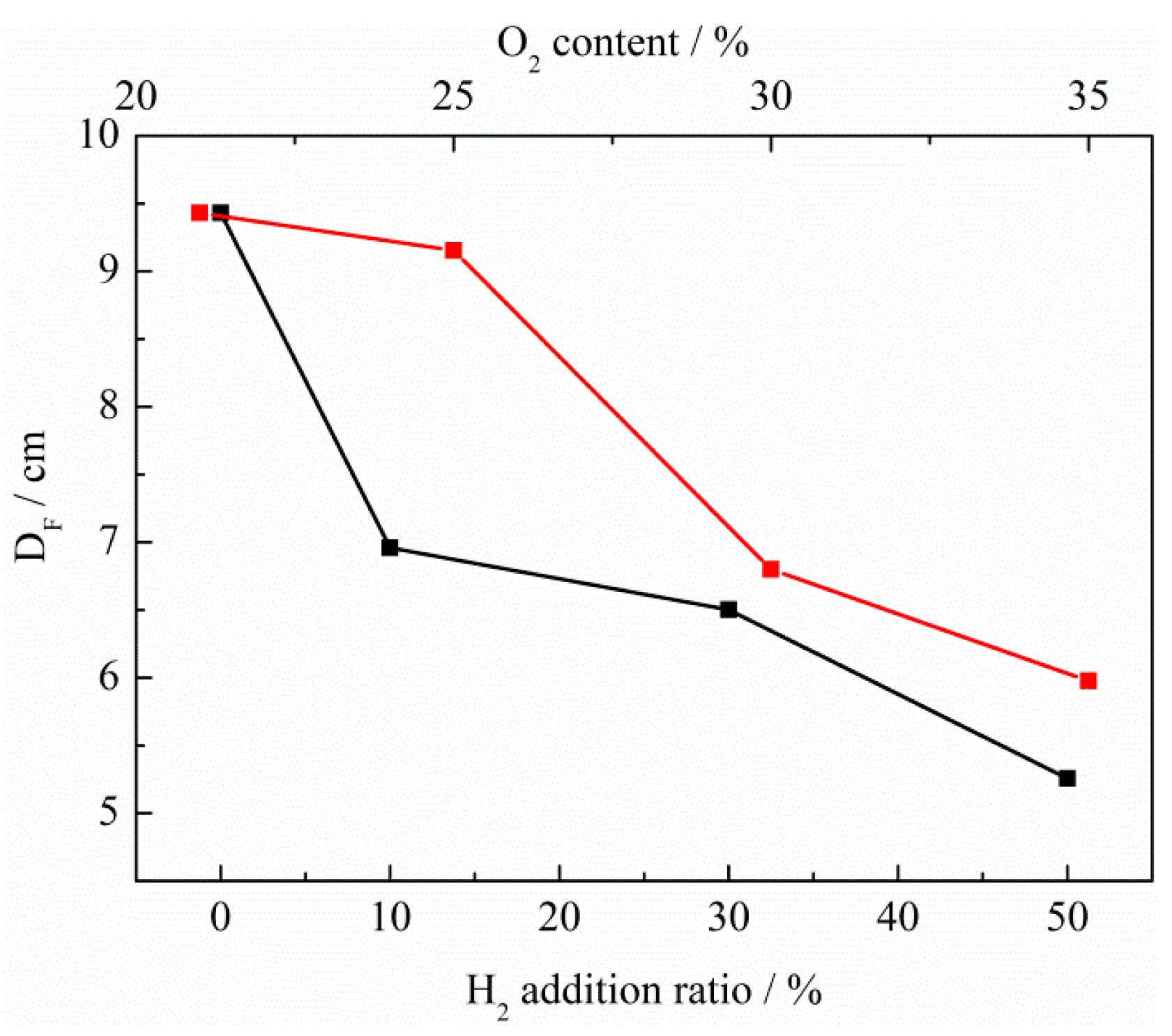
3. Discussion
4. Conclusions
- (1)
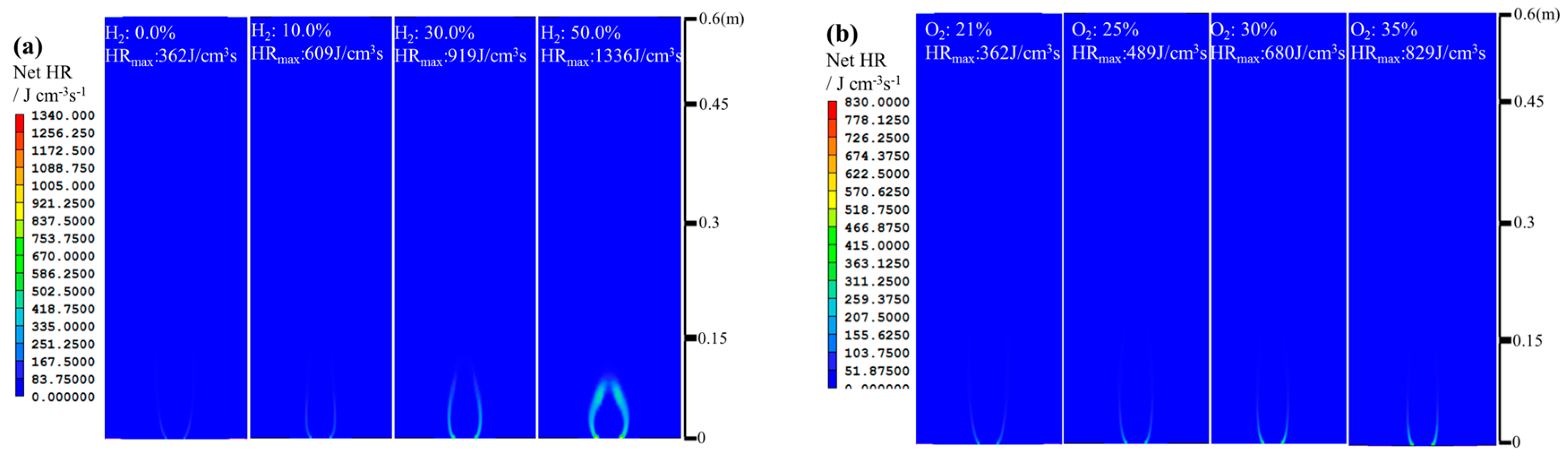
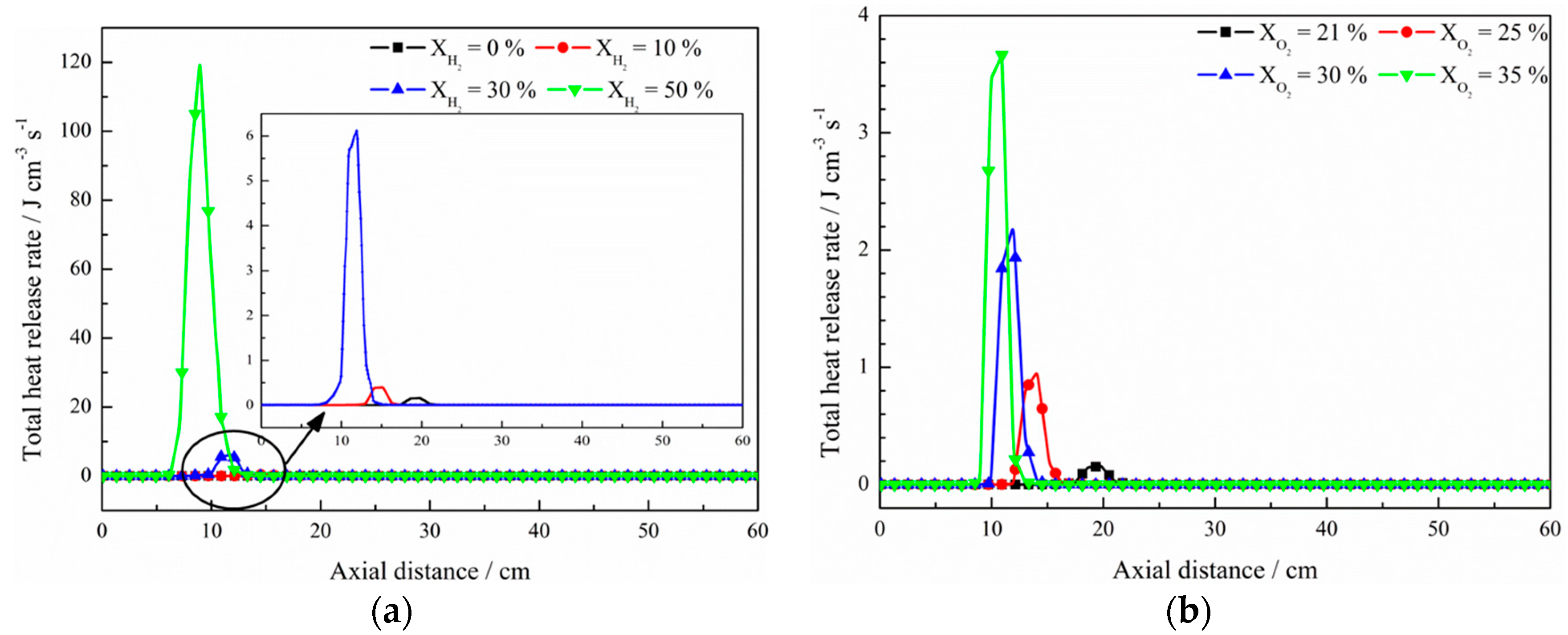
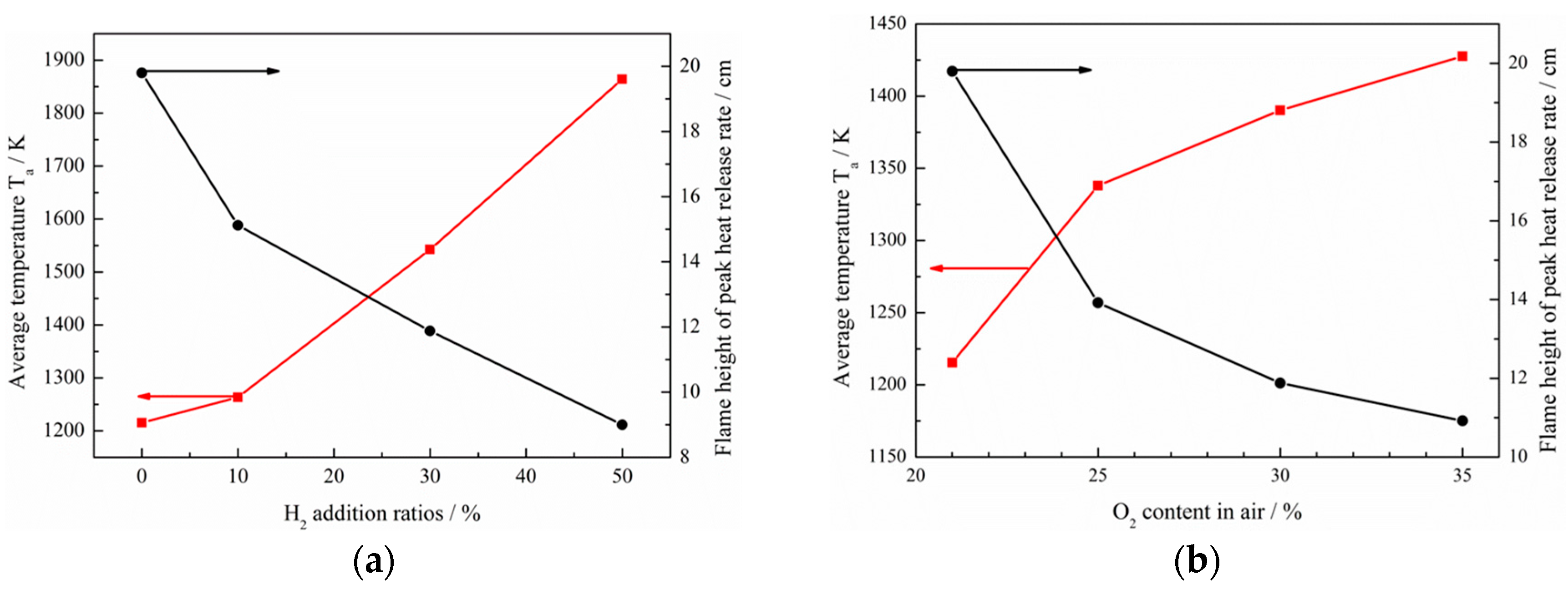
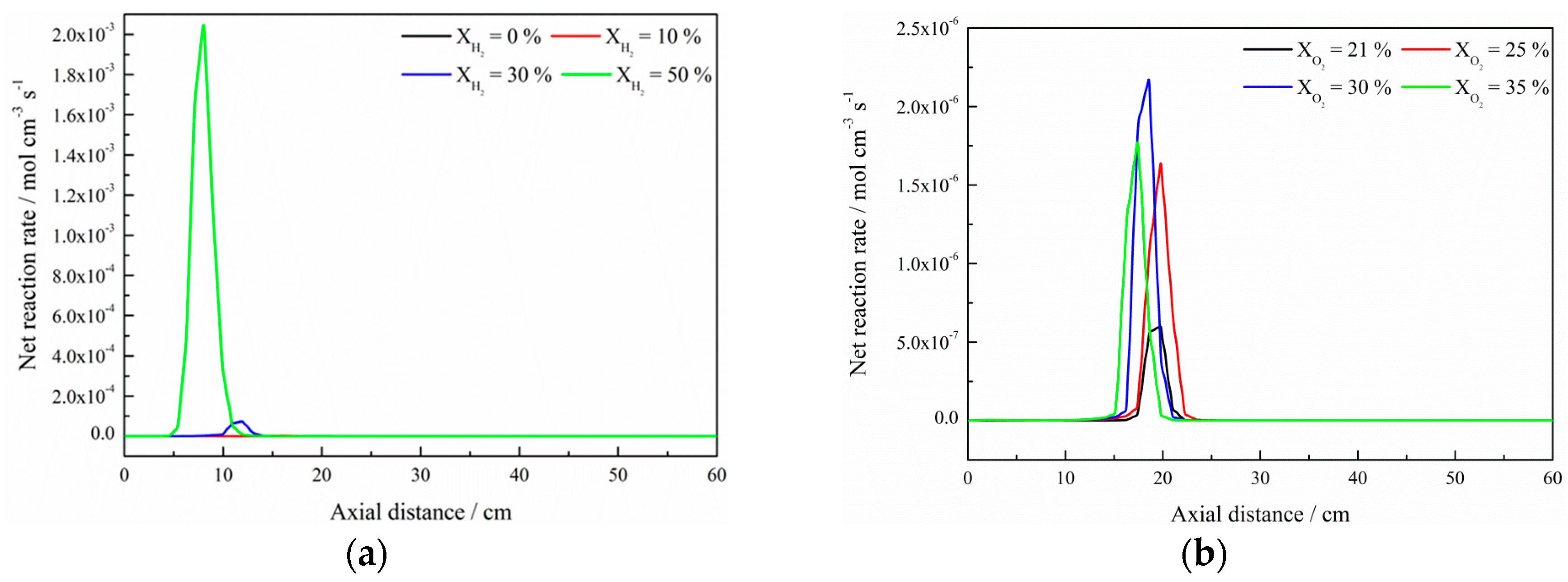
- The net reaction rate of biogas increases with increasing hydrogen addition ratio and oxygen levels, leading to a higher net heat release rate of biogas flame;
- (2)
- The formation of free radicals, such as H, O, and particularly OH, are enhanced with the increase in hydrogen addition ratio and oxygen levels;
- (3)
- Flames with enhanced heat release rates are formed under H2-enriched and O2-enriched conditions. Therefore, H2-enriched and O2-enriched combustion is beneficial to the improvement of combustion and heat release characteristics of biogas in practical application.
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| H2 addition ratio to fuel (based on low heat value) | |
| Flow rate of H2 (m·s−1) | |
| Flow rate of CH4 (m·s−1) | |
| Low heat value of H2 (kJ·mol−1) | |
| Low heat value of CH4 (kJ·mol−1) | |
| O2-enriched level (-) | |
| Flow rate of O2 (m·s−1) | |
| Flow rate of N2 (m·s−1) | |
| Density (kg·m−3) | |
| General dependent variable (-) | |
| Velocity vector (m·s−1) | |
| associated transport coefficient of Y (-) | |
| The source term (-) | |
| Mean absorption coefficient of species i (-) | |
| Formation enthalpy of specie j (J·mol−1) | |
| Enthalpy change of reaction i (J·mol−1) | |
| The reaction rate of reaction i (mol·cm−3·s−1) | |
| Heat release rate of reaction i (J·cm−3·s−1) | |
| Total heat release rate of all reaction (J·cm−3·s−1) | |
| Flame thickness (cm) | |
| The maximum temperature (K) | |
| The unburned temperature (K) | |
| The peak gradient in temperature (K·cm−1) |
References
- Divya, D.; Gopinath, L.R.; Christy, P.M. A review on current aspects and diverse prospects for enhancing biogas production in sustainable means. Renew. Sustain. Energy Rev. 2015, 42, 690–699. [Google Scholar] [CrossRef]
- Pazera, A.; Slezak, R.; Krzystek, L.; Ledakowicz, S.; Bochmann, G.; Gabauer, W.; Helm, S.; Reitmeier, S.; Marley, L.; Gorga, F.; et al. Biogas in Europe: Food and Beverage (FAB) Waste Potential for Biogas Production. Energy Fuels 2015, 29, 4011–4021. [Google Scholar] [CrossRef]
- Papurello, D.; Soukoulis, C.; Schuhfried, E.; Cappellin, L.; Gasperi, F.; Silvestri, S.; Santarelli, M.; Biasioli, F. Monitoring of volatile compound emissions during dry anaerobic digestion of the organic fraction of municipal solid waste by proton transfer reaction time-of-flight mass spectrometry. Bioresour. Technol. 2012, 126, 245–265. [Google Scholar] [CrossRef] [PubMed]
- Papurello, D.; Lanzini, A.; Tognana, L.; Silvestri, S.; Santarelli, M. Waste to energy: Exploitation of biogas from organic waste in a 500 Wel solid oxide fuel cell (SOFC) stack. Energy 2015, 85, 145–158. [Google Scholar] [CrossRef]
- Papurello, D.; Silvestri, S.; Tomasi, L.; Belcari, I.; Biasioli, F.; Santarelli, M. Biowaste for SOFCs. Energy Procedia 2016, 101, 424–431. [Google Scholar] [CrossRef]
- Bensaid, S.; Russo, N.; Fino, D. Power and hydrogen co-generation from biogas. Energy Fuels 2010, 24, 4743–4747. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, Z.; Liu, J.; Lv, Y.; Ye, X. Enhancing the storage stability of petroleum coke slurry by producing biogas from sludge fermentation. Energy 2016, 113, 319–327. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Y.; Cai, X.; Nie, Y.; Peng, C.; Huang, Z. Effect of H2O addition on the flame front evolution of syngas spherical propagation flames. Combust. Sci. Technol. 2016, 188, 1054–1072. [Google Scholar] [CrossRef]
- Park, C.; Park, S.; Lee, Y.; Kim, C.; Lee, S.; Moriyoshi, Y. Performance and emission characteristics of a SI engine fueled by low calorific biogas blended with hydrogen. Int. J. Hydrogen Energy 2011, 36, 10080–10088. [Google Scholar] [CrossRef]
- Fagbenle, R.; Oguaka, A.; Olakoyejo, O. A thermodynamic analysis of a biogas-fired integrated gasification steam injected gas turbine (BIG/STIG) plant. Appl. Therm. Eng. 2007, 27, 2220–2225. [Google Scholar] [CrossRef]
- Galvagno, A.; Chiodo, V.; Urbani, F.; Freni, F. Biogas as hydrogen source for fuel cell applications. Int. J. Hydrogen Energy 2013, 38, 3913–3920. [Google Scholar] [CrossRef]
- Shan, X.; Qian, Y.; Zhu, L.; Lu, X. Effects of EGR rate and hydrogen/carbon monoxide ratio on combustion and emission characteristics of biogas/diesel dual fuel combustion engine. Fuel 2016, 181, 1050–1057. [Google Scholar] [CrossRef]
- Cacua, K.; Amell, A.; Cadavid, F. Effects of oxygen enriched air on the operation and performance of a diesel-biogas dual fuel engine. Biomass Bioenergy 2012, 45, 159–167. [Google Scholar] [CrossRef]
- Baratieri, M.; Baggio, P.; Bosio, B.; Grigiante, M.; Longo, G. The use of biomass syngas in IC engines and CCGT plants: A comparative analysis. Appl. Therm. Eng. 2009, 29, 3309–3318. [Google Scholar] [CrossRef]
- Fischer, M.; Jiang, X. An investigation of the chemical kinetics of biogas combustion. Fuel 2015, 150, 711–720. [Google Scholar] [CrossRef]
- Somehsaraei, H.; Majoumerd, M.; Breuhaus, P.; Assadi, M. Performance analysis of a biogas-fueled micro gas turbine using a validated thermodynamic model. Appl. Therm. Eng. 2014, 66, 181–190. [Google Scholar] [CrossRef]
- Hosseini, S.; Bagheri, G.; Wahid, M. Numerical investigation of biogas flameless combustion. Energy Convers. Manag. 2014, 81, 41–50. [Google Scholar] [CrossRef]
- Zhen, H.; Leung, C.; Cheung, C. Effects of hydrogen addition on the characteristics of a biogas diffusion flame. Int. J. Hydrogen Energy 2013, 38, 6874–6881. [Google Scholar] [CrossRef]
- Wei, Z.; Leung, C.; Cheung, C.; Huang, Z. Effects of equivalence ratio, H2, and CO2 addition on the heat release characteristics of premixed laminar biogas-hydrogen flame. Int. J. Hydrogen Energy 2016, 41, 6567–6580. [Google Scholar] [CrossRef]
- Mameri, A.; Tabet, F. Numerical investigation of counter-flow diffusion flame of biogas-hydrogen blends: Effects of biogas composition, hydrogen enrichment and scalar dissipation rate on flame structure and emissions. Int. J. Hydrogen Energy 2016, 41, 2011–2022. [Google Scholar] [CrossRef]
- Zhen, H.; Leung, C.; Cheung, C.; Huang, Z. Combustion characteristic and heating performance of stoichiometric biogas-hydrogen-air flame. Int. J. Heat Mass Transf. 2016, 92, 807–814. [Google Scholar] [CrossRef]
- Zhen, H.; Leung, C.; Cheung, C.; Huang, Z. Characterization of biogas-hydrogen premixed flames using Bunsen burner. Int. J. Hydrogen Energy 2014, 39, 13292–13299. [Google Scholar] [CrossRef]
- Barlow, R. Radiation Models, Radiation, 2003. Available online: http://www.ca.sandia.gov/TNF/radiation.html (accessed on 10 February 2016).
- Spalding, D. New Developments and Computed Results, Imperial College CFDU Report HTS/81/1; Imperial College: London, UK, 1980. [Google Scholar]
- Patankar, S. Numerical Heat Transfer and Fluid Flow; Hemisphere, CRC press: New York, NY, USA, 1980. [Google Scholar]
- Smith, G. GRI-Mech. 1999. Available online: http://combustion.berkeley.edu/gri-mech/ (accessed on 23 November 2015).
- Wu, L.; Kobayashi, N.; Li, Z.; Huang, H.; Li, J. Emission and heat transfer characteristics of methane-hydrogen hybrid fuel laminar diffusion flame. Int. J. Hydrogen Energy 2015, 40, 9579–9589. [Google Scholar] [CrossRef]
- Tang, C.; Huang, Z.; Law, C. Determination, correlation, and mechanistic interpretation of effects of hydrogen addition on laminar flame speeds of hydrocarbon-air mixtures. Proc. Combust. Inst. 2011, 33, 921–928. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Kobayashi, N.; Wang, C.; Yuan, H. Numerical study on laminar burning velocity and ignition delay time of ammonia flame with hydrogen addition. Energy 2017, 126, 769–809. [Google Scholar] [CrossRef]
- Li, Z.; Han, W.; Liu, D.; Chen, Z. Laminar flame propagation and ignition properties of premixed iso-octane/air with hydrogen. Fuel 2015, 158, 443–450. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, S.; Li, Q.; Pan, X.; Liao, S.; Wang, H.; Yang, C.; Wei, S. Experimental investigation on the effects of hydrogen addition on thermal characteristics of methane/air premixed flame. Fuel 2014, 115, 232–240. [Google Scholar] [CrossRef]
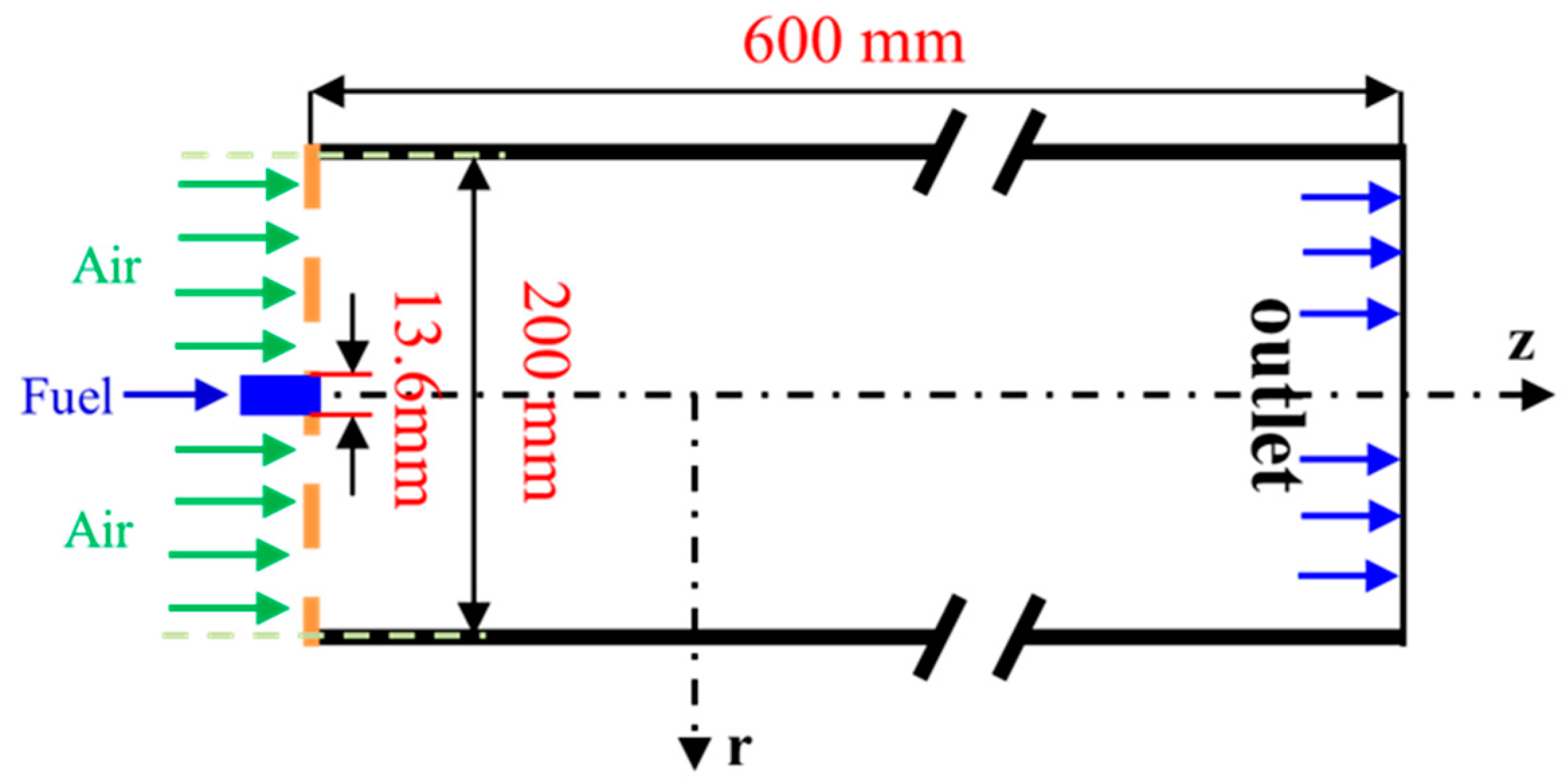
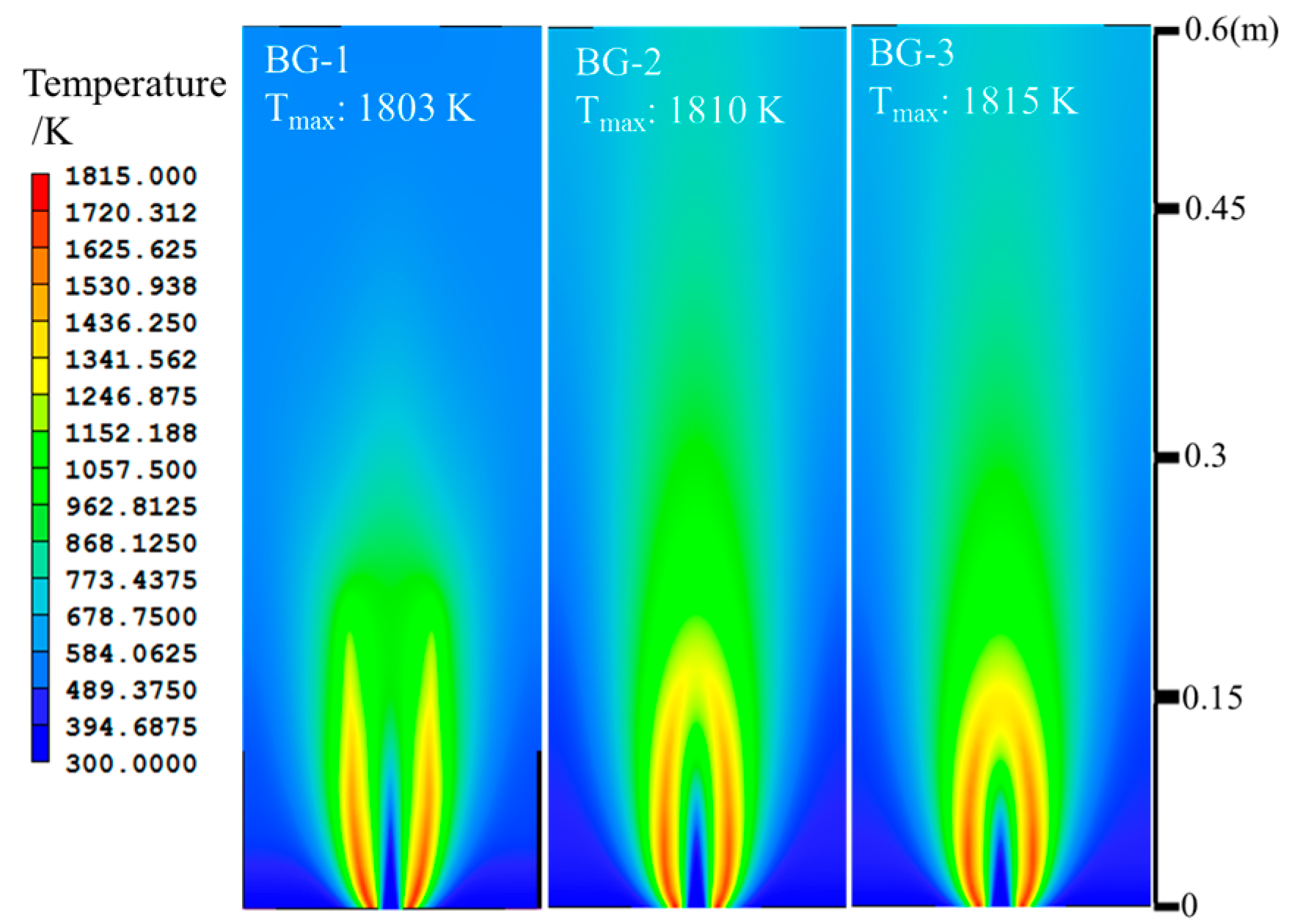

| Items | CH4/L·min−1 | CO2/L·min−1 | H2/L·min−1 | O2/L·min−1 | Air/L·min−1 | Power/kW |
|---|---|---|---|---|---|---|
| BG-1 | 1.829 | 1.220 | - | - | 18.340 | 1 |
| BG-2 | 1.829 | 0.784 | - | - | 18.340 | 1 |
| BG-3 | 1.829 | 0.457 | - | - | 18.340 | 1 |
| H2 10% | 1.647 | 1.098 | 0.602 | - | 18.014 | 1 |
| H2 30% | 1.281 | 0.854 | 1.805 | - | 17.361 | 1 |
| H2 50% | 0.915 | 0.610 | 3.008 | - | 16.709 | 1 |
| O2 25% | 1.829 | 1.220 | - | 0.780 | 14.626 | 1 |
| O2 30% | 1.829 | 1.220 | - | 1.463 | 11.376 | 1 |
| O2 35% | 1.829 | 1.220 | - | 1.950 | 9.054 | 1 |
| Coefficient | CO2 | H2O | CH4 | CO (T < 750 K) | CO (T > 750 K) |
|---|---|---|---|---|---|
| a0 | 18.741 | −0.23093 | 6.6334 | 4.7869 | 10.09 |
| a1 | −121.31 | −1.1239 | −3.5686 × 10−3 | −0.06953 | −0.01183 |
| a2 | 273.5 | 9.4153 | 1.6682 × 10−8 | 2.95775 × 10−4 | 4.7753 × 10−6 |
| a3 | −194.05 | −2.9988 | 2.5611 × 10−10 | −4.25732 × 10−7 | −5.87209 × 10−10 |
| a4 | 56.31 | 0.51382 | −2.6558 × 10−14 | 2.02894× 10−10 | −2.5334 × 10−14 |
| a5 | −5.8169 | −1.884 × 10−5 | 0 | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Huang, H.; Huhetaoli; Osaka, Y.; Bai, Y.; Kobayashi, N.; Chen, Y. Combustion and Heat Release Characteristics of Biogas under Hydrogen- and Oxygen-Enriched Condition. Energies 2017, 10, 1200. https://doi.org/10.3390/en10081200
Li J, Huang H, Huhetaoli, Osaka Y, Bai Y, Kobayashi N, Chen Y. Combustion and Heat Release Characteristics of Biogas under Hydrogen- and Oxygen-Enriched Condition. Energies. 2017; 10(8):1200. https://doi.org/10.3390/en10081200
Chicago/Turabian StyleLi, Jun, Hongyu Huang, Huhetaoli, Yugo Osaka, Yu Bai, Noriyuki Kobayashi, and Yong Chen. 2017. "Combustion and Heat Release Characteristics of Biogas under Hydrogen- and Oxygen-Enriched Condition" Energies 10, no. 8: 1200. https://doi.org/10.3390/en10081200




