Numerical Simulation and Optimization of the Melting Process of Phase Change Material inside Horizontal Annulus
Abstract
:1. Introduction
2. Numerical Study
2.1. Physical and Numerical Model
2.2. Governing Equations
2.3. Dimensionless Analysis
- Fourier number, the dimensionless time:where R is the characteristic length, which is the shell diameter.
- The dimensionless temperature:where TM = 302 K is the average phase-change temperature and TW is the boundary temperature.
- Stefan number:where L is the phase-change enthalpy. The Stefan number varies with the shell temperature and thus can be defined as the dimensionless boundary temperature.
- Degree of sub-cooling:where Tini is the initial (sub-cooled) temperature of the paraffin.
- The dimensionless offset Xoff and inner-tube diameter Xr:Xoff = Δ/Dwhere Δ is the relative offset of the centers, d0 is the inner-tube diameter and D is the constant shell diameter.Xr = d0/D
- Since in the investigated system have no heat loss, its energy efficiency will always be 100%. Thus the energy efficiency is not a suitable index of performance measurement. Instead, the exergy efficiency ηex was used:where is the change in exergy of the system, and is the exergy input from the high temperature shell, both of which satisfy the exergy balance.where I is the exergy destruction, which can be determined with,where Π is the entropy production, which satisfies the entropy balance:where is the heat transfer through the shell over the ith time step, and the summation item is the total change in entropy at the shell from the start of the simulation to time step t. is the change in entropy of the system, which can be determined by:where , , and are the heat transferred into the PCM region, the air region, and the water region, respectively; , , and are the mass weighted average temperature of the PCM region, the air region, and the water region, respectively.
2.4. Parametric Variables
3. Results and Discussion
3.1. Default Configuration
3.2. Eccentricity
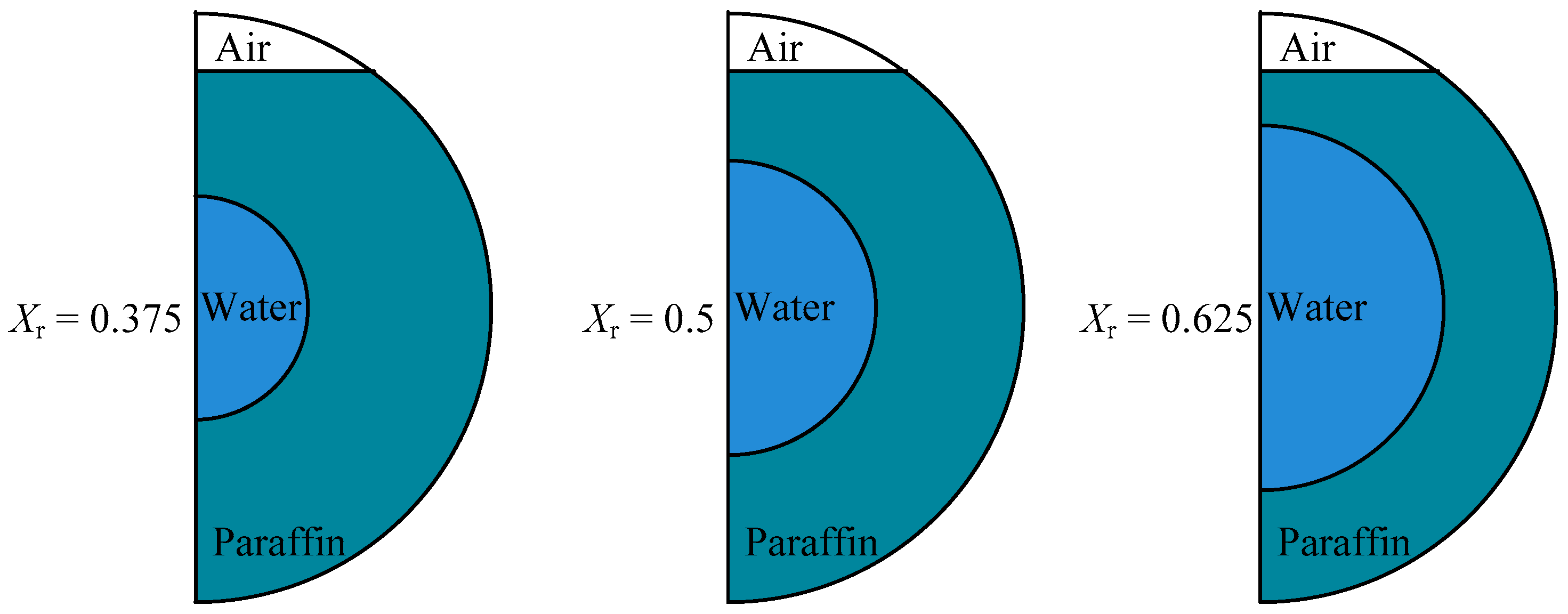
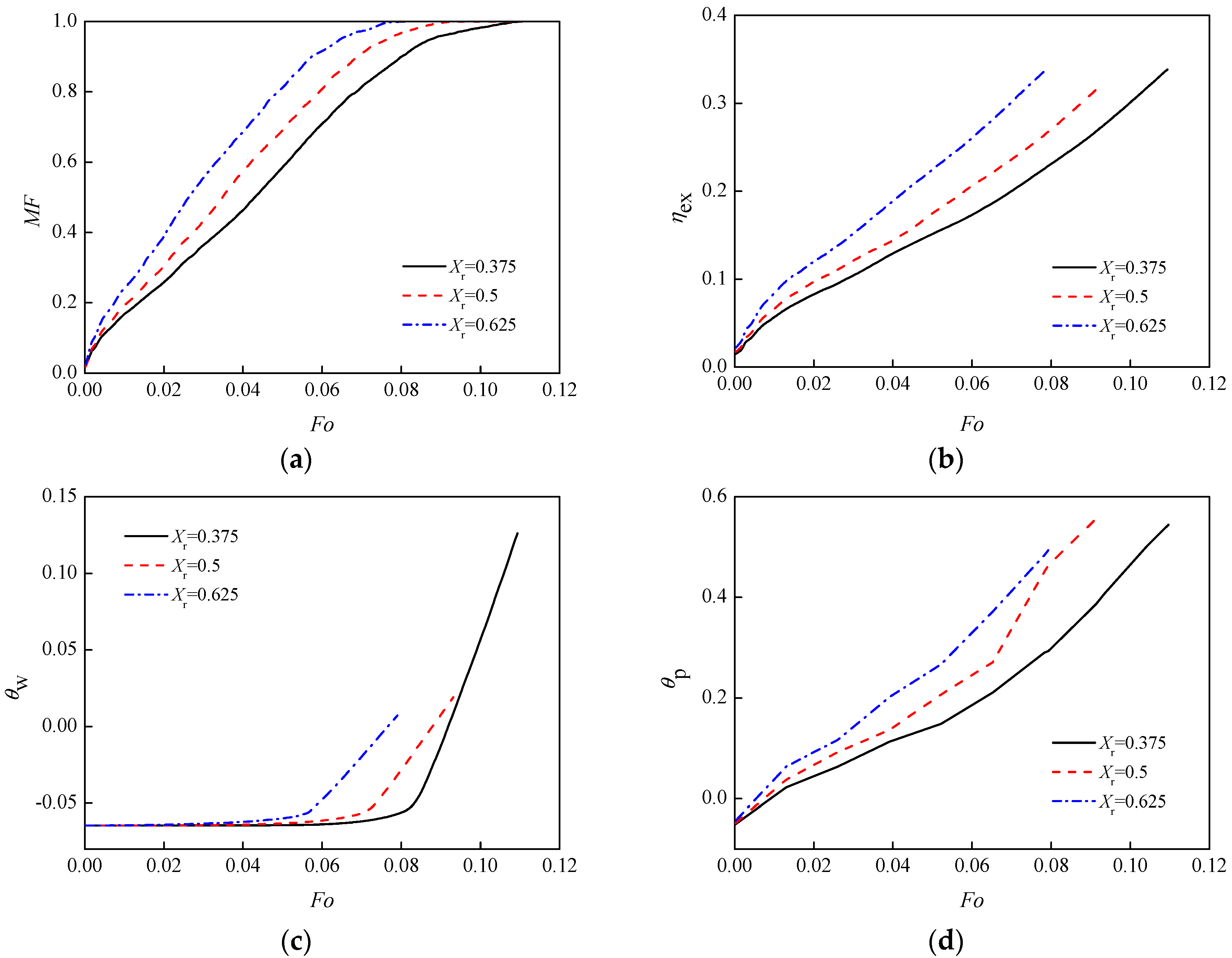
3.3. Diameter of Inner Tube
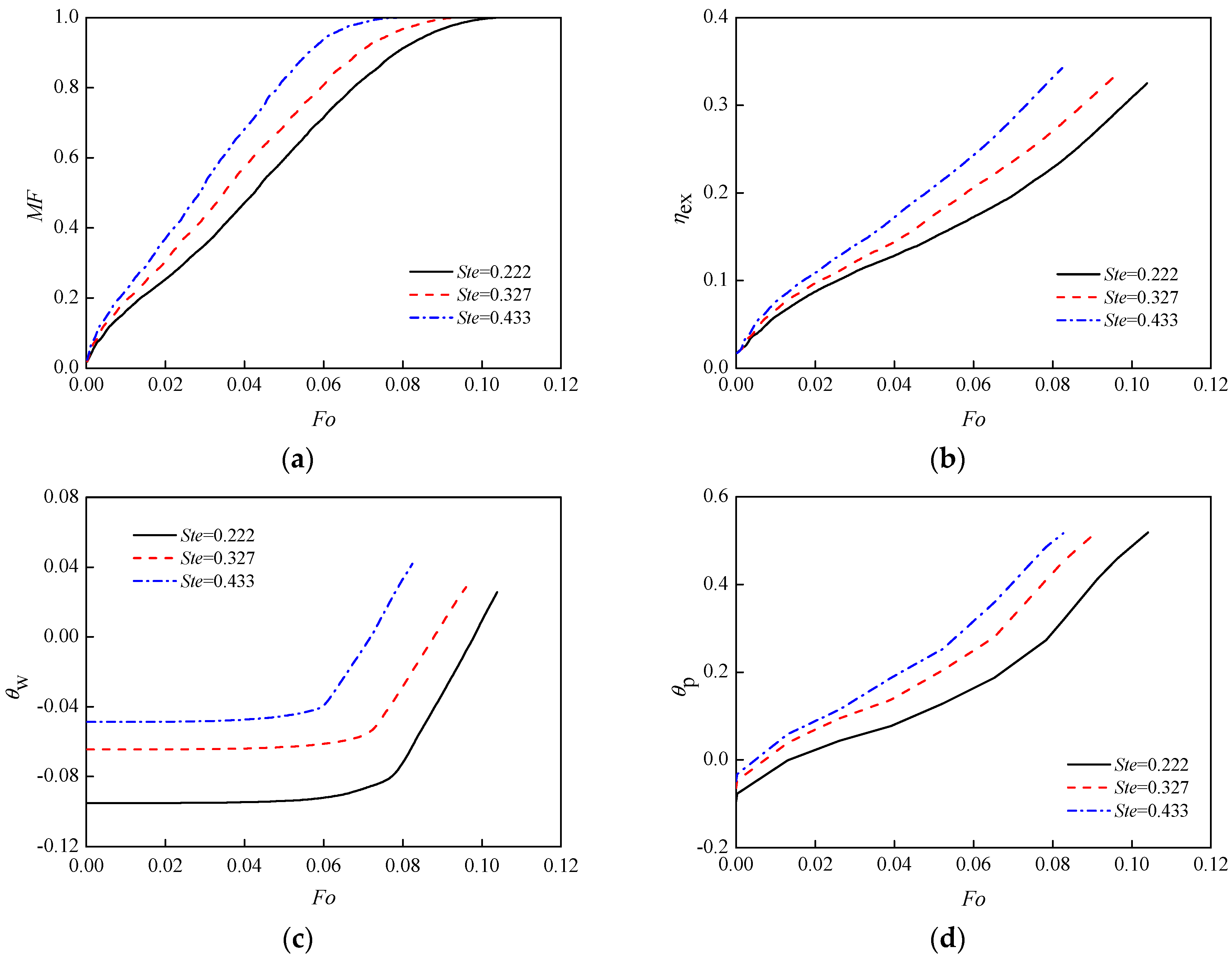
3.4. Surface Temperature
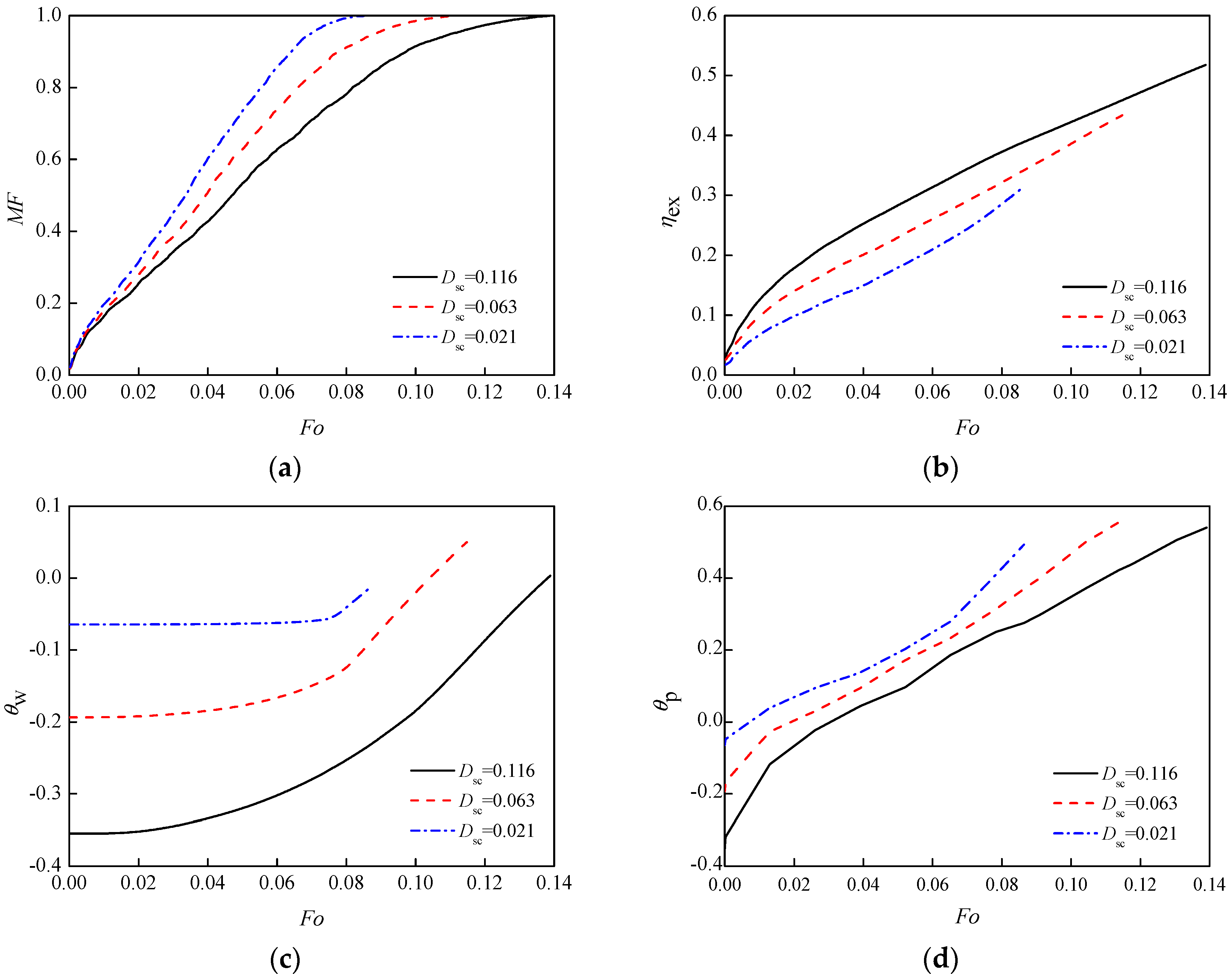
3.5. Sub-Cooling Degree
4. Conclusions
- When the boundary temperature, degree of sub-cooling, and paraffin filling amount is consistent, tube position has only a slight impact on the speed of heat-exchange process. Melting fraction, exergy efficiency, and the temperature change of the external paraffin under conditions of three different eccentricities are similar;
- For the changing inner tube diameters, with Xr = 0.5 as a reference, when inner tube diameter increases to Xr = 0.625, the melting time is 8.98% faster, and the time is slowed by 25.45% for Xr = 0.375;
- The changes of boundary temperature and sub-cooling degree directly affect the temperature difference between the PCM and boundary temperature. The larger the temperature difference, the higher the heat transfer efficiency, the shorter time of the solid phase fully melted. Also, the proportion of heat convection and conduction in the system changes. For a higher shell-PCM temperature difference, the natural convection is stronger and the melting rate is faster. Fast temperature change makes the exergy efficiency rise faster and to a higher level. However, at the end of complete melting, the exergy efficiencies were close.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, X.; Li, Y.; Sun, Z.; Zhou, J. Dynamic measurement of the thermal conductivity of phase change materials in the liquid phase near the melting point. Int. J. Heat Mass Transf. 2017, 111, 631–641. [Google Scholar] [CrossRef]
- Hosseini, M.; Rahimi, M.; Bahrampoury, R. Experimental and computational evolution of a shell and tube heat exchanger as a PCM thermal storage system. Int. Commun. Heat Mass Transf. 2014, 50, 128–136. [Google Scholar] [CrossRef]
- Li, Q.; Tehrani, S.S.M.; Taylor, R.A. Techno-economic analysis of a concentrating solar collector with built-in shell and tube latent heat thermal energy storage. Energy 2017, 121, 220–237. [Google Scholar] [CrossRef]
- Tehrani, S.S.M.; Taylor, R.A.; Saberi, P.; Diarce, G. Design and feasibility of high temperature shell and tube latent heat thermal energy storage system for solar thermal power plants. Renew. Energy 2016, 96, 120–136. [Google Scholar] [CrossRef]
- Bareiss, M.; Beer, H. An analytical solution of the heat transfer process during melting of an unfixed solid phase change material inside a horizontal tube. Int. J. Heat Mass Transf. 1984, 27, 739–746. [Google Scholar] [CrossRef]
- Chen, W.; Cheng, S.; Luo, Z.; Gu, W. Study of contact melting inside isothermally heated vertical cylindrical capsules. J. Therm. Sci. 1993, 2, 190. [Google Scholar] [CrossRef]
- Bejan, A.; Ziaei, S.; Lorente, S. The S curve of energy storage by melting. J. Appl. Phys. 2014, 116, 114902. [Google Scholar] [CrossRef]
- Lorente, S.; Bejan, A.; Niu, J. Phase change heat storage in an enclosure with vertical pipe in the center. Int. J. Heat Mass Transf. 2014, 72, 329–335. [Google Scholar] [CrossRef]
- Al-Abidi, A.A.; Mat, S.; Sopian, K.; Sulaiman, M.Y.; Mohammad, A.T. Experimental study of melting and solidification of PCM in a triplex tube heat exchanger with fins. Energy Build. 2014, 68, 33–41. [Google Scholar] [CrossRef]
- Yazici, M.Y.; Avci, M.; Aydin, O.; Akgun, M. On the effect of eccentricity of a horizontal tube-in-shell storage unit on solidification of a PCM. Appl. Therm. Eng. 2014, 64, 1–9. [Google Scholar] [CrossRef]
- Rathod, M.K.; Banerjee, J. Thermal performance enhancement of shell and tube Latent Heat Storage Unit using longitudinal fins. Appl. Therm. Eng. 2015, 75, 1084–1092. [Google Scholar] [CrossRef]
- Gasia, J.; Tay, N.H.S.; Belusko, M.; Cabeza, L.F.; Bruno, F. Experimental investigation of the effect of dynamic melting in a cylindrical shell-and-tube heat exchanger using water as PCM. Appl. Energy 2017, 185, 136–145. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Chen, Y.; Sun, Z. The melting of phase change material in a cylinder shell with hierarchical heat sink array. Appl. Therm. Eng. 2014, 73, 975–983. [Google Scholar] [CrossRef]
- Seddegh, S.; Wang, X.; Henderson, A.D. Numerical investigation of heat transfer mechanism in a vertical shell and tube latent heat energy storage system. Appl. Therm. Eng. 2015, 87, 698–706. [Google Scholar] [CrossRef]
- Darzi, A.A.R.; Jourabian, M.; Farhadi, M. Melting and solidification of PCM enhanced by radial conductive fins and nanoparticles in cylindrical annulus. Energy Convers. Manag. 2016, 118, 253–263. [Google Scholar] [CrossRef]
- Fornarelli, F.; Camporeale, S.M.; Fortunato, B.; Torresi, M.; Oresta, P.; Magliocchetti, L.; Miliozzi, A.; Santo, G. CFD analysis of melting process in a shell-and-tube latent heat storage for concentrated solar power plants. Appl. Energy 2016, 164, 711–722. [Google Scholar] [CrossRef]
- Tao, Y.; Carey, V.P. Effects of PCM thermophysical properties on thermal storage performance of a shell-and-tube latent heat storage unit. Appl. Energy 2016, 179, 203–210. [Google Scholar] [CrossRef]
- Tabassum, T.; Hasan, M.; Begum, L. 2-D numerical investigation of melting of an impure PCM in the arbitrary-shaped annuli. Int. J. Therm. Sci. 2017, 114, 296–319. [Google Scholar] [CrossRef]
- Riahi, S.; Saman, W.Y.; Bruno, F.; Belusko, M.; Tay, N.H.S. Impact of periodic flow reversal of heat transfer fluid on the melting and solidification processes in a latent heat shell and tube storage system. Appl. Energy 2017, 191, 276–286. [Google Scholar] [CrossRef]
- Kuboth, S.; König-Haagen, A.; Brüggemann, D. Numerical Analysis of Shell-and-Tube Type Latent Thermal Energy Storage Performance with Different Arrangements of Circular Fins. Energies 2017, 10, 274. [Google Scholar] [CrossRef]
- Sun, W.; Zhao, Z.; Wang, Y. Thermal Analysis of a Thermal Energy Storage Unit to Enhance a Workshop Heating System Driven by Industrial Residual Water. Energies 2017, 10, 219. [Google Scholar] [CrossRef]
- Assis, E.; Ziskind, G.; Letan, R. Numerical and experimental study of solidification in a spherical shell. J. Heat Transf. 2009, 131, 024502. [Google Scholar] [CrossRef]
- Assis, E.; Katsman, L.; Ziskind, G.; Letan, R. Numerical and experimental study of melting in a spherical shell. Int. J. Heat Mass Transf. 2007, 50, 1790–1804. [Google Scholar] [CrossRef]
- Zhang, P.; Xiao, X.; Ma, Z. A review of the composite phase change materials: Fabrication, characterization, mathematical modeling and application to performance enhancement. Appl. Energy 2016, 165, 472–510. [Google Scholar] [CrossRef]
- Das, N.; Takata, Y.; Kohno, M.; Harish, S. Effect of carbon nano inclusion dimensionality on the melting of phase change nanocomposites in vertical shell-tube thermal energy storage unit. Int. J. Heat Mass Transf. 2017, 113, 423–431. [Google Scholar] [CrossRef]
- Das, N.; Kohno, M.; Takata, Y.; Patil, D.V.; Harish, S. Enhanced melting behavior of carbon based phase change nanocomposites in horizontally oriented latent heat thermal energy storage system. Appl. Therm. Eng. 2017, 125, 880–890. [Google Scholar] [CrossRef]
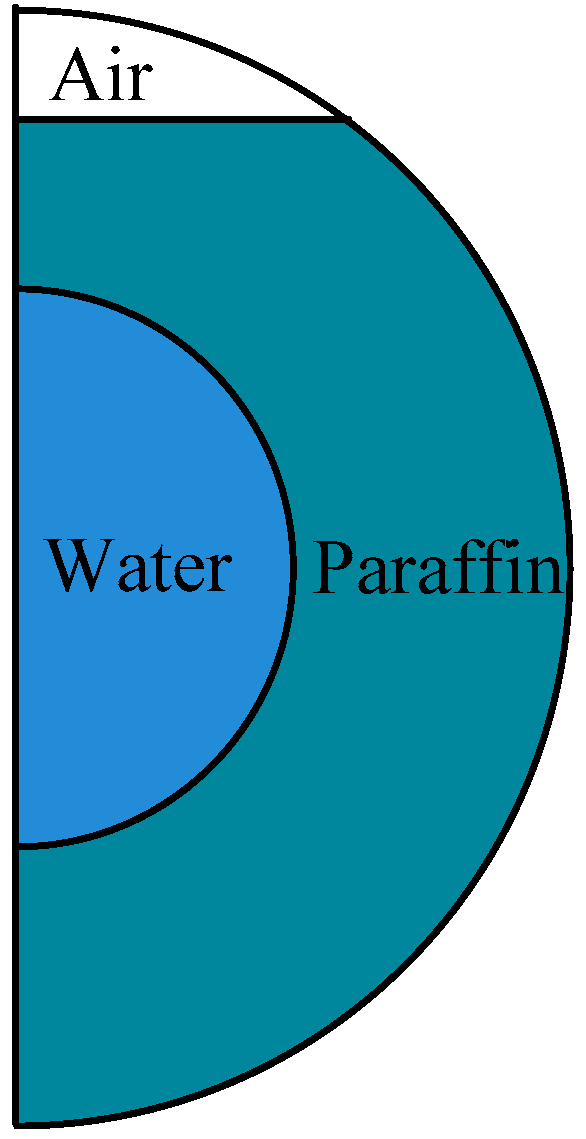
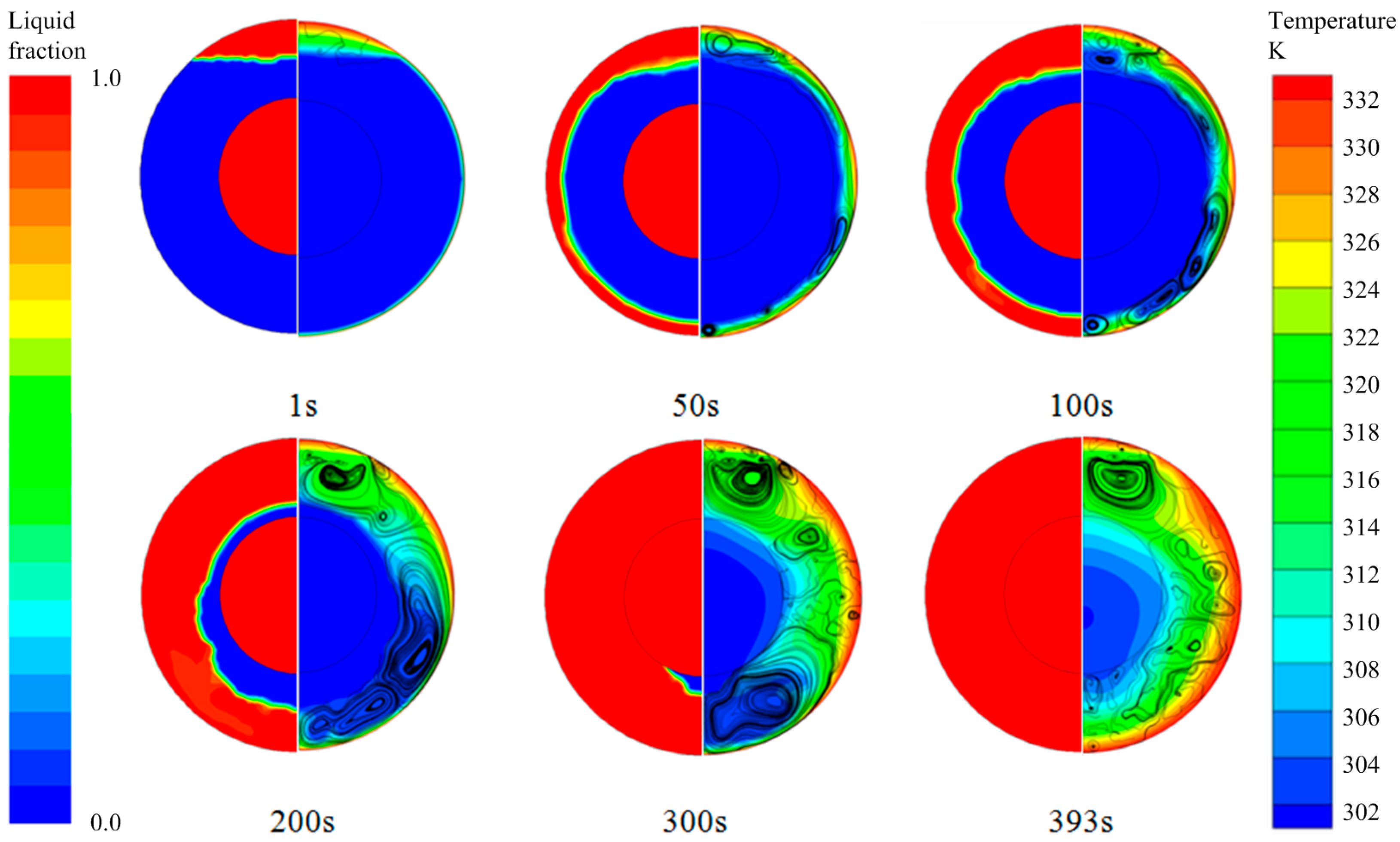
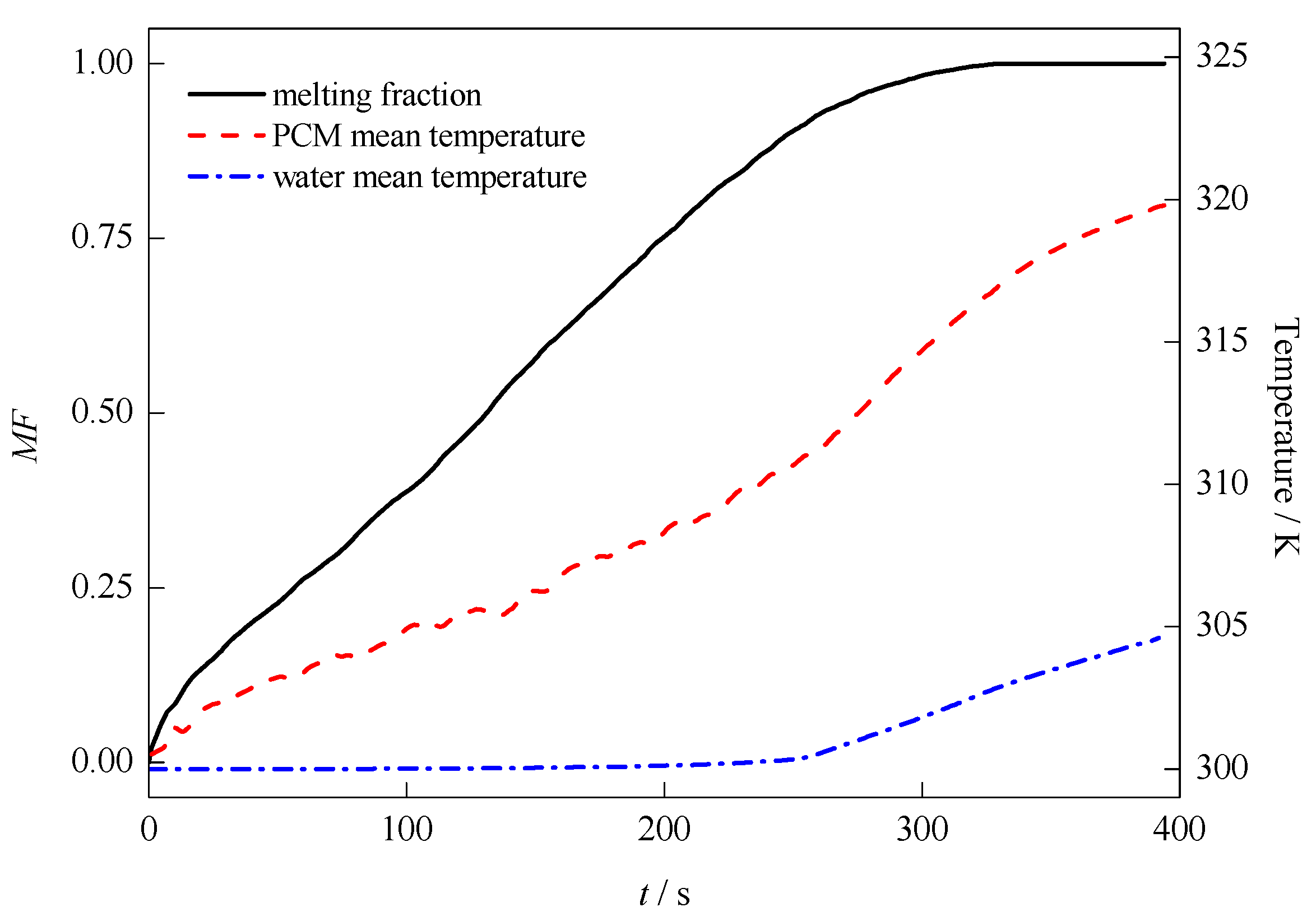
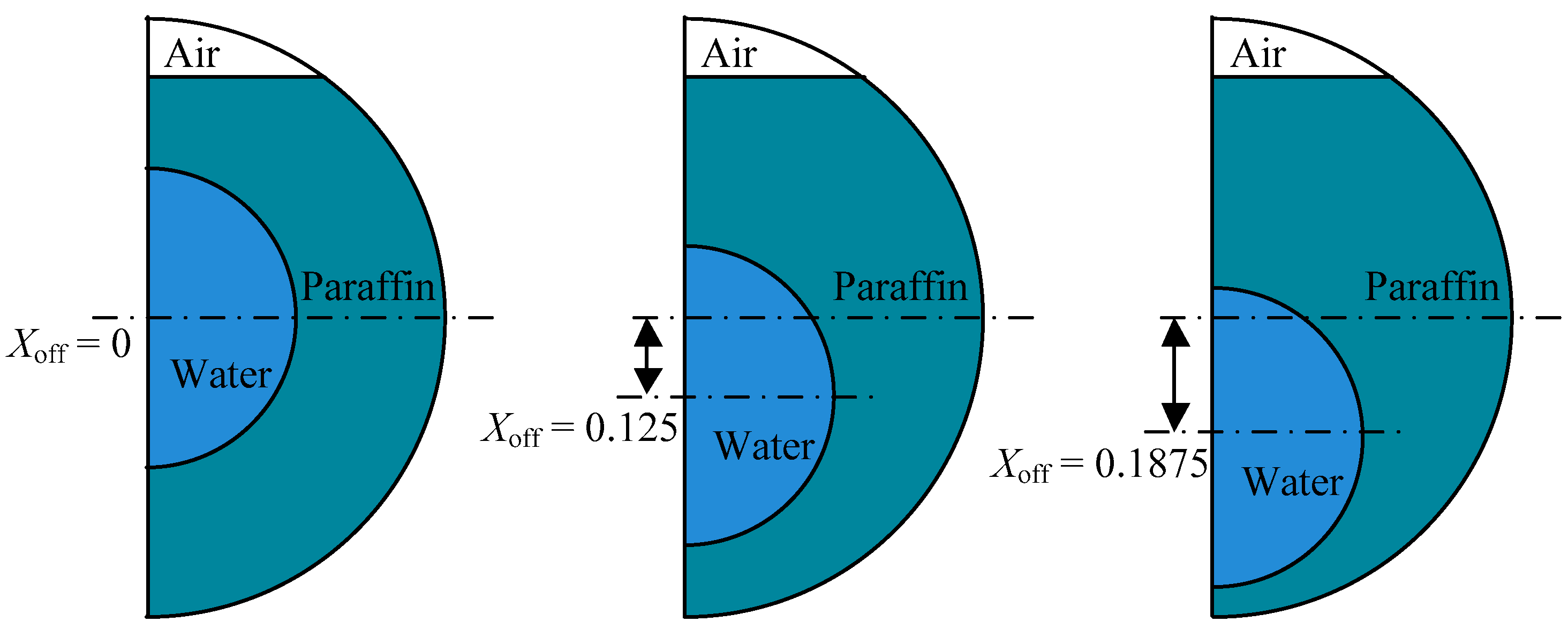

| Properties | Values | ||
|---|---|---|---|
| Solid PCM | Liquid PCM | Air | |
| density, kg/m3 | 870 | 760 1 | 1.225 2 |
| specific heat, J/(kg·K) | 2400 | 1890 | 1006.43 |
| thermal conductivity, W/(m·K) | 0.24 | 0.15 | 0.0242 |
| dynamic viscosity, Pa·s | - | 0.0032 | 1.789 × 105 |
| volumetric expansion coefficient, 1/K | - | 0.0009 | - |
| latent heat, J/kg | - | 179,000 | - |
| Factors | Values | Dimensionless Form | Dimensionless Values | ||||
|---|---|---|---|---|---|---|---|
| inner tube center offset, mm | 0 | 5 | 7.5 | Xoff | 0 | 0.125 | 0.1875 |
| inner tube diameter, mm | 15 | 20 | 25 | Xr | 0.375 | 0.5 | 0.625 |
| shell temperature, K | 323 | 333 | 343 | Ste | 0.222 | 0.327 | 0.433 |
| initial temperature, K | 291 | 296 | 300 | Dsc | 0.116 | 0.063 | 0.021 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Chen, Y.; Sun, Z. Numerical Simulation and Optimization of the Melting Process of Phase Change Material inside Horizontal Annulus. Energies 2017, 10, 1249. https://doi.org/10.3390/en10091249
Li S, Chen Y, Sun Z. Numerical Simulation and Optimization of the Melting Process of Phase Change Material inside Horizontal Annulus. Energies. 2017; 10(9):1249. https://doi.org/10.3390/en10091249
Chicago/Turabian StyleLi, Saiwei, Yu Chen, and Zhiqiang Sun. 2017. "Numerical Simulation and Optimization of the Melting Process of Phase Change Material inside Horizontal Annulus" Energies 10, no. 9: 1249. https://doi.org/10.3390/en10091249




