Biodiesel Production Potential from Littered Edible Oil Fraction Using Directly Synthesized S-TiO2/MCM-41 Catalyst in Esterification Process via Non-Catalytic Subcritical Hydrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of S-TiO2/MCM-41
2.2.2. Reaction Process
3. Results and Discussion
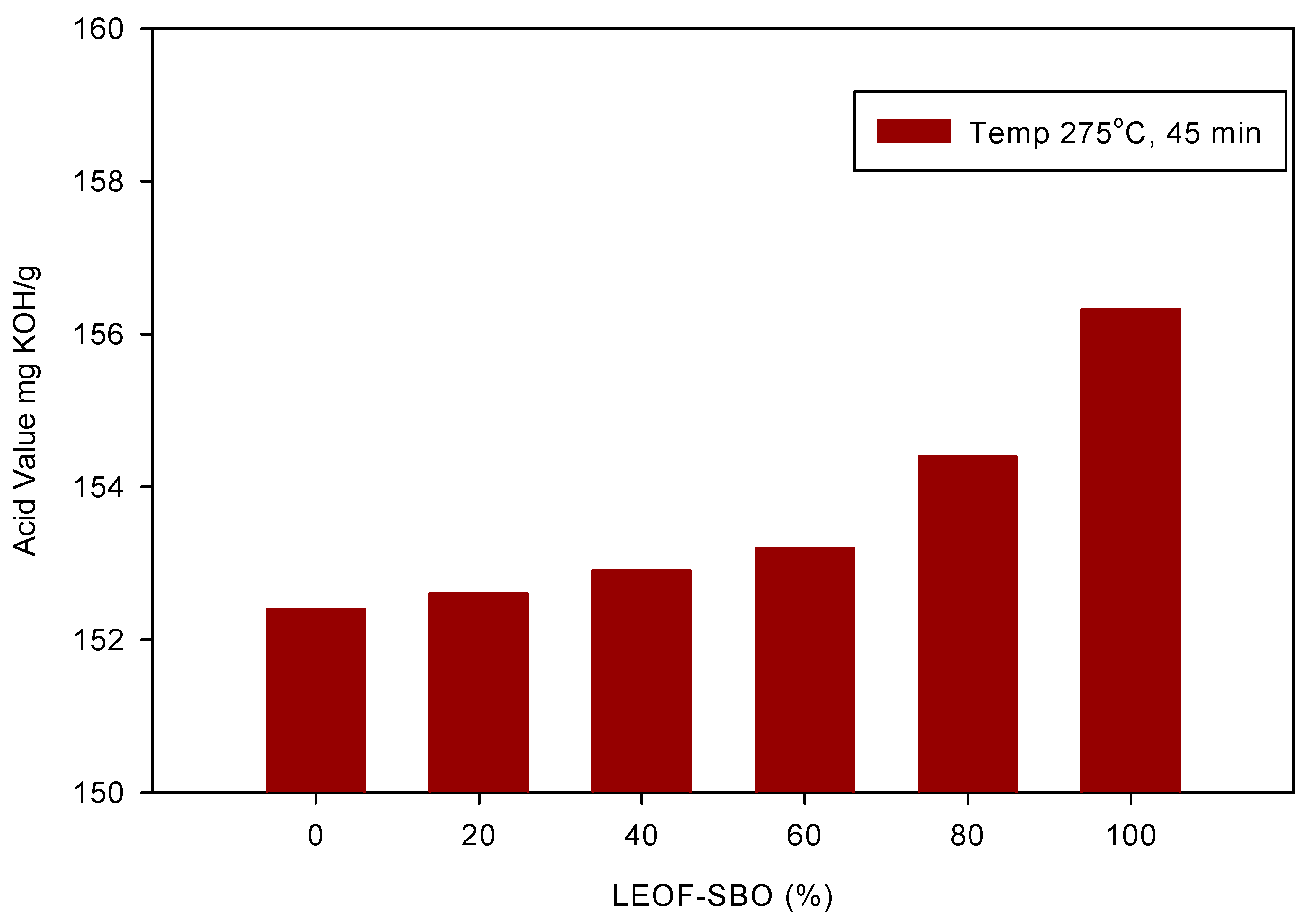
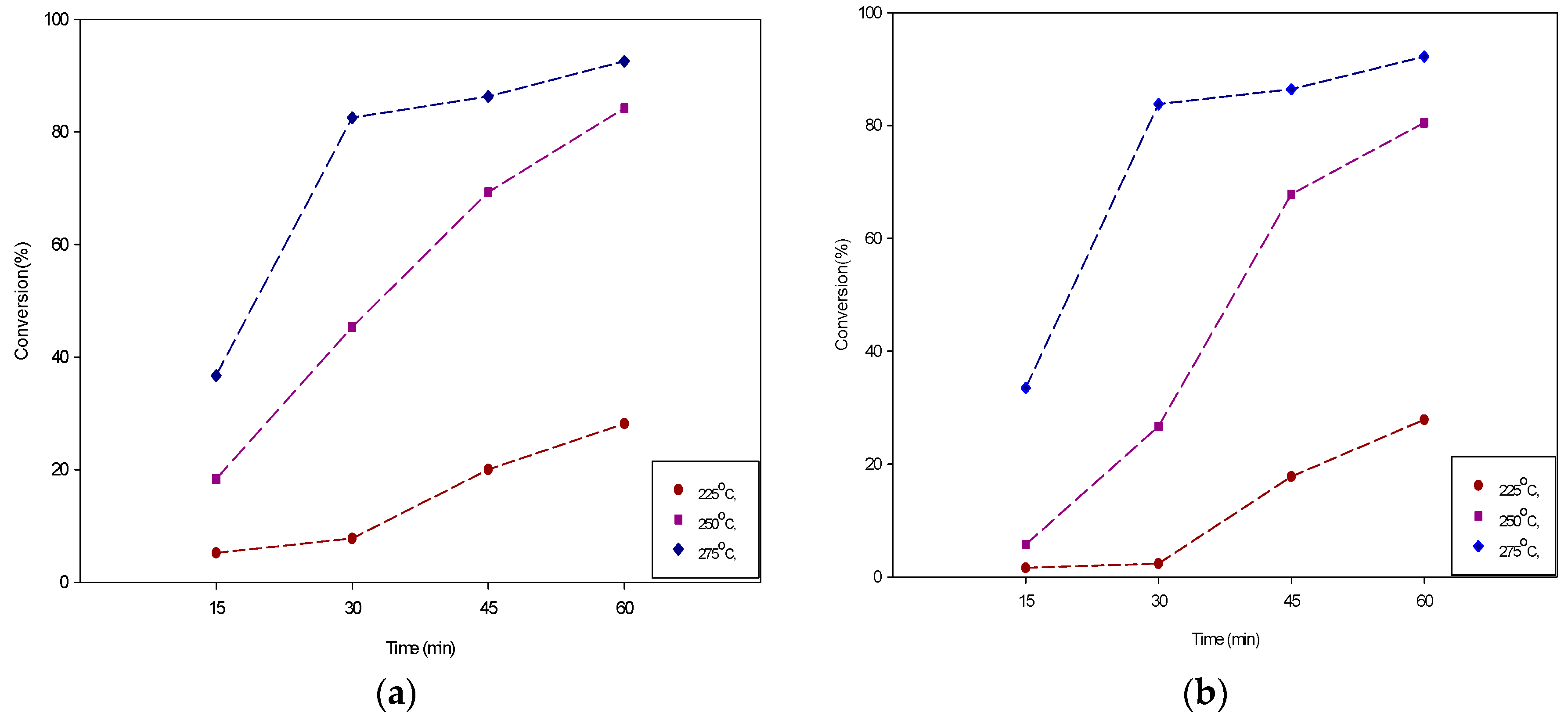
3.1. Hydrolysis of Triglycerides in Subcritical Water
3.1.1. Effect of Reaction Temperature
3.1.2. Effect on Reaction Time
3.2. Catalyst Characterization
3.2.1. X-ray Diffraction
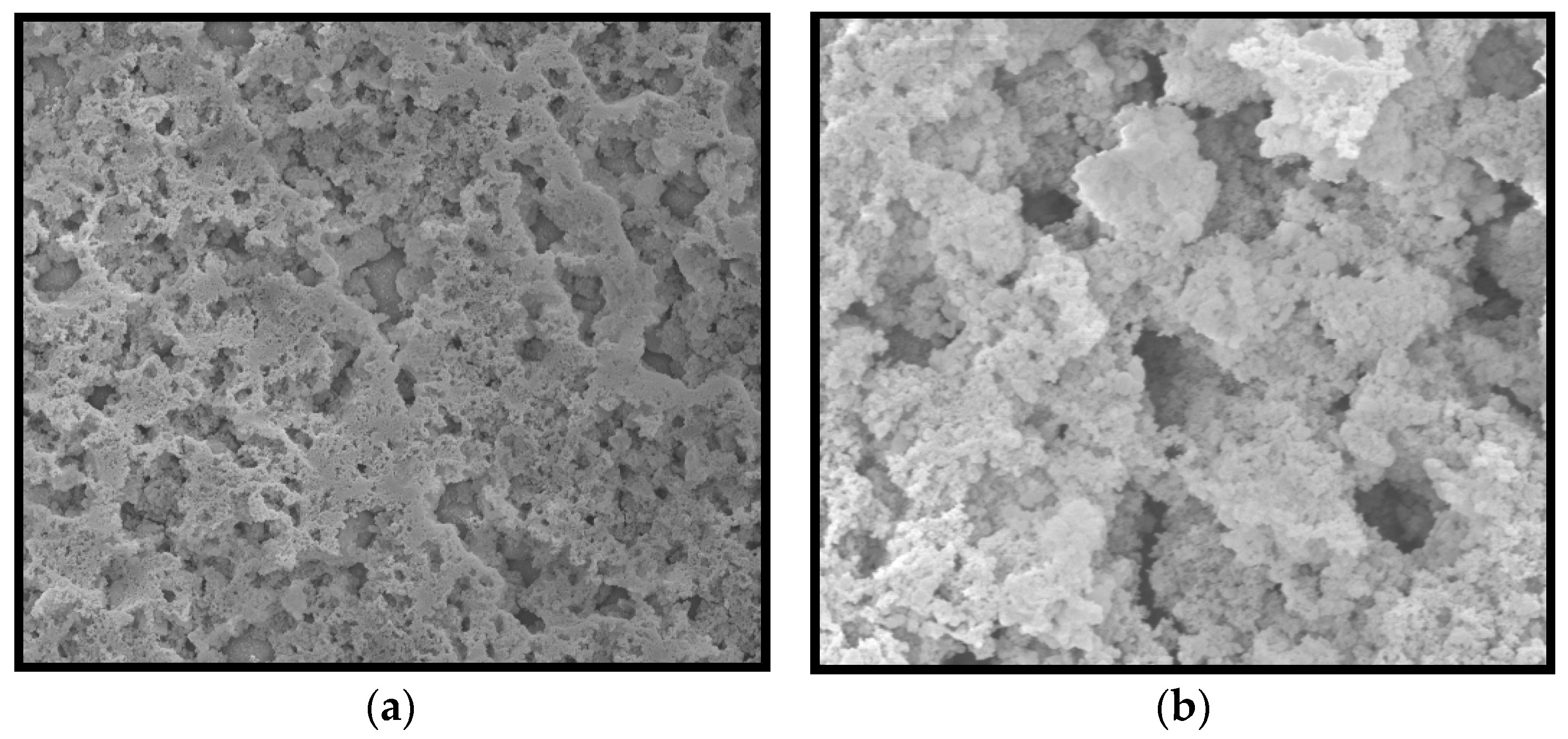
3.2.2. SEM Analysis
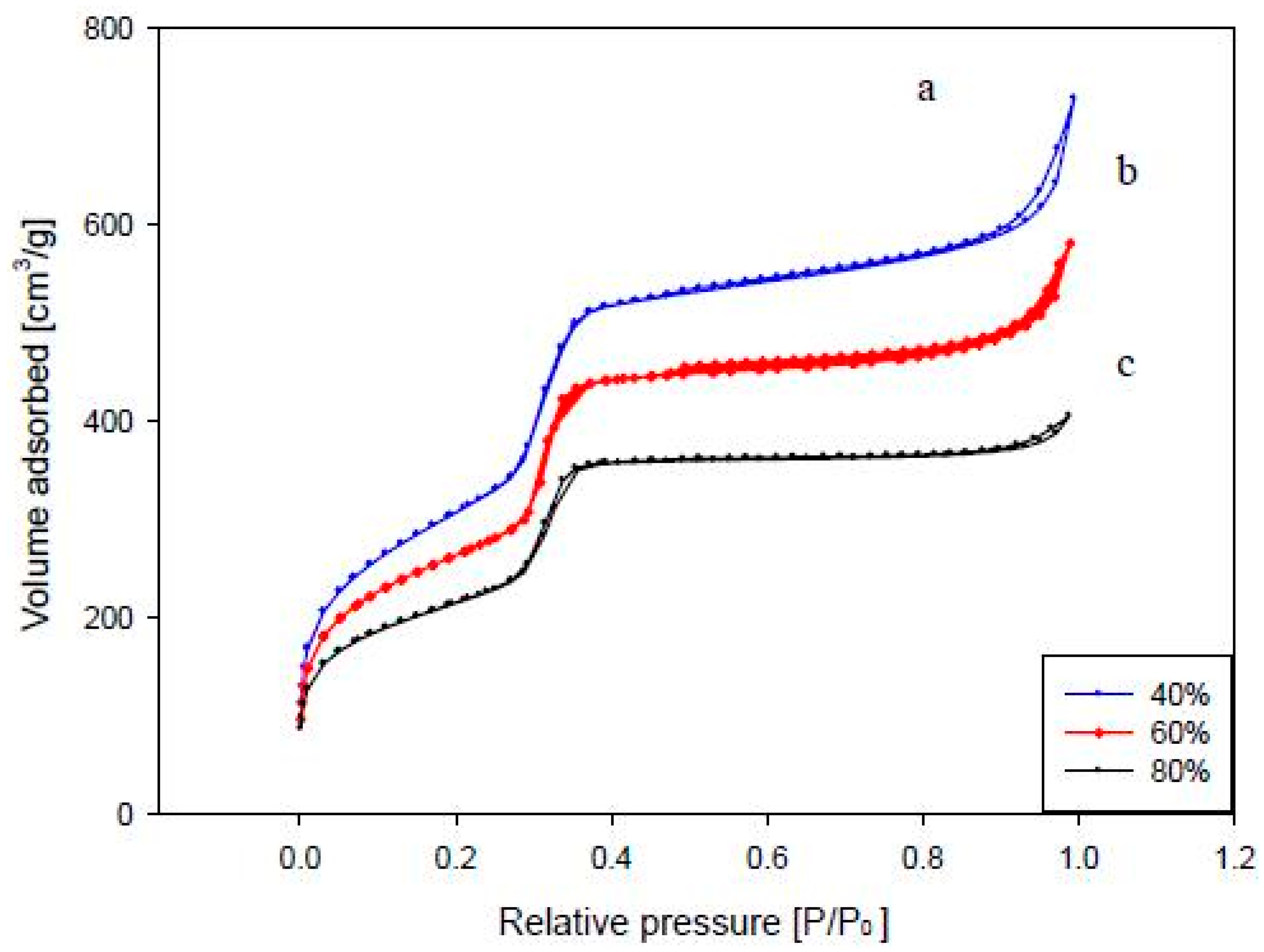
3.2.3. Physical Properties of the Catalyst
3.3. Catalytic Activity of S-TiO2/MCM-41 in Esterification of FFA in Supercritical and Subcritical Methanol
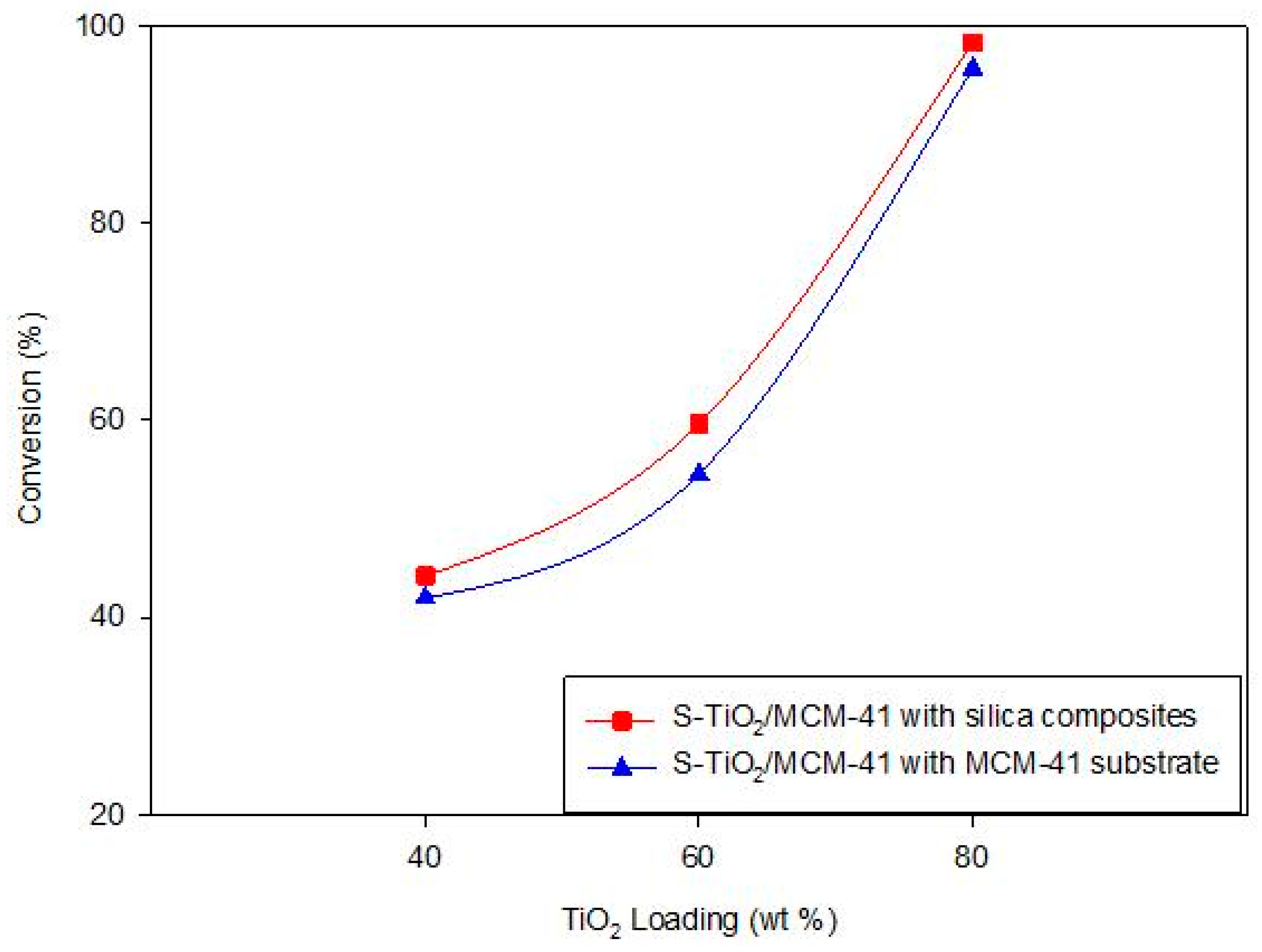
3.3.1. Effect of TiO2 Loading
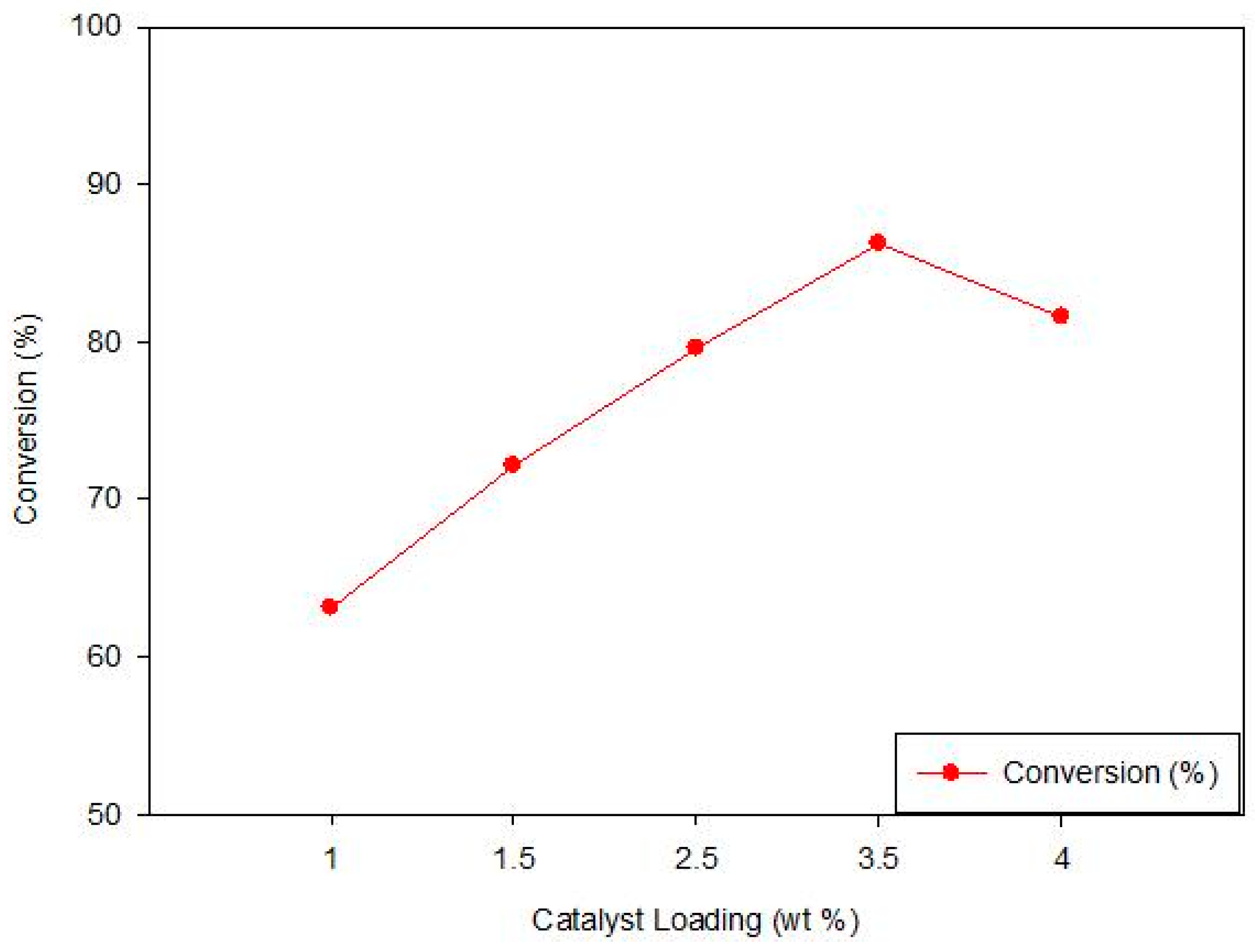
3.3.2. Effect of the Concentration of H2SO4 on the of S-TiO2/MCM-41
3.3.3. Effect of the Calcination Temperature on the Conversion of FFA
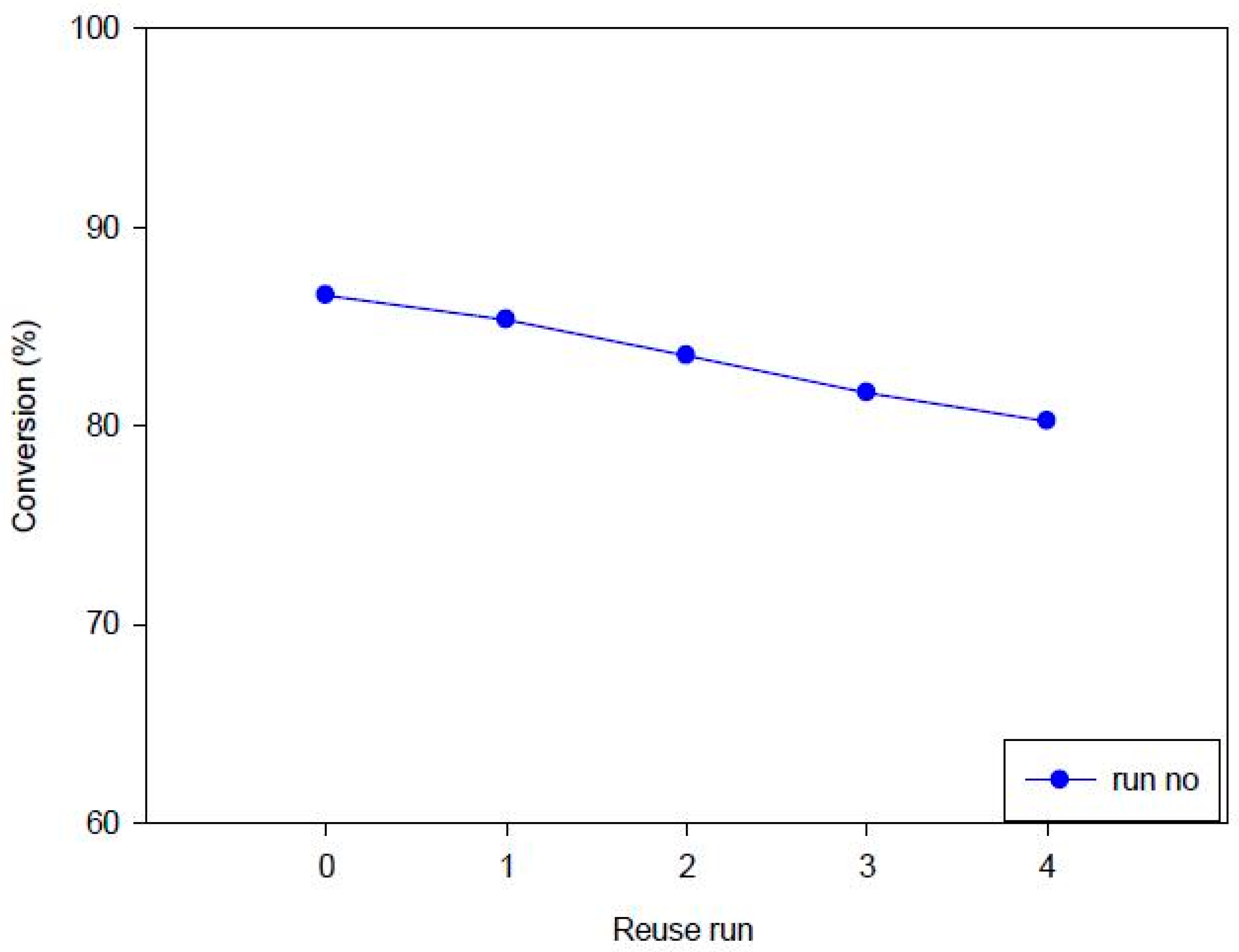
3.3.4. Stability
3.3.5. Effect of Different Catalysts for the Yield of Biodiesel
3.4. Mathematical Calculations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Macor, A.; Pavanello, P. Performance and emissions of biodiesel in a boiler for residential heating. Energy 2009, 34, 2025–2032. [Google Scholar] [CrossRef]
- Jahirul, M.; Brown, R.; Senadeera, W.; O’Hara, I.; Ristovski, Z. The Use of Artificial Neural Networks for Identifying Sustainable Biodiesel Feedstocks. Energies 2013, 6, 3764–3806. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.H.; Kalam, M.A.; Atabani, A.E. Evaluation of biodiesel blending, engine performance and emissions characteristics of Jatropha curcas methyl ester: Malaysian perspective. Energy 2013, 55, 879–887. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.H.; Kalam, M.A.; Atabani, A.E.; Fattah, I.M.R.; Mobarak, H.M. Comparative evaluation of performance and emission characteristics of Moringa oleifera and Palm oil based biodiesel in a diesel engine. Ind. Crops Prod. 2014, 53, 78–84. [Google Scholar] [CrossRef]
- Rahman, M.; Rasul, M.; Hassan, N.; Hyde, J. Prospects of Biodiesel Production from Macadamia Oil as an Alternative Fuel for Diesel Engines. Energies 2016, 9, 403. [Google Scholar] [CrossRef]
- Canakci, M.; Van Gerpen, J. A pilot plant to produce biodiesel from high free fatty acid feedstocks. Trans. ASAE 2003, 46, 945–954. [Google Scholar] [CrossRef]
- Escobar, J.C.; Lora, E.S.; Venturini, O.J.; Yáñez, E.E.; Castillo, E.F.; Almazan, O. Biofuels: Environment, technology and food security. Renew. Sustain. Energy Rev. 2009, 13, 1275–1287. [Google Scholar] [CrossRef]
- Corral Bobadilla, M.; Lostado Lorza, R.; Escribano García, R.; Somovilla Gómez, F.; Vergara González, E. An Improvement in Biodiesel Production from Waste Cooking Oil by Applying Thought Multi-Response Surface Methodology Using Desirability Functions. Energies 2017, 10, 130. [Google Scholar] [CrossRef]
- De Santoli, L.; Mancini, F.; Nastasi, B.; Piergrossi, V. Building integrated bioenergy production (BIBP): Economic sustainability analysis of Bari airport CHP (combined heat and power) upgrade fueled with bioenergy from short chain. Renew. Energy 2015, 81, 499–508. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Dalai, A.K. Waste cooking oil an economical source for biodiesel: A review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Muniyappa, P.R.; Brammer, S.C.; Noureddini, H. Improved conversion of plant oils and animal fats into biodiesel and co-product. Bioresour. Technol. 1996, 56, 19–24. [Google Scholar] [CrossRef]
- Dorado, M.P.; Ballesteros, E.; López, F.J.; Mittelbach, M. Optimization of Alkali-Catalyzed Transesterification ofBrassicaCarinataOil for Biodiesel Production. Energy Fuels 2004, 18, 77–83. [Google Scholar] [CrossRef]
- Antolín, G.; Tinaut, F.V.; Briceño, Y.; Castaño, V.; Pérez, C.; Ramírez, A.I. Optimisation of biodiesel production by sunflower oil transesterification. Bioresour. Technol. 2002, 83, 111–114. [Google Scholar] [CrossRef]
- Patil, P.; Deng, S.; Isaac Rhodes, J.; Lammers, P.J. Conversion of waste cooking oil to biodiesel using ferric sulfate and supercritical methanol processes. Fuel 2010, 89, 360–364. [Google Scholar] [CrossRef]
- Chhetri, A.B.; Watts, K.C.; Islam, M.R. Waste Cooking Oil as an Alternate Feedstock for Biodiesel Production. Energies 2008, 1, 3–18. [Google Scholar] [CrossRef]
- Pinnarat, T.; Savage, P.E. Noncatalytic esterification of oleic acid in ethanol. J. Supercrit. Fluids 2010, 53, 53–59. [Google Scholar] [CrossRef]
- Kusdiana, D.; Saka, S. Two-step preparation for catalyst-free biodiesel fuel production: Hydrolysis and methyl esterification. Appl. Biochem. Biotechnol. 2004, 113–116, 781–791. [Google Scholar] [CrossRef]
- Bhargava, A.K.; Sharma, C.P. Mechanical Behaviour and Testing of Materials; PHI Learning Private Limited: Delhi, India, 2011. [Google Scholar]
- Wan Omar, W.N.N.; Saidina Amin, N.A. Optimization of heterogeneous biodiesel production from waste cooking palm oil via response surface methodology. Biomass Bioenergy 2011, 35, 1329–1338. [Google Scholar] [CrossRef]
- Lotero, E.; Liu, Y.; Lopez, D.E.; Suwannakarn, K.; Bruce, D.A.; Goodwin, J.G. Synthesis of biodiesel via acid catalysis. Ind. Eng. Chem. Res. 2005, 44, 5353–5363. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Lewis acids: From conventional homogeneous to green homogeneous and heterogeneous catalysis. Chem. Rev. 2003, 103, 4307–4365. [Google Scholar] [CrossRef] [PubMed]
- Okuhara, T. Water-tolerant solid acid catalysts. Chem. Rev. 2002, 102, 3641–3665. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.L.; Tan, Z.Y.; Liao, Y.T.; Feng, Y.J. Application of SO42−/TiO2 solid superacid in decontaminating radioactive pollutants. J. Environ. Radioact. 2006, 87, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, P.; Fan, M.; Yu, W.; Jing, X.; Zhang, M.; Duan, X. Preparation and characterization of novel magnetic ZrO2/TiO2/Fe3O4 solid superacid. Mater. Lett. 2007, 61, 2235–2238. [Google Scholar] [CrossRef]
- Wen, Z.; Yu, X.; Tu, S.-T.; Yan, J.; Dahlquist, E. Biodiesel production from waste cooking oil catalyzed by TiO2-MgO mixed oxides. Bioresour. Technol. 2010, 101, 9570–9576. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, R.M.; Noda, L.K.; Gonçalves, N.S.; Meneghetti, S.M.P.; Meneghetti, M.R. Transesterification reaction of vegetable oils, using superacid sulfated TiO2-base catalysts. Appl. Catal. A Gen. 2008, 347, 100–105. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.-D.; Sun, L.; Xu, M.; Zhou, W.-G.; Liang, X.-H. Solid superacid catalyzed fatty acid methyl esters production from acid oil. Appl. Energy 2010, 87, 2369–2373. [Google Scholar] [CrossRef]
- Wang, J.-H.; Mou, C.-Y. Characterizations of aluminum-promoted sulfated zirconia on mesoporous MCM-41 silica: Butane isomerization. Microporous Mesoporous Mater. 2008, 110, 260–270. [Google Scholar] [CrossRef]
- Wang, Y.; Gan, Y.; Whiting, R.; Lu, G. Synthesis of sulfated titania supported on mesoporous silica using direct impregnation and its application in esterification of acetic acid and n-butanol. J. Solid State Chem. 2009, 182, 2530–2534. [Google Scholar] [CrossRef]
- Yadav, G.D.; Nair, J.J. Sulfated zirconia and its modified versions as promising catalysts for industrial processes. Microporous Mesoporous Mater. 1999, 33, 1–48. [Google Scholar] [CrossRef]
- Minami, E.; Saka, S. Kinetics of hydrolysis and methyl esterification for biodiesel production in two-step supercritical methanol process. Fuel 2006, 85, 2479–2483. [Google Scholar] [CrossRef]
- Hu, X.; Wei, T.; Liao, A.; Tong, Z. Use of acid cation-exchange resin for catalytic conversion of soybean acid oil to biodiesel. J. Mater. Cycles Waste Manag. 2016, 18, 123–131. [Google Scholar] [CrossRef]




| Reaction Conditions | LEOF | SBO | |||
|---|---|---|---|---|---|
| XTG (%) (Avg) | XTG (%) (Avg) | ||||
| t (min) | T (°C) | 1:50 o/w | 1:75 o/w | 1:50 o/w | 1:75 o/w |
| 15 | 225 | 5.19 | 4.85 | 0.86 | 1.34 |
| 15 | 250 | 18.32 | 25.95 | 3.74 | 4.59 |
| 15 | 275 | 36.71 | 54.91 | 27.92 | 49.95 |
| 30 | 225 | 7.78 | 7.57 | 2.37 | 1.70 |
| 30 | 250 | 45.35 | 31.02 | 26.68 | 24.50 |
| 30 | 275 | 82.53 | 74.26 | 83.79 | 77.44 |
| 45 | 225 | 25.33 | 17.40 | 17.79 | 16.73 |
| 45 | 250 | 74.07 | 61.77 | 64.69 | 65.28 |
| 45 | 275 | 84.50 | 80.36 | 86.41 | 83.88 |
| 60 | 225 | 28.15 | 21.89 | 27.86 | 21.51 |
| 60 | 250 | 78.60 | 82.57 | 80.49 | 72.47 |
| 60 | 275 | 92.55 | 91.79 | 89.54 | 91.85 |
| Loading of TiO2 | BET Surface Area (m2/g) | 2θ | d(100) (nm) |
|---|---|---|---|
| 40% TiO2 | 877.0704 | 2.35 | 3.754 |
| 60% TiO2 | 863.7089 | 2.36 | 3.739 |
| 80% TiO2 | 714.9370 | 2.63 | 3.355 |
| H2SO4 Concentration (M) | Conversion of FFA (%) |
|---|---|
| 0.25 | 58.6% |
| 0.50 | 72.5% |
| 0.75 | 83.4% |
| 1.0 | 96.78% |
| 1.25 | 73.58% |
| Calcination Temperature (°C) | Specific Surface Area (m2/g) | Conversion (%) |
|---|---|---|
| 450 | 645.527 | 80% |
| 500 | 680.355 | 86% |
| 550 | 714.937 | 98% |
| 600 | 654.326 | 82% |
| Catalyst | Surface Area (m2/g) | Conversion (%) |
|---|---|---|
| TiO2 | 56.05 | 51.23 |
| MCM-41 | 789.0642 | 49.05 |
| SBA-15 | 1035.06 | 46.88 |
| Ti(SO4)2 | - | 65.32 |
| H2SO4 | - | 52.12 |
| S-TiO2 | 48.63 | 56.31 |
| 1% S-TiO2/MCM-41 traditional | 691.29 | 63.09 |
| 1% S-TiO2/MCM-41 (40% TiO2) direct | 842.31 | 44.25 |
| 1% S-TiO2/MCM-41 (60% TiO2) direct | 810.32 | 59.26 |
| 1% S-TiO2/MCM-41 (80% TiO2) direct | 714.94 | 64.25 |
| 3.5% S-TiO2/MCM-41 (80% TiO2) direct | 714.94 | 86.18 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhuyan, M.S.U.S.; Alam, A.H.M.A.; Chu, Y.; Seo, Y.C. Biodiesel Production Potential from Littered Edible Oil Fraction Using Directly Synthesized S-TiO2/MCM-41 Catalyst in Esterification Process via Non-Catalytic Subcritical Hydrolysis. Energies 2017, 10, 1290. https://doi.org/10.3390/en10091290
Bhuyan MSUS, Alam AHMA, Chu Y, Seo YC. Biodiesel Production Potential from Littered Edible Oil Fraction Using Directly Synthesized S-TiO2/MCM-41 Catalyst in Esterification Process via Non-Catalytic Subcritical Hydrolysis. Energies. 2017; 10(9):1290. https://doi.org/10.3390/en10091290
Chicago/Turabian StyleBhuyan, Md Sufi Ullah Siddik, Abul Hasnat Md Ashraful Alam, Younghwan Chu, and Yong Chan Seo. 2017. "Biodiesel Production Potential from Littered Edible Oil Fraction Using Directly Synthesized S-TiO2/MCM-41 Catalyst in Esterification Process via Non-Catalytic Subcritical Hydrolysis" Energies 10, no. 9: 1290. https://doi.org/10.3390/en10091290