A New Approach for Retaining Mercury in Energy Generation Processes: Regenerable Carbonaceous Sorbents
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of the Carbon Support
2.2. Impregnation with Gold
2.3. Characterization of the Sorbent
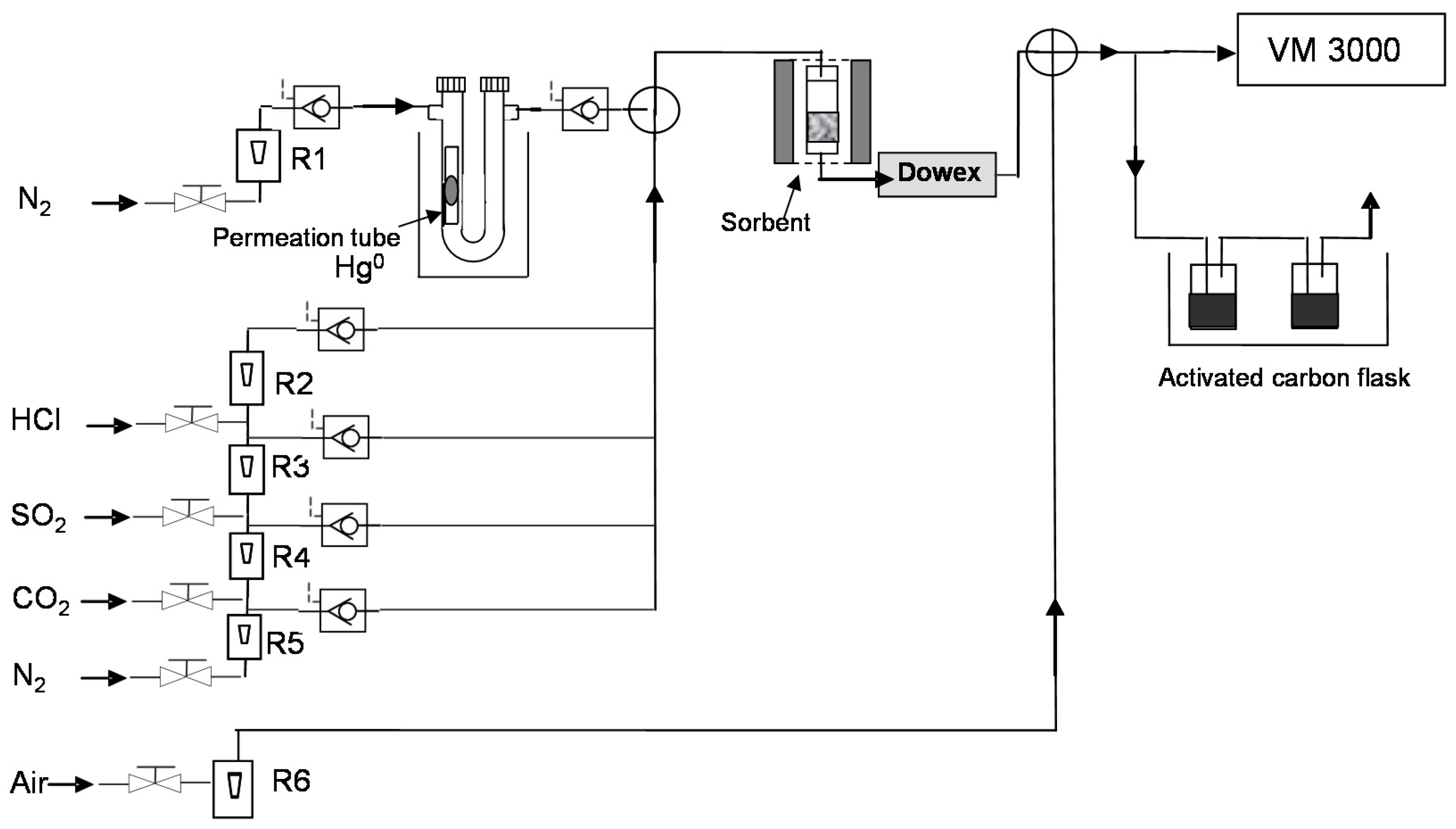
2.4. Hg Retention/Regeneration Device
3. Results and Discussion
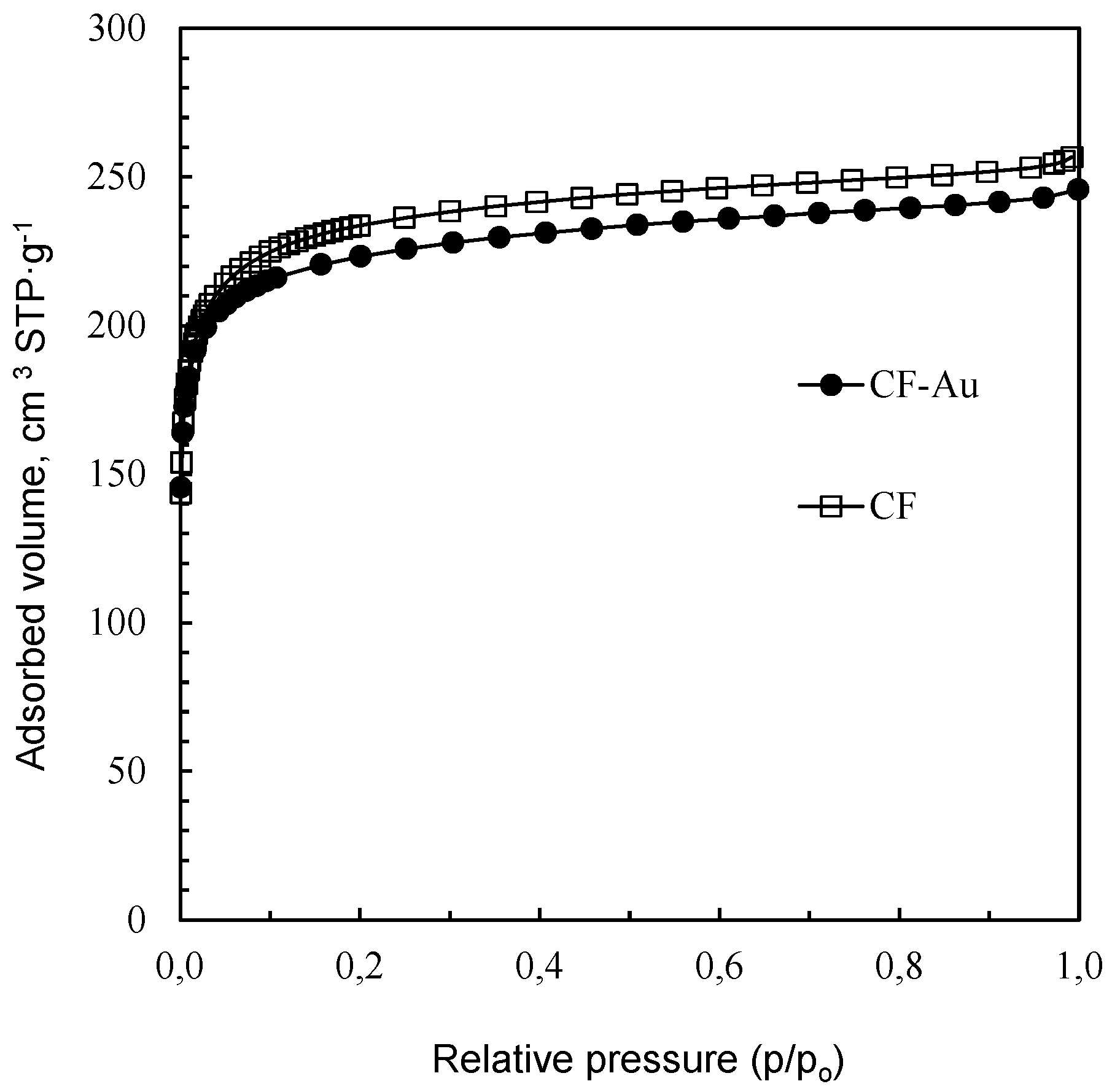
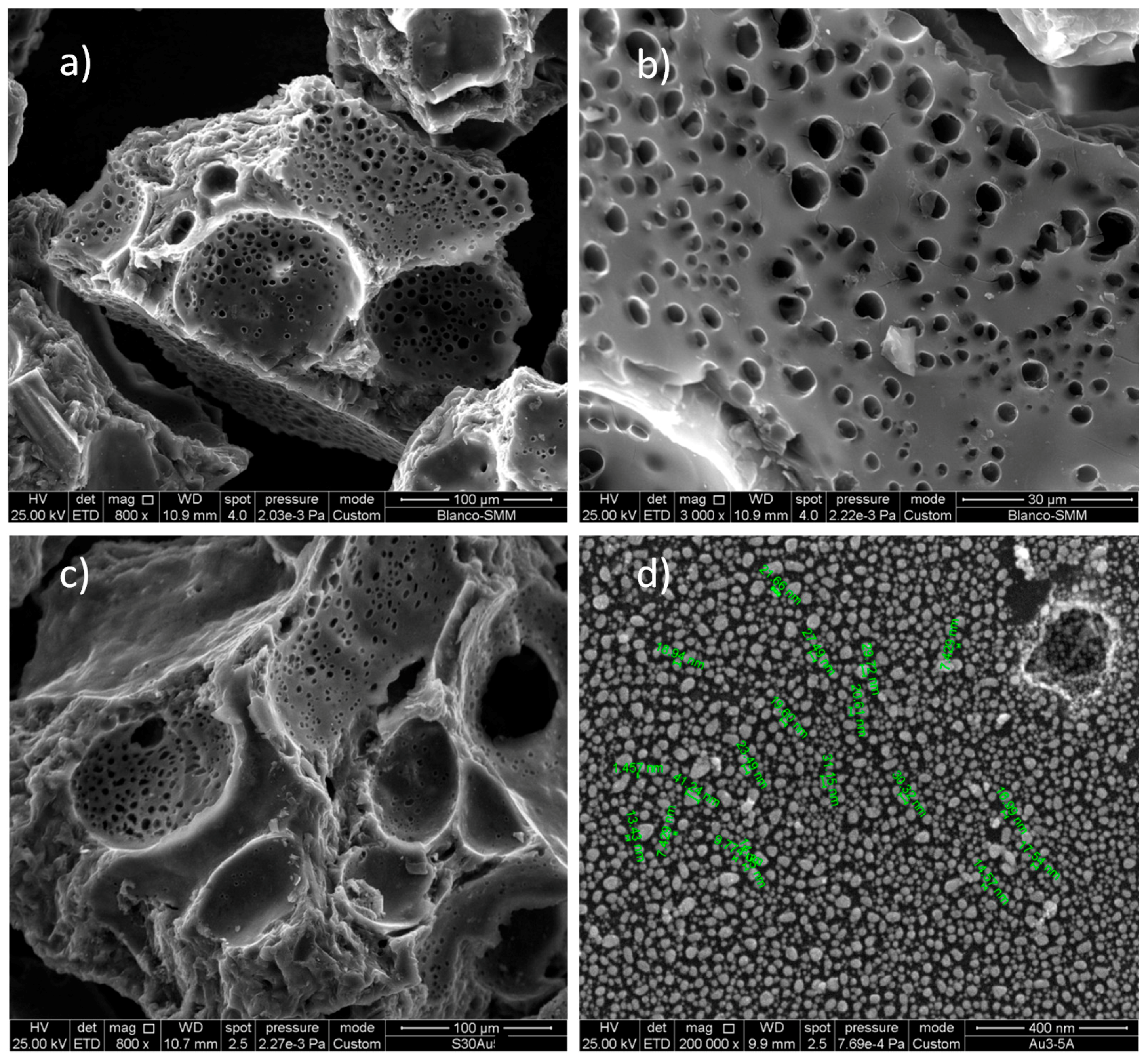
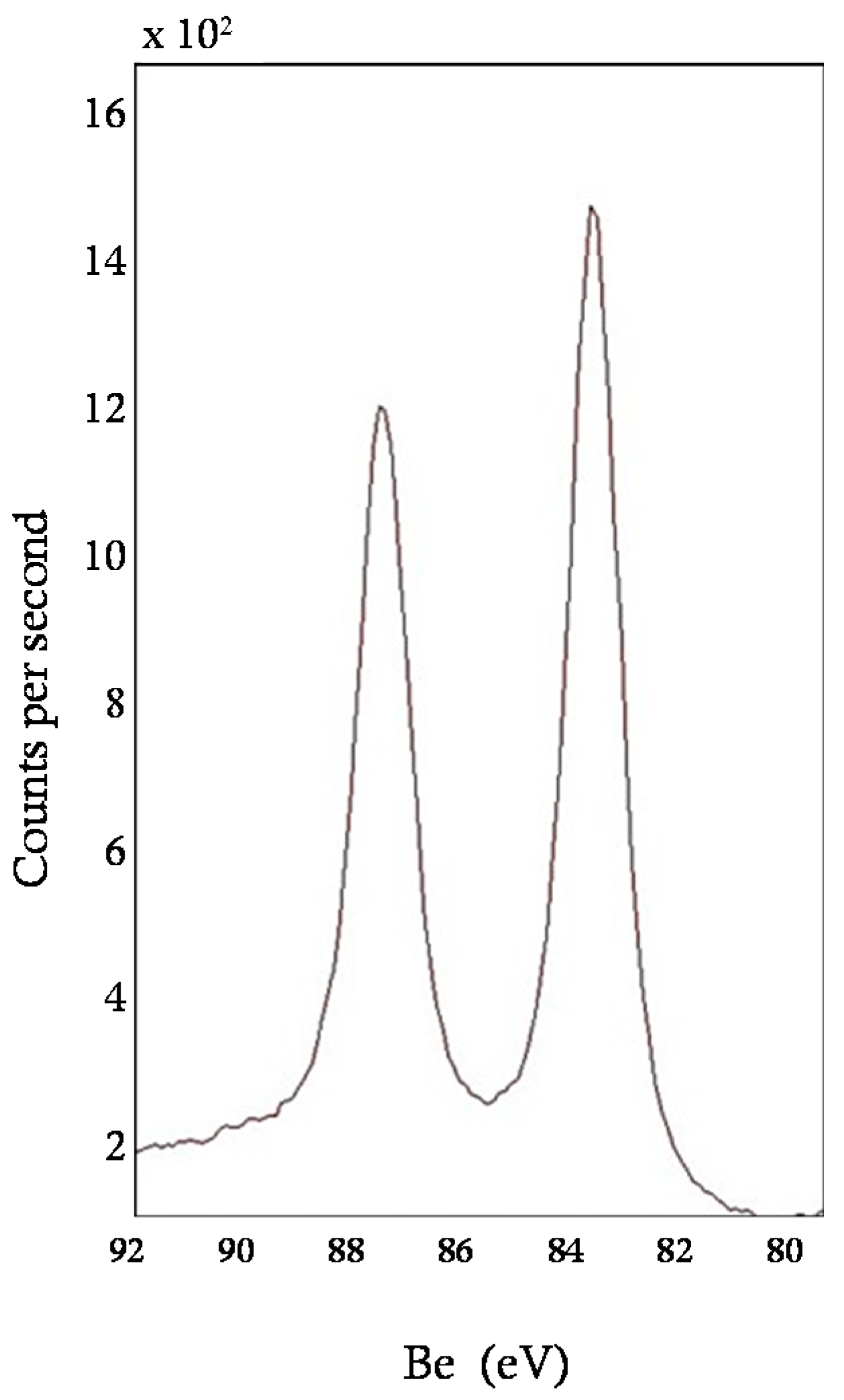
3.1. Characterization of the Sorbent
3.2. Hg Retention
3.2.1. Effect of Impregnation with Gold
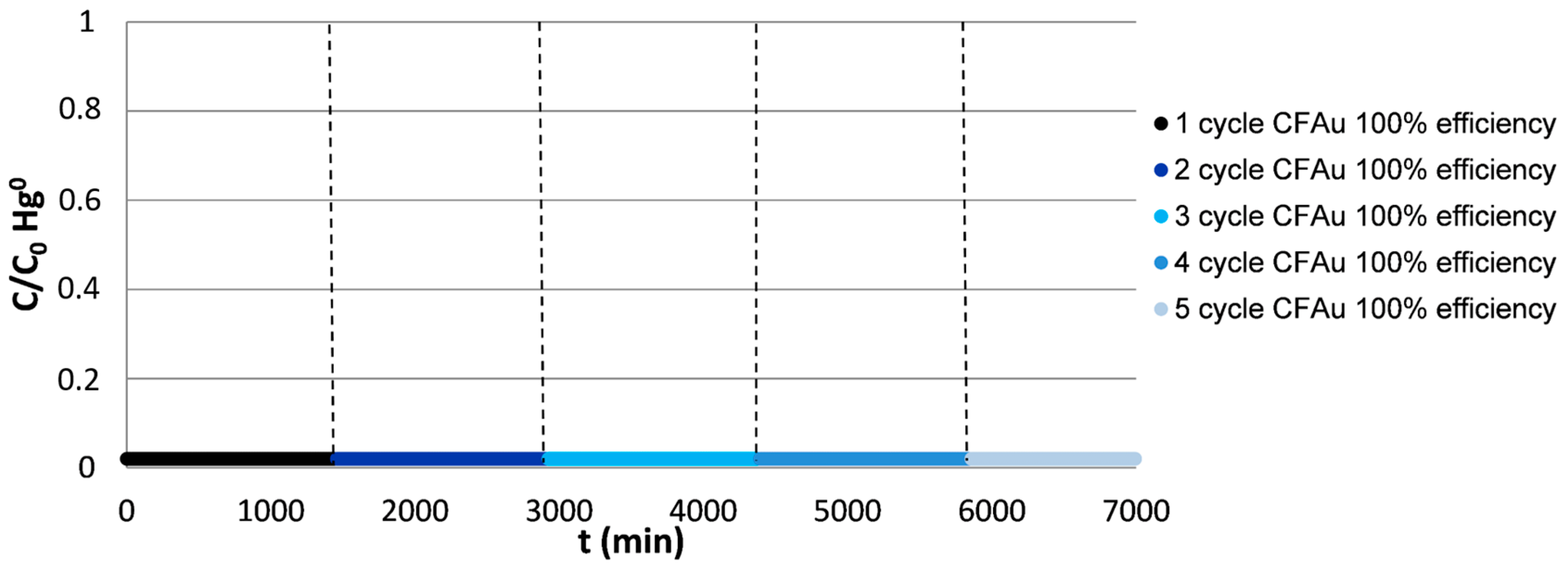
3.2.2. Effect of the Gas Composition
- In contrast to what was observed in the atmosphere containing only CO2 and N2 where hardly any oxidized mercury was detected at the exit of the reactor, in the atmosphere containing HCl and SO2 an increase in Hg2+ was produced during the regeneration cycles of the CF-Au foam (Table 2).
- In the presence of acid gases, approximately 7% of the desorbed mercury was retained in the Dowex resin evidencing the formation of Hg2+.
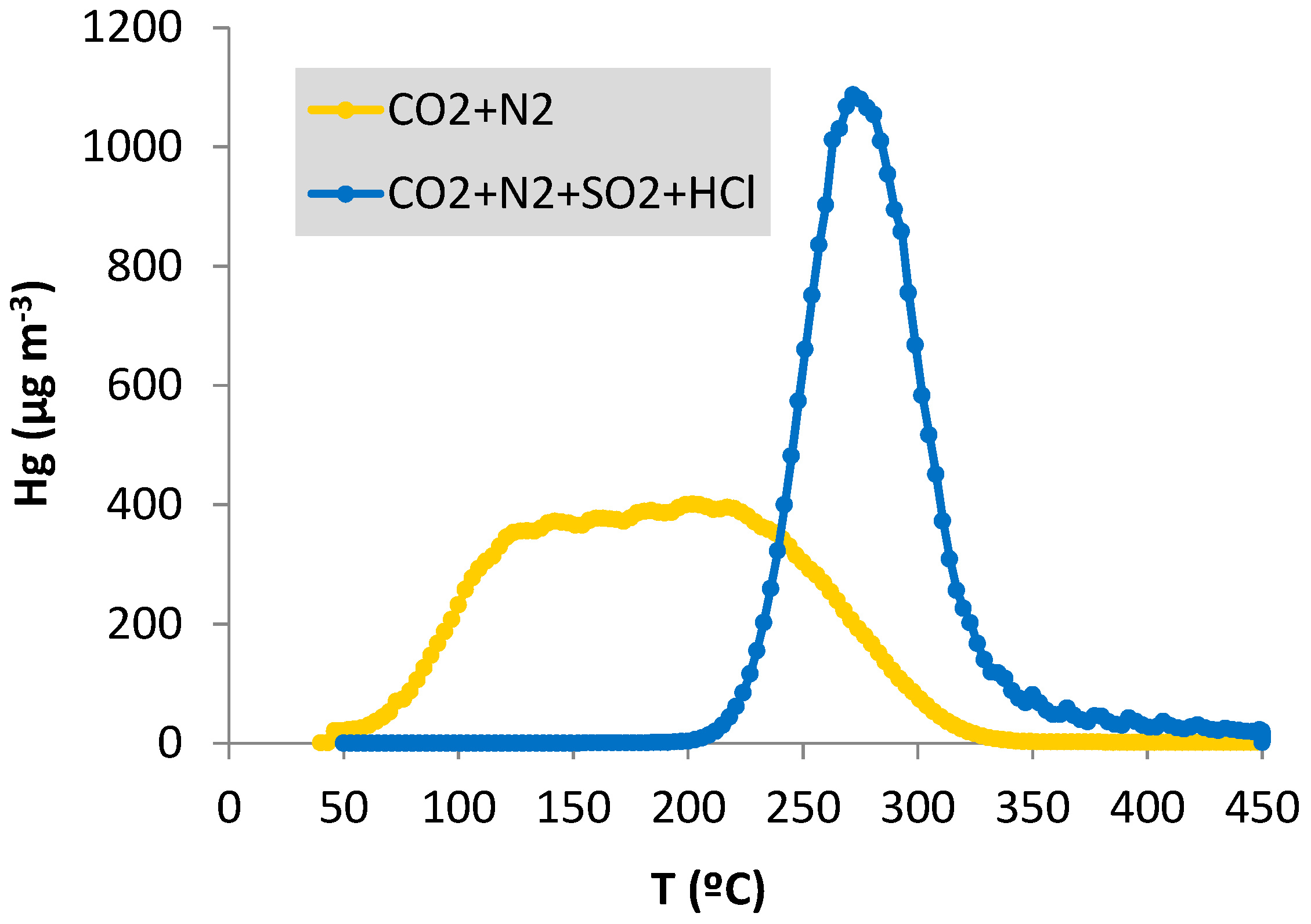
- Moreover, the mercury desorption profiles of a regeneration cycle of the CF-Au sorbent after adsorption in the atmospheres containing CO2 and N2 differ significantly from those obtained with CO2, N2, HCl and SO2 in the gas stream (Figure 7). The profiles obtained in the absence of HCl and SO2 show a wide band, with mercury desorption taking place in the 60 to 325 °C range, while that obtained in the presence of these gases is narrower (200 °C–350 °C) with a maximum peak of desorption at 275 °C.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- International Energy Agency (IEA). Key World Energy Statistics; International Energy Agency: Paris, France, 2016; 80p. [Google Scholar]
- International Energy Agency (IEA). Energy and Climate Change; International Energy Agency: Paris, France, 2015; 200p. [Google Scholar]
- European Environment Agency (EEA). Emissions of Primary PM2.5 and PM10 Particulate Matter; European Environment Agency: Copenhagen, Denmark, 2014; 20p. [Google Scholar]
- International Energy Agency (IEA). CO2 Emissions from Fuel Combustion; International Energy Agency: Paris, France, 2016; 166p. [Google Scholar]
- U.S. Energy Information Administration (EIA). Independent Statistics Analysis; U.S. Energy Information Administration: Washington, DC, USA, 2013.
- United Nations Environment Programme (UNEP). Global Mercury Assessment 2013: Sources, Emissions, Releases and Environmental Transport; UNEP Chemicals Branch: Geneva, Switzerland, 2013; 44p.
- The European Pollutant Release and Transfer Register (E-PRTR). Registro Europeo de Emisiones y Transferencia de Contaminantes. 2014. Available online: http://prtr.ec.europa.eu/#/pollutantreleases (accessed on 31 August 2017).
- Feeley, T.; O’Palko, B.; Jones, A. Developing mercury control technology for coal-fired power plants—From concept to commercial reality. Main Group Chem. 2008, 7, 169–179. [Google Scholar] [CrossRef]
- Pavlish, J.H.; Hamre, L.L.; Zhuang, Y. Mercury control technologies for coal combustion and gasification systems. Fuel 2010, 89, 838–847. [Google Scholar] [CrossRef]
- Sloss, L.L. Economics of Mercury Control; CCC/134; IEA Clean Coal Centre: London, UK, 2008; p. 51. [Google Scholar]
- Sundseth, K.; Pacyna, J.M.; Pacyna, E.G.; Munthe, J.; Belhaj, M.; Astrom, S. Economic Benefits from Decreased Mercury Emissions: Projections for 2020. J. Clean. Prod. 2010, 18, 386–394. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). Minamata Convention on Mercury. October 2013. Available online: http://www.mercuryconvention.org/Portals/11/documents/Booklets/Minamata%20Convention%20on%20Mercury_booklet_English.pdf (accessed on 31 August 2017).
- Bustard, J.; Durham, M.; Starns, T.; Lindsey, C.; Martin, C.; Schlager, R.; Baldrey, L. Full-scale evaluation of sorbent injection for mercury control on coal-fired power plants. Fuel Process. Technol. 2004, 85, 549–562. [Google Scholar] [CrossRef]
- Sjostrom, S.; Durham, M.; Bustard, C.J.; Martin, C. Activated carbon injection for mercury control: Overview. Fuel 2010, 89, 1320–1322. [Google Scholar] [CrossRef]
- Pfughoeft-Hassett, D.F.; Hassett, D.J.; Buckley, T.D.; Heebink, L.V.; Pavlish, J.H. Activated carbon for mercury control: Implications for fly ash management. Fuel Process. Technol. 2009, 90, 1430–1434. [Google Scholar] [CrossRef]
- Senior, C.; Bustard, C.J.; Durham, M.; Baldrey, K.; Michaud, D. Characterization of fly ash from full-scale demonstration of sorbent injection for mercury control on coal-fired power plants. Fuel Process. Technol. 2004, 85, 601–612. [Google Scholar] [CrossRef]
- Granite, E.J.; Pennline, H.W.; Hargis, R.A. Novel sorbents for mercury removal from flue gas. Ind. Eng. Chem. Res. 2000, 39, 1020–1029. [Google Scholar] [CrossRef]
- Butz, J.R.; Turchi, C.; Broderick, T.E.; Albiston, J. Options for mercury removal from coal-fired flue gas streams: Pilot-scale research on activated carbon, alternative and regenerables sorbents. In Proceedings of the 17th International Pittsburgh Coal Conference, Pittsburgh, PA, USA, 5–8 September 2000. [Google Scholar]
- López-Antón, M.A.; Fernandez-Miranda, N.; Martínez-Tarazona, M.R. The application of regenerable sorbents for mercury capture in gas phase. Environ. Sci. Pollut. Res. 2016, 23, 24495–24503. [Google Scholar] [CrossRef] [PubMed]
- Ballestero, D.; Gómez-Giménez, C.; García-Díez, E.; Juan, R.; Rubio, B.; Izquierdo, M.T. Influence of temperature and regeneration cycles on Hg capture and efficiency by structured Au/C regenerable sorbents. J. Hazard. Mater. 2013, 260, 247–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Giménez, C.; Ballestero, D.; Juan, R.; Rubio, B.; Izquierdo, M.T. Mercury capture by a regenerable sorbent under oxycoal combustion conditions: Effect of SO2 and O2 on capture efficiency. Chem. Eng. Sci. 2015, 122, 232–239. [Google Scholar] [CrossRef]
- Izquierdo, M.T.; Ballestero, D.; Juan, R.; García-Díez, E.; Rubio, B.; Ruiz, C.; Pino, M.R. Tail-end Hg capture on Au/carbon-monolith regenerable sorbents. J. Hazard. Mater. 2011, 193, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Murphy, P.D.; Wu, C.Y. Removal of elemental mercury form simulated coal-combustion flue gas using SiO2-TiO2 nanocomposite. Fuel Process. Technol. 2008, 89, 567–573. [Google Scholar] [CrossRef]
- Liu, Y.; Kelly, D.J.A.; Yang, H.; Lin, C.C.H.; Kuznicki, S.M.; Xu, Z. Novel regenerable sorbent for mercury capture from flue gases of coal-fired power plant. Environ. Sci. Technol. 2008, 42, 6205–6210. [Google Scholar] [CrossRef] [PubMed]
- Scala, F.; Anacleria, C.; Cimino, S. Characterization of a regenerable sorbent for high temperature elemental mercury capture from flue gas. Fuel 2013, 108, 13–18. [Google Scholar] [CrossRef]
- Baltrus, J.P.; Granite, E.J.; Pennline, H.W.; Stanko, D.C.; Hamilton, H.; Rowsell, L.; Poulston, S.; Smith, A.; Chu, W. Characterization of a regenerable sorbent for high temperature elemental mercury capture from flue gas. Fuel 2010, 89, 1323–1325. [Google Scholar] [CrossRef]
- Baltrus, J.P.; Granite, E.J.; Rupp, E.C.; Stanko, D.C.; Howard, B.; Pennline, H.W. Effect of Dispersion on the Capture of Toxic Elements from Fuel Gas by Palladium-Alumina Sorbents. Fuel 2011, 90, 1992–1998. [Google Scholar] [CrossRef]
- Granite, E.J.; Pennline, H.W. Method for High Temperature Mercury Capture from Gas Streams. U.S. Patent 7033419, 25 April 2006. [Google Scholar]
- Battistoni, C.; Bemporad, E.; Galdikas, A.; Kaciulis, S.; Mattogno, G.; Mickevicius, S.; Olevano, V. Interaction of mercury vapor with thin films of gold. Appl. Surf. Sci. 1996, 103, 107–111. [Google Scholar] [CrossRef]
- Kobiela, T.; Nowakowski, B.; Dus, R. The influence of gas phase composition on the process of Au-Hg amalgam formation. Appl. Surf. Sci. 2003, 206, 78–89. [Google Scholar] [CrossRef]
- Levlin, M.; Ikavalko, E.; Laitinen, T. Adsorption of mercury on gold and silver surfaces. Fresenius J. Anal. Chem. 1999, 365, 577–586. [Google Scholar] [CrossRef]
- Dong, J.; Xu, Z.; Kuznicki, S.M. Mercury removal from flue gases by novel regenerable magnetic nanocomposite sorbents. Environ. Sci. Technol. 2009, 43, 3266–3271. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, J.; López-Antón, M.A.; Díaz-Somoano, M.; García, R.; Martínez-Tarazona, M.R. Development of gold nanoparticles-doped activated carbon sorbent for elemental mercury. Energy Fuel 2011, 25, 2022–2027. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, J.; López-Antón, M.A.; Díaz-Somoano, M.; García, R.; Martínez-Tarazona, M.R. Regenerable sorbents for mercury capture in simulated coal combustion flue gas. J. Hazard. Mater. 2013, 260, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Portzer, J.W.; Albritton, J.R.; Allen, C.C.; Gupta, R.P. Development of novel sorbents for mercury control at elevated temperatures in coal-derived syngas: Results of initial screening of candidate materials. Fuel Process. Technol. 2004, 85, 621–630. [Google Scholar] [CrossRef]
- Rodríguez, E.; García, R. Microporosity development in coal-based carbon foams. Energy Fuel 2012, 26, 3703–3710. [Google Scholar] [CrossRef]
- Rodríguez, E.; García, R. Low-cost hierarchical micro/macroporous carbon foams as efficient sorbents for CO2 capture. Fuel Process. Technol. 2017, 156, 235–245. [Google Scholar] [CrossRef]
- Fernandez-Miranda, N.; Rumayor, M.; López-Antón, M.A.; Díaz-Somoano, M.; Martínez-Tarazona, M.R. Mercury retention by fly ashes from oxy-fuel processes. Energy Fuel 2015, 29, 2227–2233. [Google Scholar] [CrossRef]
- Fuente-Cuesta, A.; Díaz-Somoano, M.; Lopez-Anton, M.A.; Martinez-Tarazona, M.R. Oxidised mercury determination from combustion gases using an ionic exchanger. Fuel 2014, 122, 218–222. [Google Scholar] [CrossRef]
- Fernández-Miranda, N.; Lopez-Anton, M.A.; Díaz-Somoano, M.; Martinez-Tarazona, M.R. Effect of oxy-combustion flue gas on mercury oxidation. Environ. Sci. Technol. 2014, 48, 7164–7170. [Google Scholar] [CrossRef] [PubMed]
- Ghorishi, S.B.; Keeney, R.M.; Serre, S.D.; Gullent, B.K.; Jozewicz, W.S. Development of a Cl-impregnated activated carbon for entrained-flow capture of elemental mercury. Environ. Sci. Technol. 2002, 36, 4454–4459. [Google Scholar] [CrossRef] [PubMed]
- Norton, G.A.; Yang, H.; Brown, R.C.; Laudal, D.L.; Dunham, G.E.; Erjavec, J. Heterogeneous oxidation of mercury in simulated post combustion conditions. Fuel 2003, 82, 107–116. [Google Scholar] [CrossRef]
- Olson, E.S.; Azenkeng, A.; Laumb, J.D.; Jensen, R.R.; Bensen, S.A.; Hoffmann, M.R. New developments in the theory and modeling of mercury oxidation and binding on activated carbons in flue gas. Fuel Process. Technol. 2009, 90, 1360–1363. [Google Scholar] [CrossRef]
- Nowakowski, R.; Kobiela, T.; Wolfram, Z.; Duces, R. Atomic force microscopy of Au/Hg alloy formation on thin Au films. Appl. Surf. Sci. 1997, 115, 217–231. [Google Scholar] [CrossRef]

| Method of Analysis | Parameters | CF | CF-Au |
|---|---|---|---|
| Adsorption N2 | SBET (m2·g−1) | 880 | 850 |
| VT (cm3·g−1) | 0.39 | 0.37 | |
| VDR-N2 (cm3·g−1) | 0.36 | 0.35 | |
| VMeso (cm3·g−1) | 0.03 | 0.02 | |
| ICP-MS | Au (wt %) | - | 1.8 |
| No. Cycle | R (µg g−1) | % Hgret | % Hg2+ |
|---|---|---|---|
| 1 | 637 | 99.7 | 0.3 |
| 2 | 601 | 99.7 | 0.3 |
| 3 | 627 | 98.2 | 1.8 |
| 4 | 594 | 95.7 | 4.3 |
| 5 | 615 | 93.7 | 6.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Miranda, N.; Rodríguez, E.; Lopez-Anton, M.A.; García, R.; Martínez-Tarazona, M.R. A New Approach for Retaining Mercury in Energy Generation Processes: Regenerable Carbonaceous Sorbents. Energies 2017, 10, 1311. https://doi.org/10.3390/en10091311
Fernández-Miranda N, Rodríguez E, Lopez-Anton MA, García R, Martínez-Tarazona MR. A New Approach for Retaining Mercury in Energy Generation Processes: Regenerable Carbonaceous Sorbents. Energies. 2017; 10(9):1311. https://doi.org/10.3390/en10091311
Chicago/Turabian StyleFernández-Miranda, Nuria, Elena Rodríguez, Maria Antonia Lopez-Anton, Roberto García, and Maria Rosa Martínez-Tarazona. 2017. "A New Approach for Retaining Mercury in Energy Generation Processes: Regenerable Carbonaceous Sorbents" Energies 10, no. 9: 1311. https://doi.org/10.3390/en10091311





