Evolving Microbial Communities in Cellulose-Fed Microbial Fuel Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. MFC Construction and Operation
2.2. DNA Extraction, Polymerase Chain Reaction (PCR) and Illumina Sequencing
2.3. Determination of Volatile Acids Production
3. Results
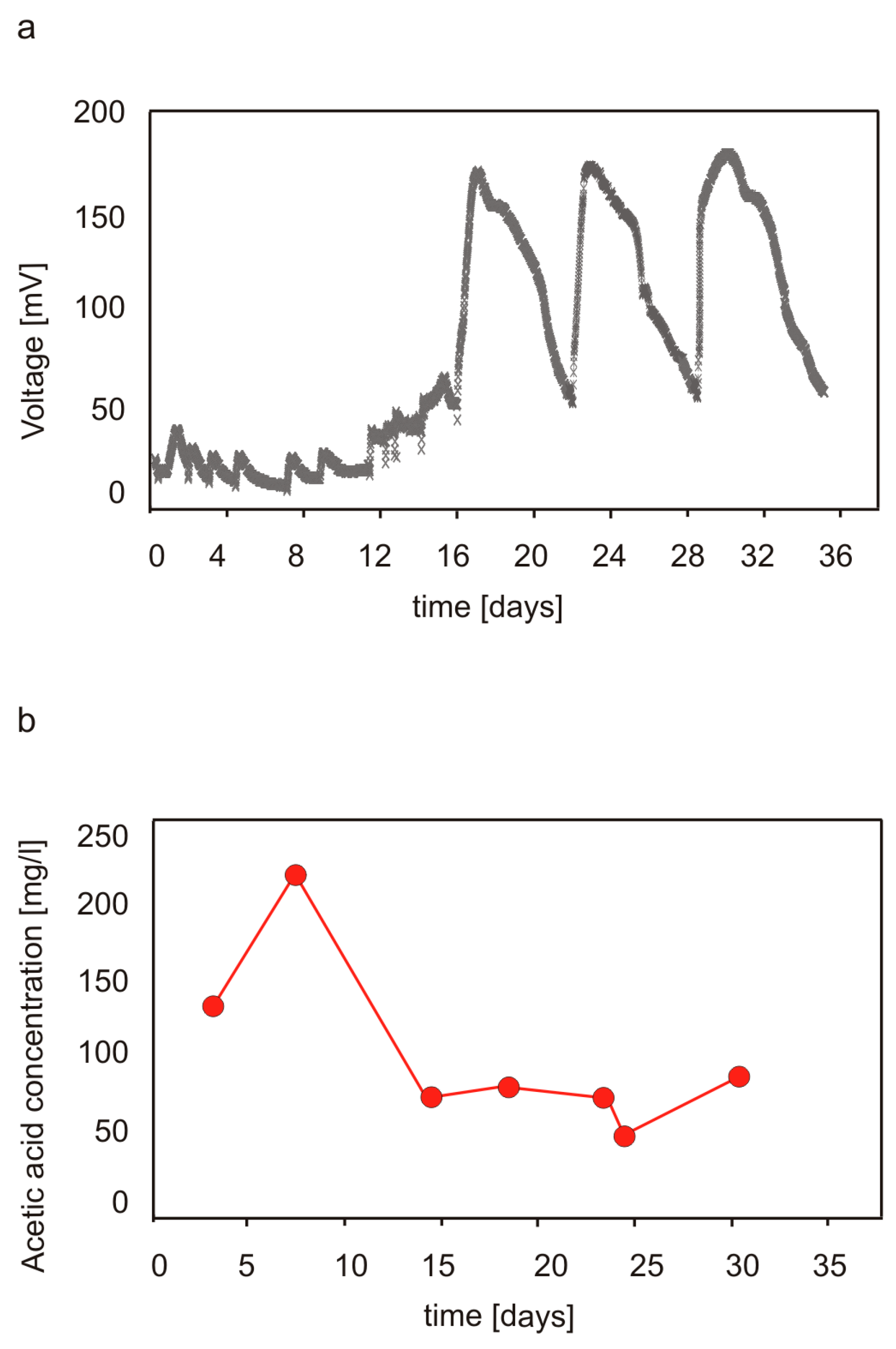
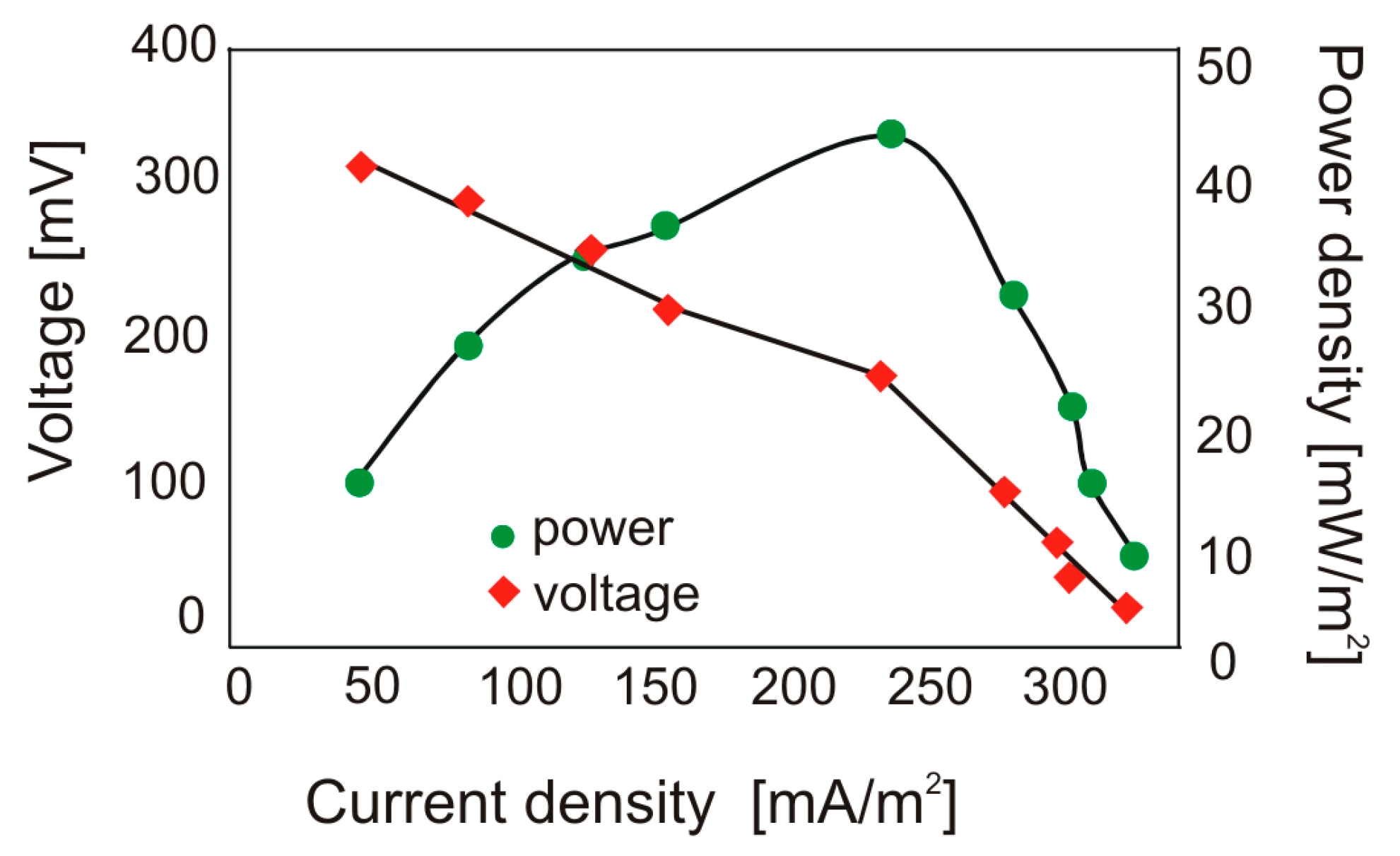
3.1. Performance of MFCs Fed with Cellulose and Production of Volatile Acids
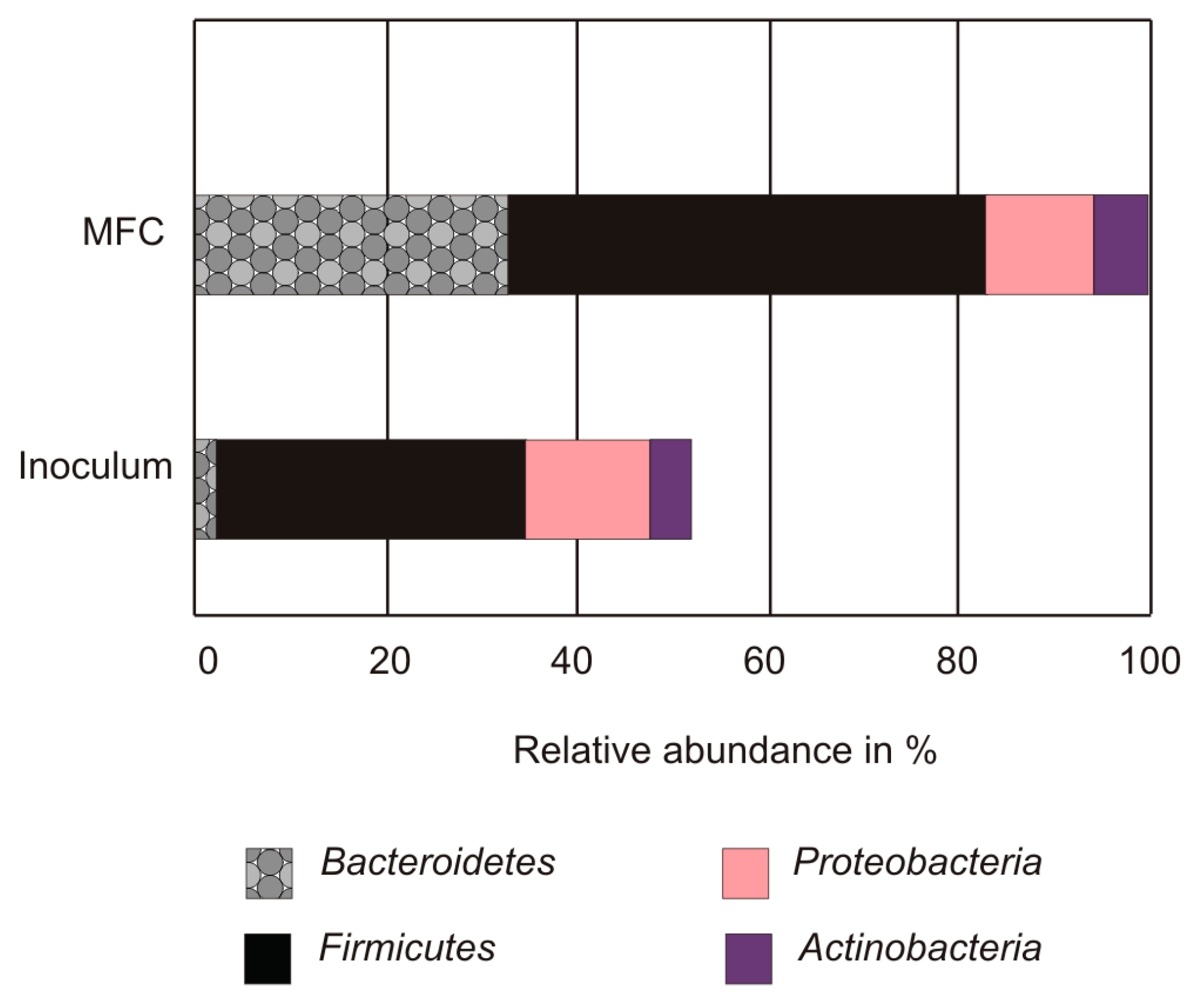
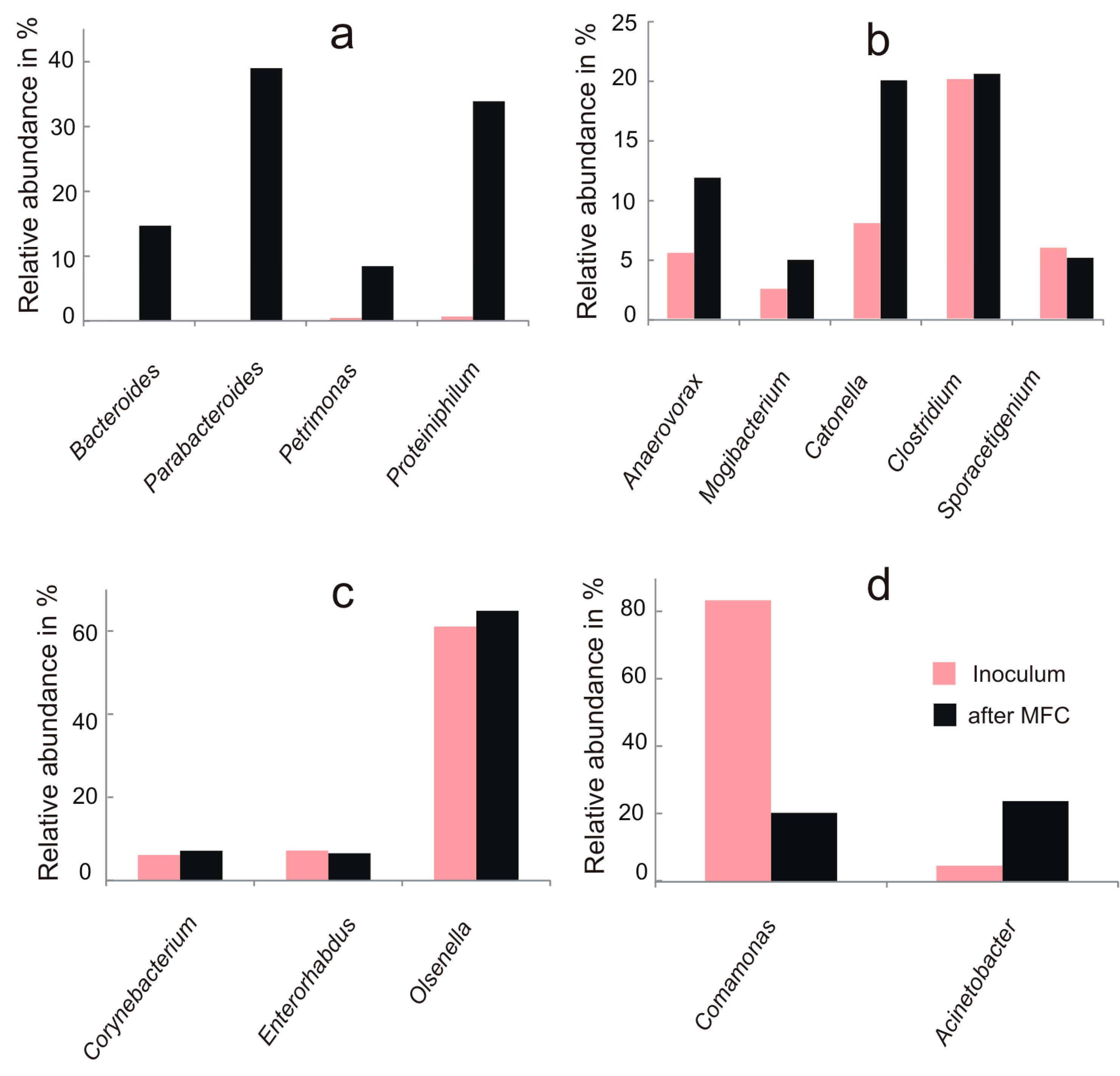
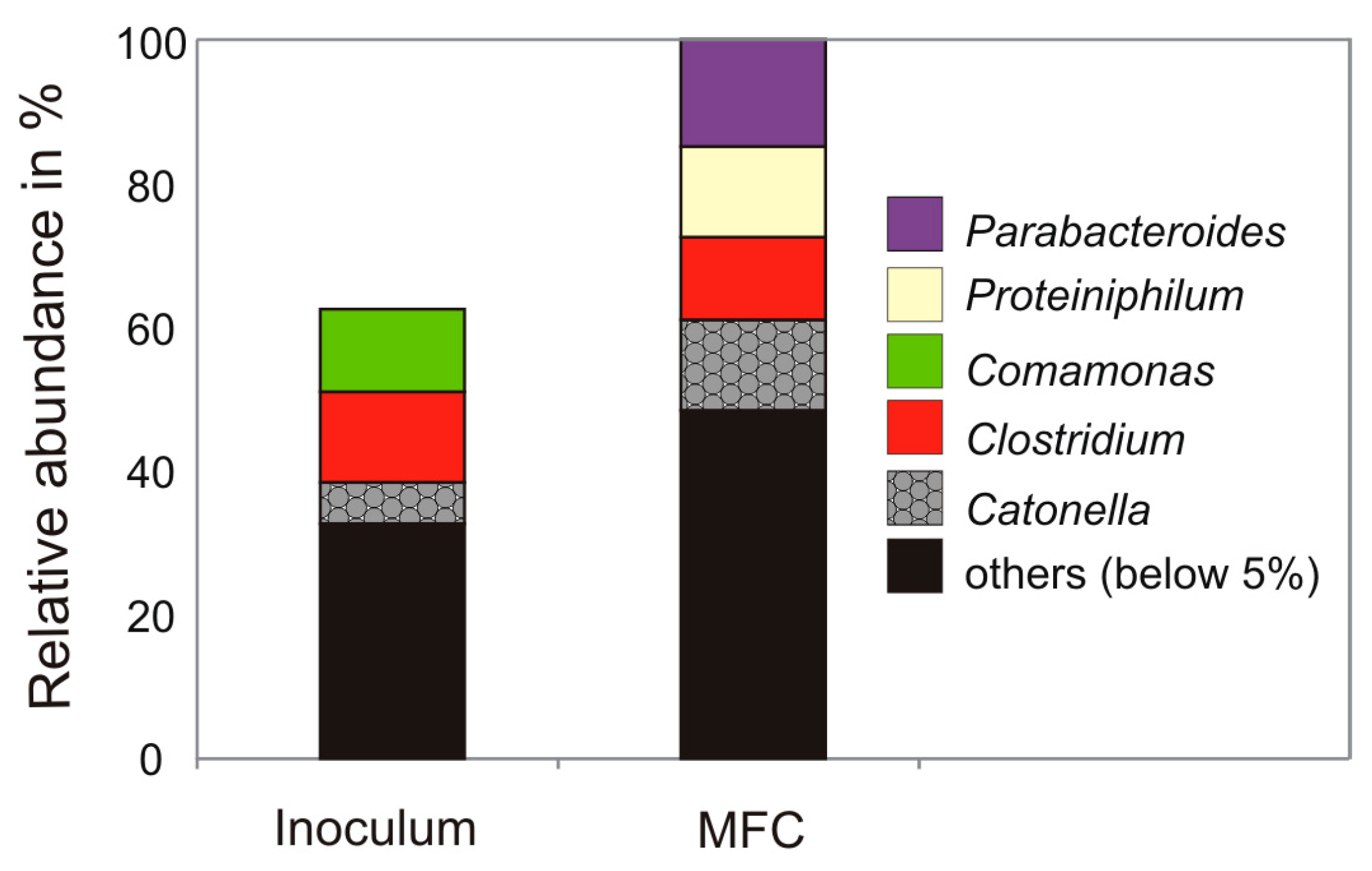
3.2. Microbial Community Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009, 7, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Yu, H.Q.; He, Z. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 2014, 7, 911–924. [Google Scholar] [CrossRef]
- Toczyłowska-Mamińska, R. Limits and perspectives of pulp and paper industry wastewater treatment—A review. Renew. Sustain. Energy Rev. 2017, 78, 764–772. [Google Scholar] [CrossRef]
- Ahmad, F.; Atiyeh, M.N.; Pereira, B.; Stephanopoulos, G.N. A review of cellulosic microbial fuel cells: Performance and challenges. Biomass Bioenergy 2013, 56, 179–188. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Khawdas, W.; Aso, Y.; Ohara, H. Microbial fuel cells using Cellulomonas spp. with cellulose as fuel. J. Biosci. Bioeng. 2017, 123, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Toczyłowska-Mamińska, R.; Szymona, K.; Madej, H.; Wong, W.Z.; Bala, A.; Brutkowski, W.; Krajewski, K.; H’ng, P.S.; Mamiński, M. Cellulolytic and electrogenic activity of Enterobacter cloacae in mediatorless microbial fuel cell. Appl. Energy 2015, 160, 88–93. [Google Scholar] [CrossRef]
- Hassan, S.H.A.; Kim, Y.S.; Oh, S.E. Power generation from cellulose using mixed and pure cultures of cellulose-degrading bacteria in a microbial fuel cell. Enzyme Microb. Technol. 2012, 51, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Xing, D.; Wagner, R.; Regan, J.M.; Richard, T.L.; Logan, B.E. Simultaneous cellulose degradation and electricity production by Enterobacter cloacae in a microbial fuel cell. Appl. Environ. Microbiol. 2009, 75, 3673–3678. [Google Scholar] [CrossRef] [PubMed]
- Kouzuma, A.; Kato, S.; Watanabe, K. Microbial interspecies interactions: Recent findings in syntrophic cosortia. Front. Microbiol. 2015, 6, 454–477. [Google Scholar] [CrossRef] [PubMed]
- Velasquez-Orta, S.B.; Head, I.M.; Curtis, T.P.; Scott, K. Factors affecting current production in microbial fuel cells using different industrial wastewaters. Bioresour. Technol. 2011, 102, 5105–5112. [Google Scholar] [CrossRef] [PubMed]
- Chae, K.J.; Choi, M.J.; Lee, J.W.; Kim, K.Y.; Kim, I.S. Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour. Technol. 2009, 100, 3518–3525. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Ward, T.E.; Regan, J.M. Electricity production from cellulose in a microbial fuel cell using a defined binary culture. Environ. Sci. Technol. 2007, 14, 4781–4786. [Google Scholar] [CrossRef]
- Ren, Z.; Steinberg, L.M.; Regan, J.M. Electricity production and microbial biofilm characterization in cellulose-fed microbial fuel cells. Water Sci. Technol. 2008, 58, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Rismani-Yazdi, H.; Christy, A.D.; Dehority, B.A.; Morrison, M.; Yu, Z.; Tuovinen, O.H. Electricity generation from cellulose by rumen microorganisms in microbial fuel cells. Biotechnol. Bioeng. 2007, 97, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Min, B.; Huang, L.; Angelidaki, I. Generation of electricity and analysis of microbial communities in wheat straw biomass-powered microbial fuel cells. Appl. Environ. Microbiol. 2009, 75, 3389–3395. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Richard, T.L.; Logan, B.E. Enzymatic hydrolysis of cellulose coupled with electricity generation in a microbial fuel cell. Biotechnol. Bioeng. 2008, 101, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Shimoyama, T.; Hotta, Y.; Watanabe, K. Characterization of a filamentous biofilm community established in a cellulose-fed microbial fuel cell. BMC Microbiol. 2008, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Y.; Wang, H.; Qu, Y.; Yu, Y.; Ren, N.; Li, N.; Wang, E.; Lee, H.; Logan, B.E. Bioaugmentation for electricity generation from corn stover biomass using microbial fuel cells. Environ. Sci. Technol. 2009, 43, 6088–6093. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Maness, P.C.; Logan, B.E. Electricity production from steam-exploded corn stover biomass. Energy Fuels 2006, 20, 1716–1721. [Google Scholar] [CrossRef]
- Huang, L.; Logan, B.E. Electricity generation and treatment of paper recycling wastewater using a microbial fuel cell. Appl. Microbiol. Biotechnol. 2008, 80, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Kiely, P.; Logan, B.E. Pre-acclimation of a wastewater inoculum to cellulose in an aqueous–cathode MEC improves power generation in air-cathode MFCs. Bioresour. Technol. 2011, 102, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Kiely, P.D.; Rader, G.; Regan, J.M.; Logan, B.E. Long-term cathode performance and the microbial communities that develop in microbial fuel cells fed different fermentation endproducts. Bioresour. Technol. 2011, 102, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Kiely, P.D.; Regan, J.M.; Logan, B.E. The electric picnic: Synergistic requirements for exoelectrogenic microbial communities. Curr. Opin. Biotechnol. 2011, 22, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Borjas, Z.; Ortez, J.M.; Aldaz, A.; Feliu, J.; Núñez-Esteve, A. Strategies for reducing the start-up operation of microbial electrochemical treatments of urban wastewater. Energies 2015, 8, 14064–14077. [Google Scholar] [CrossRef] [Green Version]
- Kiely, P.D.; Cusick, R.; Call, D.F.; Selembo, P.A.; Regan, J.M.; Logan, B.E. Anode microbial communities produced by changing from microbial fuel cell to microbial electrolysis cell operation using two different wastewaters. Bioresour. Technol. 2011, 102, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Cheng, S.; Watson, V.; Estadt, G. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kim, K.Y.; Saikaly, P.E.; Logan, B.E. The impact of new cathode materials relative to baseline performance of microbial fuel cells all with the same architecture and solution chemistry. Energy Environ. Sci. 2017, 10, 1025–1033. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, H.; Logan, B.E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006, 8, 489–494. [Google Scholar] [CrossRef]
- Morais, J.P.S.; Rosa, M.D.F.; De Souza Filho, M.D.S.M.; Dias Nascimento, L.; Do Nascimento, D.M.; Casales, A.R. Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohydr. Polym. 2013, 91, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Gliniewicz, K.; Gerritsen, A.T.; McDonald, A.G. Analysis of microbial community variation during the mixed culture fermentation of agricultural peel wastes to produce lactic acid. Bioresour. Technol. 2016, 208, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Herrera, D.; Pacheco-Catalan, D.; Valdez-Ojeda, R.; Canto-Canche, B.; Dom Inguez-Benetton, X.; Domínguez-Maldonado, J.; Alzate-Gaviria, L. Characterization of anode and anolyte community growth and the impact of impedance in a microbial fuel cell. BMC Biotechnol. 2014, 14, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Bermek, H.; Catal, T.; Akan, S.S.; Ulutas, M.S.; Kumru, M.; Ösgüven, M.; Liu, H.; Özçelik, B.; Akarsubasi, A.T. Olive mill wastewater treatment in single-chamber air-cathode microbial fuel cells. World J. Microbiol. Biotechnol. 2014, 30, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Ma, F.; Wei, L.; Chua, H.; Chang, C.C.; Zhang, X.J. Electricity generation from cattle dung using microbial fuel cell technology during anaerobic acidogenesis and the development of microbial populations. Waste Manag. 2012, 32, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, D.M.; Smith, A.; Dahale, S.; Stradford, J.P.; Li, J.V.; Grüning, A.; Bushell, M.E.; Marchesi, J.R.; Avignone-Rossa, C. Segregation of the anodic microbial communities in a microbial fuel cell cascade. Front. Microbiol. 2016, 7, 699–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beecroft, N.J.; Zhao, F.; Varcoe, J.R.; Slade, R.C.T.; Thumser, A.E.; Avignone-Rossa, C. Dynamic changes in the microbial community composition in microbial fuel cells fed with sucrose. Appl. Microbiol. Biotechnol. 2012, 93, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Min, B.; Huang, L.; Angelidaki, I. Electricity generation and microbial community response to substrate changes in microbial fuel cell. Bioresour. Technol. 2011, 102, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.D.; Kiely, P.D.; Call, D.F.; Rismani-Yasdi, H.; Bibby, K.; Peccia, J.; Regan, J.M.; Logan, B.E. Convergent development of bacterial communities in microbial fuel cells. ISME J. 2012, 6, 2002–2013. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, N. Carbon dioxide and acetate utilization by Clostridium klyuveri. J. Biol. Chem. 1954, 209, 597–603. [Google Scholar] [PubMed]
- Schnurer, A.; Svensson, B.; Schink, B. Enzyme activities in energetics of acetate metabolism by the mesophilic syntrophically acetate-oxidizing anaerobe Clostridium ultunense. FEMS Microbiol. Lett. 1997, 154, 331–336. [Google Scholar] [CrossRef]
- Diez-Gonzalez, F.; Russell, J.B.; Hunter, J.B. The role of NAD-independent lactate dehydrogenase and acetate in utilization of lactate by Clostridium acetobutylicum strain P262. Arch. Microbiol. 1995, 164, 36–42. [Google Scholar] [CrossRef]
- Karakashev, D.; Batstone, D.J.; Trably, E.; Angelidak, I. Acetate oxidation is the dominant methanogenic pathway from acetate in the absence of methanosaetaceae. Appl. Environ. Microbiol. 2006, 72, 5138–5141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, M.; Benno, Y. Reclassification of Bacteroides distasonis, Bacteroides goldsteinii and Bacteroides merdae as Parabacteroides distasonis gen. nov., comb. nov., Parabacteroides goldsteinii comb. nov. and Parabacteroides merdae comb. nov. Int. J. Syst. Evol. Microbiol. 2006, 56, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Ussery, D.W.; Nielsen, J.; Nookaew, I. A closer look at Bacteroides: Phylogenetic relationship and genomic implications of a life in the human gut. Microb. Ecol. 2011, 61, 473–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.H.; Park, H.S.; Kim, H.J.; Kim, G.T.; Chang, I.S.; Lee, J.; Phung, N.T. Enrichment of microbial community generating electricity using a fuel-cell-type electrochemical cell. Appl. Microbiol. Biotechnol. 2004, 63, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.T.; Webster, G.; Wimpenny, J.W.; Kim, B.H.; Kim, H.J.; Weightman, A.J. Bacterial community structure, compartmentalization and activity in a microbial fuel cell. J. Appl. Microbiol. 2006, 101, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Lovley, D.R. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Boon, N.; Siciliano, S.D.; Verhaege, M.; Verstraete, W. Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl. Environ. Microbiol. 2004, 70, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Phung, N.T.; Lee, J.; Kang, K.H.; Chang, I.S.; Kim, B.H.; Sung, H.C. Use of acetate for enrichment of electrochemically active microorganisms and their 16S rDNA analysis. FEMS Microbiol. Lett. 2003, 223, 185–191. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E.; Nevin, K. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 2004, 49, 219–286. [Google Scholar] [PubMed]
- Weber, K.A.; Achenbach, L.A.; Coates, J.D. Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 2006, 4, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Bergey, D.H.; De Vos, P.; Whitman, W.B. The Firmicutes; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Deng, H.; Xue, H.; Zhong, W. A novel exoelectrogenic bacterium phylogenetically related to Clostridium sporogenes isolated from copper contaminated soil. Electroanalysis 2017, 29, 1294–1300. [Google Scholar] [CrossRef]
- Yazdani, S.S.; Gonzalez, R. Anaerobic fermentation of glycerol: A path to economic viability for the biofuels industry. Curr. Opin. Biotechnol. 2007, 18, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, N.; Kim, J.S.; Lee, Y.Y. Fermentation of xylose into acetic acid by Clostridium thermoaceticum. Appl. Biochem. Biotechnol. 2001, 91–93, 367–376. [Google Scholar] [CrossRef]
- Broda, D.M.; Saul, D.J.; Bell, R.G.; Musgrave, D.R. Clostridium algidixylanolyticum sp. nov., a psychrotolerant, xylan-degrading, spore-forming bacterium. Int. J. Syst. Evol. Microbiol. 2000, 50, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, G. Bacterial Metabolism, 2nd ed.; Springer: New York, NY, USA, 1979. [Google Scholar]
- Heyndrickx, M.; De Vos, P.; De Ley, J. H2 production from chemostat fermentation of glucose by Clostridium butyricum and Clostridium pasteurianum in ammonium and phosphate limitation. Biotechnol. Lett. 1990, 12, 731–736. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, B.H.; Kim, H.S.; Kim, H.J.; Kim, G.T.; Kim, M.; Chang, I.S.; Park, Y.K.; Chang, H.I. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell. Anaerobe 2001, 7, 297–306. [Google Scholar] [CrossRef]
- Dobbin, P.S.; Carter, J.P.; San Juan, C.G.S.; von Hobe, M.; Powell, A.K.; Richardson, D.J. Dissimilatory Fe(III) reduction by Clostridium beijerinckii isolated from freshwater sediment using Fe(III) maltol enrichment. FEMS Microbiol. Lett. 1999, 176, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kiely, P.D.; Call, D.F.; Yates, M.D.; Regan, J.M.; Logan, B.E. Anodic biofilms in microbial fuel cells harbor low numbers of higher-power producing bacteria than abundant genera. Appl. Microbiol. Biotechnol. 2010, 88, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, D.V.P.; Jacobs, D.; Gregory, K.; Huang, J.; Hu, Y.; Vidic, R.; Yun, M. Changes in carbon electrode morphology affect microbial fuel cell performance with Shewanella oneidensis MR-1. Energies 2015, 8, 1817–1829. [Google Scholar] [CrossRef] [Green Version]
- Franks, A.F.; Nevin, K.P. Microbial fuel cells, A current review. Energies 2010, 3, 899–919. [Google Scholar] [CrossRef]
- Xing, D.; Cheng, S.; Logan, B.E.; Regan, J.M. Isolation of the exoelectrogenic denitrifying bacterium Comamonas denitrificans based on dilution to extinction. Appl. Microbiol. Biotechnol. 2010, 85, 1575–1587. [Google Scholar] [CrossRef] [PubMed]
- Grüning, A.; Beecroft, N.J.; Avignone-Rossa, C. Low-potential respirators support electricity production in microbial fuel cells. Microb. Ecol. 2015, 70, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Future shock from the microbe electric. Microb. Biotechnol. 2009, 2, 128–156. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, J.M.; Yadav, A.; Ghosh, P.C.; Adeloju, S.B. Recent advances in the development and utilization of modern anode materials for high performance microbial fuel cells. Biosens. Bioelectron. 2017, 90, 558–576. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.D.; Khan, M.; Sultana, S.; Joshi, R.; Ahmed, S.; Yu, E.; Scott, K.; Khan, M.Z. Bioelectrochemical conversion of waste to energy using microbial fuel cell technology. Process Biochem. 2017, 57, 141–158. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toczyłowska-Mamińska, R.; Szymona, K.; Król, P.; Gliniewicz, K.; Pielech-Przybylska, K.; Kloch, M.; Logan, B.E. Evolving Microbial Communities in Cellulose-Fed Microbial Fuel Cell. Energies 2018, 11, 124. https://doi.org/10.3390/en11010124
Toczyłowska-Mamińska R, Szymona K, Król P, Gliniewicz K, Pielech-Przybylska K, Kloch M, Logan BE. Evolving Microbial Communities in Cellulose-Fed Microbial Fuel Cell. Energies. 2018; 11(1):124. https://doi.org/10.3390/en11010124
Chicago/Turabian StyleToczyłowska-Mamińska, Renata, Karolina Szymona, Patryk Król, Karol Gliniewicz, Katarzyna Pielech-Przybylska, Monika Kloch, and Bruce E. Logan. 2018. "Evolving Microbial Communities in Cellulose-Fed Microbial Fuel Cell" Energies 11, no. 1: 124. https://doi.org/10.3390/en11010124



