Co-Digestion of Napier Grass and Its Silage with Cow Dung for Bio-Hydrogen and Methane Production by Two-Stage Anaerobic Digestion Process
Abstract
:1. Introduction
2. Results and Discussion
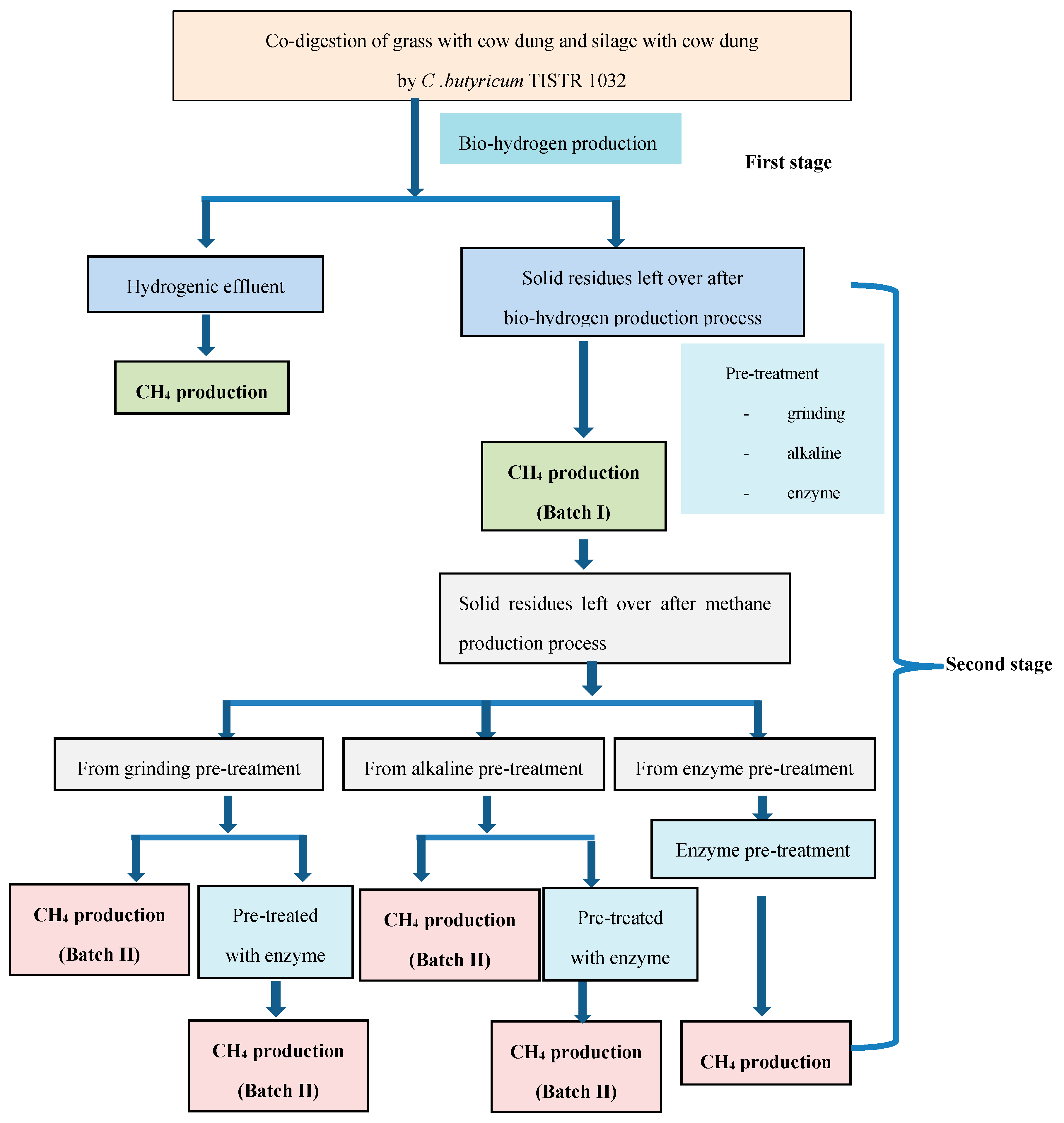
2.1. Bio-Hydrogen Production from the Co-Digestion of Grass with Cow Dung and Silage with Cow Dung by C. butyricum TISTR 1032
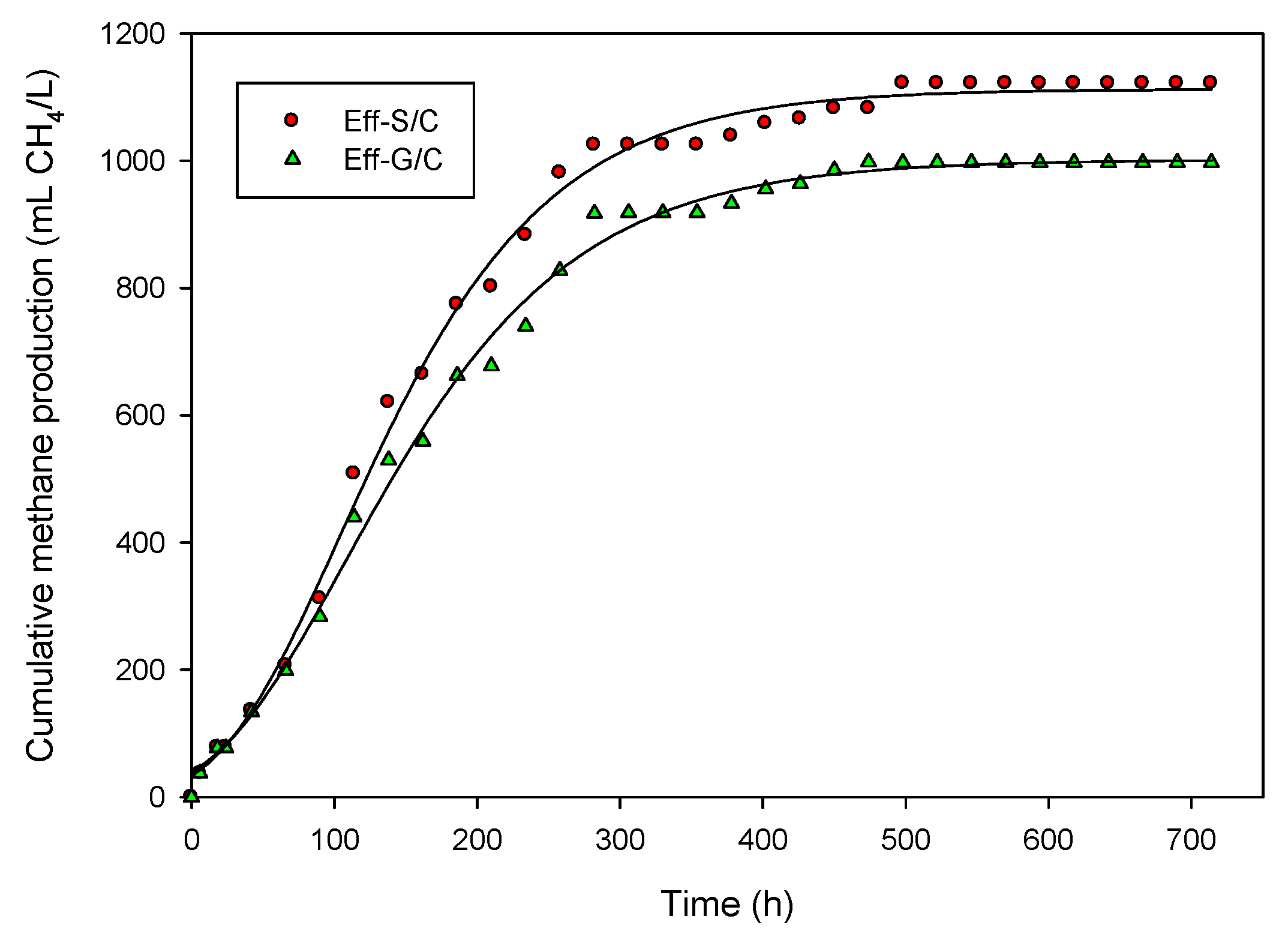
2.2. Methane Production from Hydrogenic Effluent
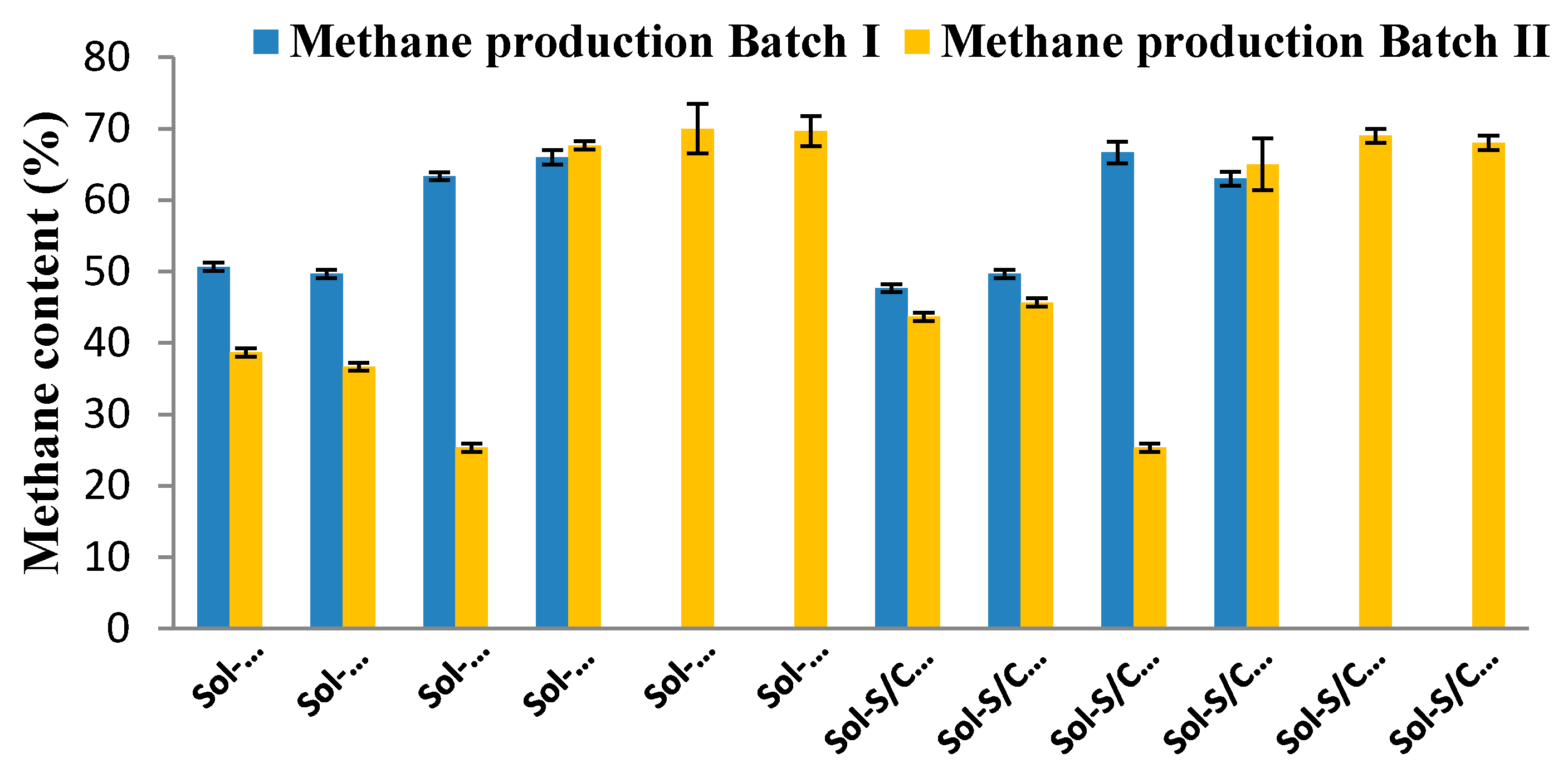
2.3. Methane Production from Solid Residues Left over after Hydrogen (Batch I) and Methane (Batch II) Fermentation Processes
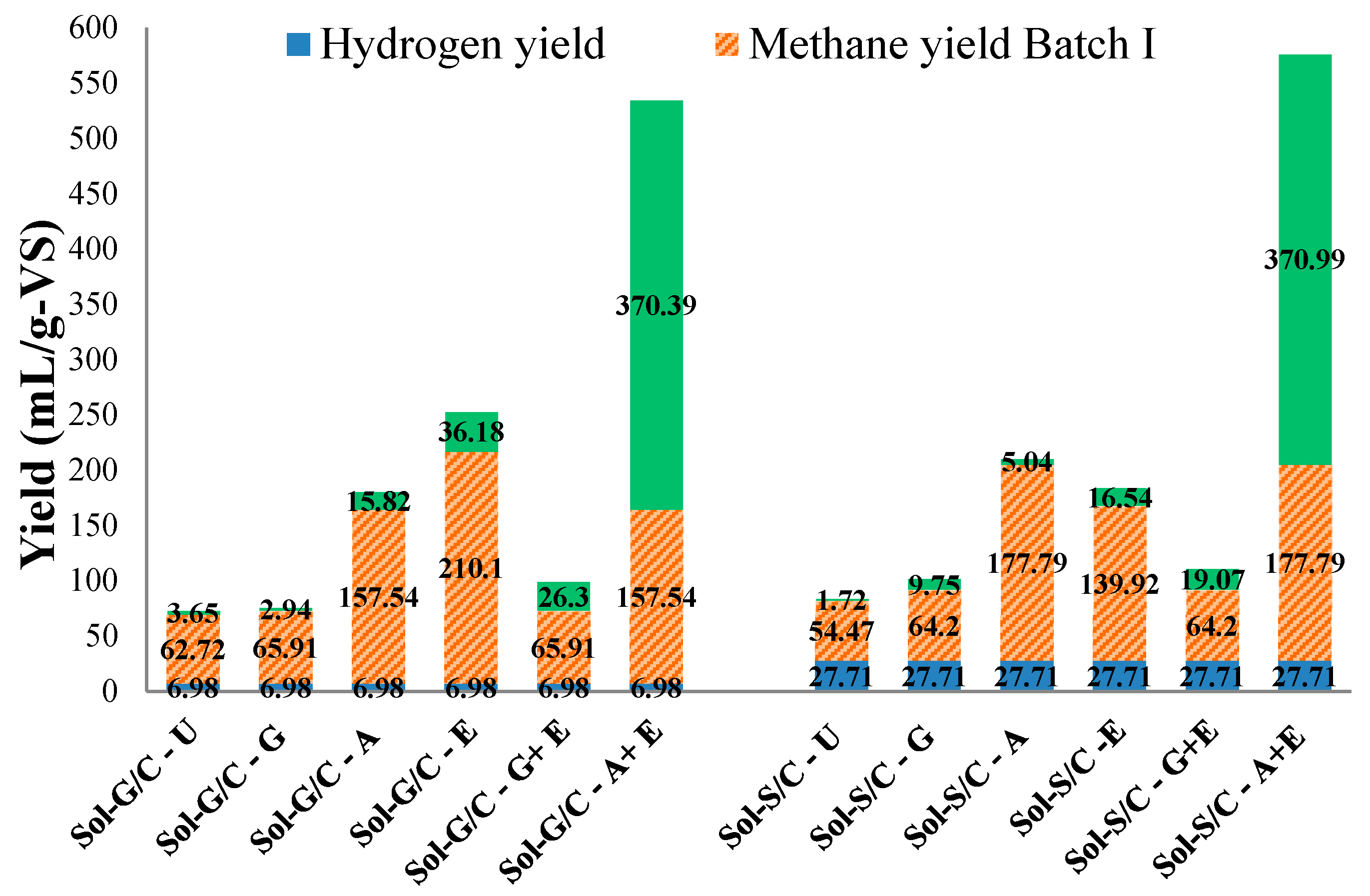
2.4. Production and Energy Yields
3. Materials and Methods
3.1. Substrates
3.2. Inoculum
3.3. Bio-Hydrogen Production from Co-Digestion of Grass and Silage with Cow Dung by C. butyricum TISTR 1032 (First Stage)
3.4. Methane Production from Hydrogenic Effluent and Solid Residues Left over after Bio-Hydrogen Production Process by Anaerobic Mixed Cultures (Second Stage)
3.5. Methane Production from Solid Residues Left over after Methane Production Process (Batch I) by Anaerobic Mixed Cultures
3.6. Pretreatment Methods for Solid Residues
3.7. Analytical Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| A | alkaline pretreatment |
| AD | anaerobic digestion |
| A+E | alkaline plus enzyme pretreatment |
| BA | basic anaerobic |
| C/N | ratio carbon to nitrogen ratio |
| COD | chemical oxygen demand |
| E | enzyme pretreatment |
| Eff-G/C | hydrogenic effluent from co-digestion of grass with cow dung |
| Eff-S/C | hydrogenic effluent from co-digestion of silage with cow dung |
| FPU | filter paper unit |
| G | grinding |
| GC | gas chromatography |
| G/C | co-digestion of grass with cow dung |
| G+E | grinding plus enzyme pretreatment |
| HP | hydrogen production potential (mL H2/L) |
| HPR | hydrogen production rate (mL H2)/L·(h) |
| HY | hydrogen yield (mL H2/g-VSadded) |
| MP | methane production (mL CH4/L) |
| MY | methane yield (mL CH4/g-VSadded) |
| Rm | methane production rate (mL CH4)/L·(h) |
| S/C | co-digestion of silage with cow dung |
| SMPs | soluble metabolite products (g/L) |
| Sol-G/C | solid residue of grass with cow dung |
| Sol-S/C | solid residue of silage with cow dung |
| TCD | thermal conductivity detector |
| TS | total solid (g/g-dry weight) |
| TSY | tryptone sucrose yeast extract |
| TVFAs | total volatile fatty acids (g/L) |
| U | untreated solid residue |
| VFAs | volatile fatty acids (g/L) |
| VS | volatile solid (g/g-dry weight) |
References
- Edwards, P.; Kuznetsov, V.; David, W.; Brandon, N. Hydrogen and fuel cells: Towards a sustainable energy future. Energy Policy 2008, 36, 4356–4362. [Google Scholar] [CrossRef]
- Liu, X.; Li, R.; Ji, M.; Han, L. Hydrogen and methane production by co-digestion of waste activated sludge and food waste in the two-stage fermentation process: Substrate conversion and energy yield. Bioresour. Technol. 2013, 146, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, G.; Gavala, H.N.; Skiadas, I.V.; Lyberatos, G. Effect of substrate concentration on fermentative hydrogen production from sweet sorghum extract. Int. J. Hydrogen Energy 2011, 36, 4843–4851. [Google Scholar] [CrossRef]
- Jung, K.W.; Kim, D.H.; Shin, H.S. Continuous fermentative hydrogen and methane production from Laminaria japonica using a two-stage fermentation system with recycling of methane fermented effluent. Int. J. Hydrogen Energy 2012, 37, 5648–5657. [Google Scholar] [CrossRef]
- Shi, X.; Kim, D.H.; Shin, H.S.; Jung, K.W. Effect of temperature on continuous fermentative hydrogen production from Laminaria japonica by anaerobic mixed cultures. Bioresour. Technol. 2013, 144, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, C.; Su, H. Dark bio-hydrogen fermentation by an immobilized mixed culture of Bacillus cereus and Brevumdimonas naejangsanensis. Renew. Energy 2017, 105, 458–464. [Google Scholar] [CrossRef]
- Maintinguer, S.I.; Lazaro, C.Z.; Pachiega, R.; Varesche, M.B.A.; Sequinel, R.; Oliveira, J.E. Hydrogen bioproduction with Enterobacter sp. isolated from brewery wastewater. Int. J. Hydrogen Energy 2017, 42, 152–160. [Google Scholar] [CrossRef]
- Wan, J.; Jing, Y.; Zhang, S.; Angelidaki, I.; Luo, G. Mesophilic and thermophilic alkaline fermentation of waste activated sludge for hydrogen production: Focusing on homoacetogenesis. Water Res. 2016, 102, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, A.; Sposito, F.; Frunzo, L.; Trably, E.; Escudié, R.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Effects of operational parameters on dark fermentative hydrogen production from biodegradable complex waste biomass. Waste Manag. 2016, 50, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ding, L.; Lin, R.; Liu, M.; Zhou, C.K. Physicochemical characterization of typical municipal solid wastes. Energy Convers. Manag. 2016, 117, 297–304. [Google Scholar] [CrossRef]
- Han, W.; Hu, Y.; Li, S.; Huang, J.; Nie, N.; Zhao, H.; Tang, J. Simultaneous dark fermentative hydrogen and ethanol production from waste bread in a mixed packed tank reactor. J. Clean. Prod. 2017, 141, 608611. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Sivagurunathan, P.; Parthiban, A.; Kim, S.H. Optimization of substrate concentration of dilute acid hydrolyzate of lignocellulosic biomass in batch hydrogen production. Int. Biodeterior. Biodegrad. 2016, 113, 22–27. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Shukla, M.; Rajulu, A.V. Chemical composition and structural characterization of Napier grass fibers. Mater. Lett. 2012, 67, 35–38. [Google Scholar] [CrossRef]
- Lu, Q.L.; Tang, L.R.; Wang, S.; Huang, B.; Chen, Y.D.; Chen, X.R. An investigation on the characteristics of cellulose nanocrystals from Pennisetum sinese. Biomass Bioenergy 2014, 70, 267–272. [Google Scholar] [CrossRef]
- Phitsuwan, P.; Charupongrat, S.; Klednark, R.; Ratanakhanokchai, K. Structural features and enzymatic digestibility of Napier grass fibre treated with aqueous ammonia. J. Ind. Eng. Chem. 2015, 32, 360–364. [Google Scholar] [CrossRef]
- Sawasdee, V.; Pisutpaisal, N. Feasibility of Biogas Production from Napier Grass. Energy Procedia 2014, 61, 1229–1233. [Google Scholar] [CrossRef]
- Dussadee, N.; Reansuwan, K.; Ramaraj, R. Potential development of compressed bio-methane Gas Production from pig farms and elephant grass silage for transportation in Thailand. Bioresour. Technol. 2014, 155, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Seppälä, M.; Laine, A.; Rintala, J. Screening of novel plants for biogas production in northern conditions. Bioresour. Technol. 2013, 139, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Zhang, D.; Singh, P.M.; Ragauskas, A.J. The new forestry biofuels sector. Biofuels Bioprod. Biorefin. 2008, 2, 58–73. [Google Scholar] [CrossRef]
- Saha, B.C. Hemicellulose bioconversion. Microbiol. Biotechnol. 2003, 30, 279–326. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Wang, A.; Cao, G.; Xu, J.; Gao, L. Bioconversion of lignocellulosic biomass to hydrogen: Potential and challenges. Biotechnol. Adv. 2009, 27, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Strezov, V.; Evans, T.J.; Hayman, C. Thermal conversion of elephant grass (Pennisetum Purpureum Schum) to bio-gas, bio-oil and charcoal. Bioresour. Technol. 2008, 99, 8394–8399. [Google Scholar] [CrossRef] [PubMed]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T. Biomass recalcitrance: Engineering plants and enzymes for biofuels yield. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Li, D.; Sun, Y.; Wang, G.; Yuan, Z.; Zhen, F.; Wang, Y. Improved biogas production from rice straw by co-digestion with kitchen waste and pig manure. Waste Manag. 2013, 33, 2653–2658. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, R.H.; Liu, X.Y.; Chen, C.; Xiao, X.; Feng, L.; He, Y.F.; Liu, G.Q. Evaluating methane production from anaerobic mono and co-digestion of kitchen waste, corn stover, and chicken manure. Energy Fuels 2013, 27, 2085–2091. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhang, D.; Li, G.; Lu, J.; Li, S. Solid state anaerobic co-digestion of tomato residues with dairy manure and corn stover for biogas production. Bioresour. Technol. 2016, 217, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.M.; Zhang, X.Q.; Dong, Z.X.; Guo, H.R. Dynamic changes of lignin contents of MT-1 elephant grass and its closely related cultivars. Biomass Bioenergy 2011, 35, 1732–1738. [Google Scholar] [CrossRef]
- Pakarinen, O.; Tahti, P.; Rintala, J. One-stage H2 and CH4 and two-stage H2 and CH4 production from grass silage and from solid and liquid fractions of NaOH pre-treated grass silage. Biomass Bioenergy 2009, 33, 1419–1427. [Google Scholar] [CrossRef]
- Cavinato, C.; Bolzonella, D.; Eusebi, A.L.; Pavan, P. Bio-hythane production by thermophilic two-phase anaerobic digestion of organic fraction of municipal solid waste: Preliminary results. In AIDIC Conference Series; AIDIC: Milan, Italy, 2009; Volume 9, p. 166. [Google Scholar]
- Surendra, K.C.; Khanal, S.K. Effects of crop maturity and size reduction on digestibility and methane yield of dedicated energy crop. Bioresour. Technol. 2015, 178, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Song, W.; Ding, L.; Xie, B.; Zhou, J.; Cen, K. Characterisation of water hyacinth with microwave-heated alkali pretreatment for enhanced enzymatic digestibility and hydrogen/methane fermentation. Bioresour. Technol. 2015, 182, 1–7. [Google Scholar] [CrossRef] [PubMed]
- He, Y.C.; Ding, Y.; Xue, Y.F.; Yang, B.; Liu, F.; Wang, C.; Zhu, Z.Z.; Qing, Q.; Wu, H.; Zhu, C.; et al. Enhancement of enzymatic saccharification of corn stover with sequential Fenton pretreatment and dilute NaOH extraction. Bioresour. Technol. 2015, 193, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Nissila, M.E.; Lay, C.; Puhakka, J. Dark fermentative hydrogen production from lignocellulosic hydrolyzates. A review. Biomass Bioenergy 2014, 67, 145–159. [Google Scholar] [CrossRef]
- Rabemanolontsoa, H.; Saka, S. Various pretreatments of lignocellulosics. Bioresour. Technol. 2016, 199, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Bizukoj, M.; Ledakowicz, S. Pretreatment of energy crops with sodium hydroxide and cellulolytic enzymes to increase biogas production. Biomass Bioenergy 2015, 80, 213–221. [Google Scholar] [CrossRef]
- Kaur, K.; Phutela, U.G. Enhancement of paddy straw digestibility and biogas production by sodium hydroxide-microwave pretreatment. Renew. Energy 2016, 92, 178–184. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Poulsen, T.G.; Xia, X.; Sheng, K. Combinations of fungal and milling pretreatments for enhancing rice straw biogas production during solid-state anaerobic digestion. Bioresour. Technol. 2017, 224, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Thomas, L.; Joseph, A.; Gottumukkala, L.D. Xylanase and cellulase systems of Clostridium sp.: An insight on molecular approaches for strain improvement. Bioresour. Technol. 2014, 158, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Nualsri, C.; Reungsanga, A.; Plangklang, P. Biochemical hydrogen and methane potential of sugarcane syrup using a two-stage anaerobic fermentation process. Ind. Crops Prod. 2016, 82, 88–99. [Google Scholar] [CrossRef]
- Pattra, S.; Lay, C.H.; Lin, C.Y.; O-Thong, S.; Reungsang, A. Performance and population analysis of hydrogen production from sugarcane juice by non-sterile continuous stirred tank reactor augmented with Clostridium butyricum. Int. J. Hydrogen Energy 2011, 36, 8697–8703. [Google Scholar] [CrossRef]
- Rafieenia, R.; Chaganti, S.R. Flux balance analysis of different carbon source fermentation with hydrogen producing Clostridium butyricum using Cell Net Analyzer. Bioresour. Technol. 2015, 175, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Zhai, N.; Zhang, T.; Yin, D.; Yang, G.; Wang, X.; Ren, G.; Feng, Y. Effect of initial pH on anaerobic co-digestion of kitchen waste and cow manure. Waste Manag. 2015, 38, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, G.L.; Wang, A.J.; Ren, H.Y.; Zhang, K.; Ren, N.Q. Consolidated bioprocessing performance of Thermoanaerobacterium thermosaccharolyticum M18 on fungal pretreated cornstalk for enhanced hydrogen production. Biotechnol. Biofuels 2014, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Salehian, P.; Karimi, K. Alkali pretreatment for improvement of biogas and ethanol production from different waste parts of pine tree. Ind. Eng. Chem. Res. 2013, 52, 972–978. [Google Scholar] [CrossRef]
- Frigon, J.C.; Mehta, P.; Guiot, S.R. Impact of mechanical, chemical and enzymatic pretreatments on the methane yield from the anaerobic digestion of switch grass. Biomass Bioenergy 2012, 36, 1–11. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: Review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Prapinagsorn, W.; Sittijunda, S.; Reungsang, A. Co-Digestion of Napier Grass and Its Silage with Cow Dung for Methane Production. Energies 2017, 10, 1654. [Google Scholar] [CrossRef]
- Fangkum, A.; Reungsang, A. Biohydrogen production from mixed xylose/arabinose at thermophilic temperature by anaerobic mixed cultures in elephant dung. Int. J. Hydrogen Energy 2011, 36, 13928–13938. [Google Scholar] [CrossRef]
- Owen, W.; Stuckey, C.; Healy, J.; Young, L.; McCarty, P. Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1979, 13, 485–492. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for Examination of Water and Wastewater, 18th ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2008; pp. 1–18.
- Zheng, X.J.; Yu, H.Q. Inhibitory effects of butyrate on biological hydrogen production with mixed anaerobic culturees. J. Environ. Manag. 2005, 74, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.; Dhanoa, M.S.; Dijkstra, J.; Bannink, A.; Kebreab, E.; France, J. Some methodological and analytical considerations regarding application of the gas production technique. Anim. Feed Sci. Technol. 2007, 135, 139–156. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, L.; Rombouts, F.M.; Van’t, R.K. Modeling the bacterial growth curve. Appl. Environ. Microbiol. 1990, 64, 1878–1883. [Google Scholar]
- Reungsang, A.; Pattra, S.; Sittijunda, S. Optimization of Key Factors Affecting Methane Production from Acidic Effluent Coming from the Sugarcane Juice Hydrogen Fermentation Process. Energies 2012, 5, 4746–4757. [Google Scholar] [CrossRef]
| Treatment | Final pH | HP (mL H2/L) | HPR (mL H2/(L·h)) | Lag Phase (h) | R2 | HY (mL H2/g-VSadded) | SMPs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetic Acid | Propionic Acid | Butyric Acid | TVFAs | ||||||||||
| (g/L) | (%) | (g/L) | (%) | (g/L) | (%) | (g/L) | |||||||
| G/C+ Clostridium butyricum TISTR 1032 | 5.02 | 140 | 11.35 | 1.31 | 0.98 | 6.98 | 2.64 | 67 | 0.12 | 3 | 1.20 | 30 | 3.96 |
| S/C+ Clostridium butyricum TISTR 1032 | 5.26 | 554 | 39.79 | 0.24 | 0.99 | 27.71 | 1.88 | 47 | 0.21 | 5 | 1.91 | 48 | 4.00 |
| G/C | 5.23 | 39 | 3.71 | 7.20 | 0.99 | 1.94 | 2.41 | 72 | 0.19 | 6 | 0.72 | 22 | 3.34 |
| S/C | 5.52 | 46 | 3.82 | 6.28 | 0.98 | 2.30 | 1.82 | 57 | 0.26 | 8 | 1.09 | 34 | 3.17 |
| Effluent | Final pH | MP (mL CH4/L) | Rm (mL CH4/(L·h)) | MY (mL CH4/g-CODadded) | CH4 Content (%) | Substrate Conversion (%) |
|---|---|---|---|---|---|---|
| Eff-G/C | 7.75 | 902 | 4.05 | 169.87 | 66 | 48 |
| Eff-S/C | 7.60 | 1002 | 4.94 | 141.33 | 67 | 40 |
| Treatment | Methane Production Batch I | Methane Production Batch II | ||||
|---|---|---|---|---|---|---|
| MP (mL CH4/L) | Rm (mL CH4/(L·h)) | Yield (mL CH4/g-VSadded) | MP (mL CH4/L) | Rm (mL CH4/(L·h)) | Yield (mL CH4/g-VSadded) | |
| Sol-G/C-U | 851 | 3.36 | 62.72 | 70 | 2.76 | 3.65 |
| Sol-G/C-G | 894 | 2.91 | 65.91 | 60 | 2.99 | 2.94 |
| Sol-G/C-A | 2136 | 2.86 | 157.54 | 138 | 1.79 | 15.82 |
| Sol-G/C-E | 2849 | 12.51 | 210.10 | 251 | 8.08 | 36.18 |
| Sol-G/C-G+E | - | - | - | 196 | 7.78 | 26.30 |
| Sol-G/C-A+E | - | - | - | 2113 | 8.71 | 370.39 |
| Sol-S/C-U | 1044 | 2.62 | 54.47 | 83 | 1.97 | 1.72 |
| Sol-S/C-G | 1231 | 3.15 | 64.20 | 130 | 3.02 | 9.75 |
| Sol-S/C-A | 3408 | 3.13 | 177.79 | 103 | 1.39 | 5.04 |
| Sol-S/C-E | 2682 | 11.33 | 139.92 | 170 | 8.27 | 16.54 |
| Sol-S/C-G+E | - | - | - | 185 | 7.47 | 19.07 |
| Sol-S/C-A+E | - | - | - | 2240 | 9.16 | 370.99 |
| Treatment | Bio-Hydrogen Production | Methane Production | Combined Bio-Hydrogen and Methane Production | |||||
|---|---|---|---|---|---|---|---|---|
| G/C | S/C | G/C | S/C | G/C | S/C | |||
| H2 and CH4 batch assay | ||||||||
| H2 production | ||||||||
| Energy yield (MJ/g-VSadded) | 0.09 | 0.36 | - | - | 0.09 | 0.36 | ||
| CH4 production from hydrogenic effluent | ||||||||
| Energy yield (MJ/g-VSadded) | - | - | 2.01 | 1.81 | 2.01 | 1.81 | ||
| CH4 Batch Assay (Pretreatment of Solid Residues for Methane Production) | Sol-G/C-A | Sol-G/C-A+E | Sol-S/C-A | Sol-S/C-A+E | Batch I and Batch II | Batch I and Batch II | ||
| Batch I | ||||||||
| Energy yield (MJ/g-VSadded) | 9.32 | - | 7.42 | - | 9.32 | 7.42 | ||
| Batch II | ||||||||
| Energy yield (MJ/g-VSadded) | - | 468.85 | - | 195.11 | 468.85 | 195.11 | ||
| Total Energy Yield (MJ/g-VSadded) | 480.27 | 204.70 | ||||||
| Characteristic | Grass | Silage | Cow Dung | Anaerobic Sludge |
|---|---|---|---|---|
| Total solid (TS) | 0.25 a | 0.42 a | 0.26 a | 0.07 a |
| Volatile solid (VS) | 0.21 a | 0.38 a | 0.21 a | 0.04 a |
| Moisture (%) | 76.01 | 70.65 | 79.58 | 93.49 |
| pH | 5.84 | 3.84 | 8.28 | 7.89 |
| Ash (%) | 2.49 | 3.36 | 5.05 | 2.78 |
| Cellulose (%) | 26.35 | 31.95 | 24.49 | ND |
| Hemicellulose (%) | 17.26 | 19.93 | 26.62 | ND |
| Lignin (%) | 31.25 | 32.22 | 25.73 | ND |
| Carbon (%) | 45.36 | 43.18 | 37.64 | ND |
| Nitrogen (%) | 0.78 | 0.92 | 1.81 | ND |
| C/N | 58.15 | 46.93 | 20.80 | ND |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prapinagsorn, W.; Sittijunda, S.; Reungsang, A. Co-Digestion of Napier Grass and Its Silage with Cow Dung for Bio-Hydrogen and Methane Production by Two-Stage Anaerobic Digestion Process. Energies 2018, 11, 47. https://doi.org/10.3390/en11010047
Prapinagsorn W, Sittijunda S, Reungsang A. Co-Digestion of Napier Grass and Its Silage with Cow Dung for Bio-Hydrogen and Methane Production by Two-Stage Anaerobic Digestion Process. Energies. 2018; 11(1):47. https://doi.org/10.3390/en11010047
Chicago/Turabian StylePrapinagsorn, Wipa, Sureewan Sittijunda, and Alissara Reungsang. 2018. "Co-Digestion of Napier Grass and Its Silage with Cow Dung for Bio-Hydrogen and Methane Production by Two-Stage Anaerobic Digestion Process" Energies 11, no. 1: 47. https://doi.org/10.3390/en11010047






