Improving Vegetable Oil Properties by Transforming Fatty Acid Chain Length in Jatropha Oil and Coconut Oil Blends
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. Fatty Acid Composition
2.3. Experiments
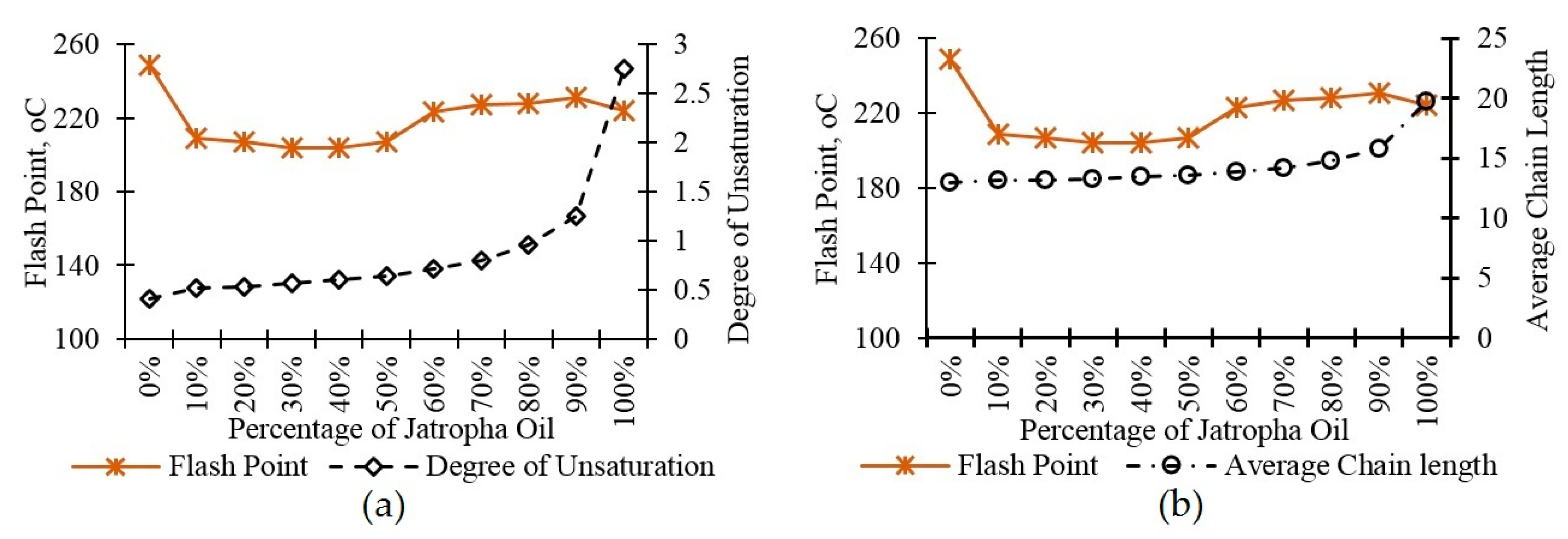
3. Results and Discussion
3.1. Fatty Acid Composition
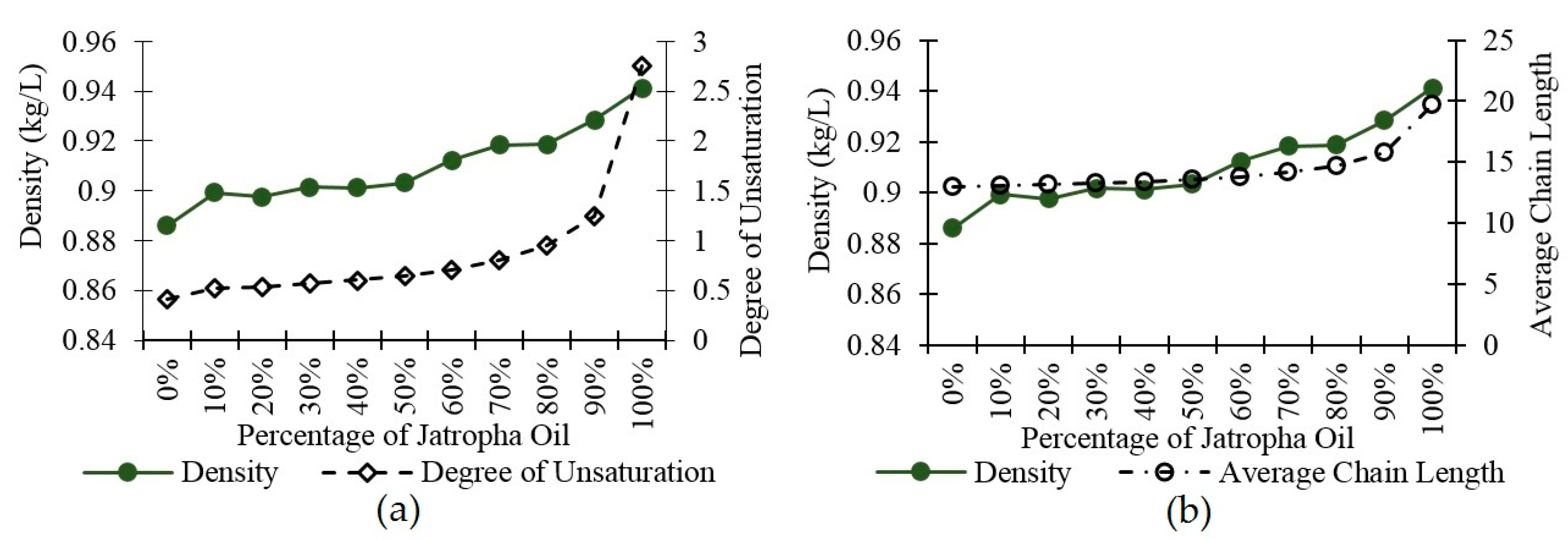
3.2. Density
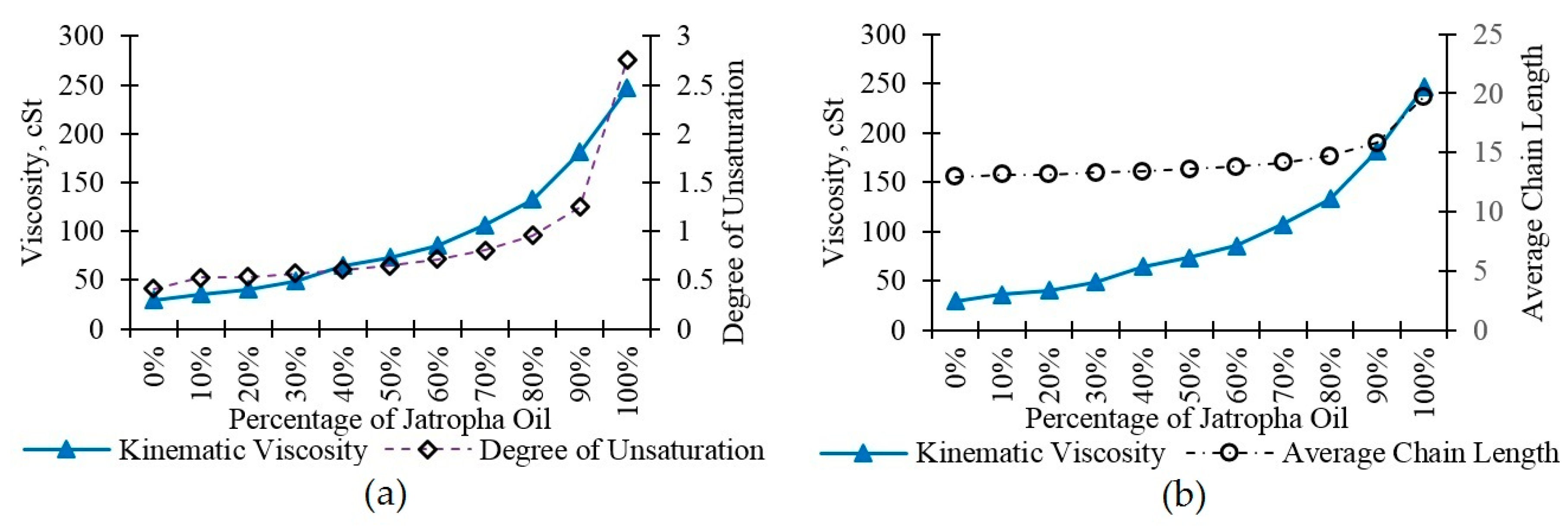
3.3. Viscosity
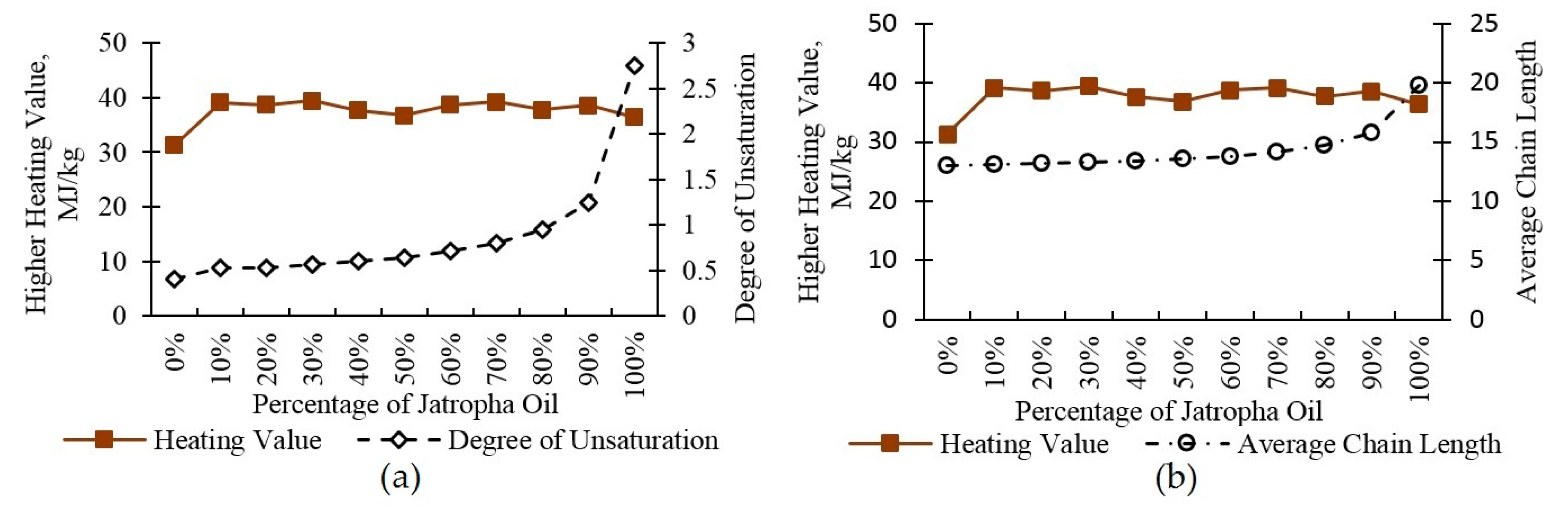
3.4. Heating Value
3.5. Flash Point
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tziourtzioumis, D.N.; Stamatelos, A.M. Experimental investigation of the effect of biodiesel blends on a di diesel engine’s injection and combustion. Energies 2017, 10, 970. [Google Scholar] [CrossRef]
- Pramanik, K. Properties and use of jatropha curcas oil and diesel fuel blends in compression ignition engine. Renew. Energy 2003, 28, 239–248. [Google Scholar] [CrossRef]
- Forson, F.K.; Oduro, E.K.; Hammond-Donkoh, E. Performance of jatropha oil blends in a diesel engine. Renew. Energy 2004, 29, 1135–1145. [Google Scholar] [CrossRef]
- Misra, R.D.; Murthy, M.S. Straight vegetable oils usage in a compression ignition engine—A review. Renew. Sustain. Energy Rev. 2010, 14, 3005–3013. [Google Scholar] [CrossRef]
- Demirbas, A. Relationships derived from physical properties of vegetable oil and biodiesel fuels. Fuel 2008, 87, 1743–1748. [Google Scholar] [CrossRef]
- Knothe, G. “Designer” biodiesel: Optimizing fatty ester composition to improve fuel properties. Energy Fuels 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Si, Z.; Zhang, X.; Wang, C.; Ma, L.; Dong, R. An overview on catalytic hydrodeoxygenation of pyrolysis oil and its model compounds. Catalysts 2017, 7, 169. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Kumar, G.; Kumar, D.; Singh, S.; Kothari, S.; Bhatt, S.; Singh, C.P. Continuous low cost transesterification process for the production of coconut biodiesel. Energies 2010, 3, 43–56. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. Kinematic viscosity of biodiesel fuel components and related compounds. Influence of compound structure and comparison to petrodiesel fuel components. Fuel 2005, 84, 1059–1065. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, M.P. Prospects of biodiesel from jatropha in india: A review. Renew. Sustain. Energy Rev. 2010, 14, 763–771. [Google Scholar] [CrossRef]
- Anastopoulos, G.; Zannikou, Y.; Stournas, S.; Kalligeros, S. Transesterification of vegetable oils with ethanol and characterization of the key fuel properties of ethyl esters. Energies 2009, 2, 362–376. [Google Scholar] [CrossRef]
- Agarwal, D.; Agarwal, A.K. Performance and emissions characteristics of jatropha oil (preheated and blends) in a direct injection compression ignition engine. Appl. Therm. Eng. 2007, 27, 2314–2323. [Google Scholar] [CrossRef]
- Moser, B.R. Biodiesel production, properties, and feedstocks. In Vitro Cell. Dev. Biol.-Plant 2009, 45, 229–266. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Fayaz, H. Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew. Sustain. Energy Rev. 2013, 18, 211–245. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Ramirez, J.A.; Brown, R.J.; Rainey, T.J. A review of hydrothermal liquefaction bio-crude properties and prospects for upgrading to transportation fuels. Energies 2015, 8, 6765–6794. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Verduzco, L.F.; Rodriguez-Rodriguez, J.E.; del Rayo Jaramillo-Jacob, A. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 2012, 91, 102–111. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. Kinematic viscosity of biodiesel components (fatty acid alkyl esters) and related compounds at low temperatures. Fuel 2007, 86, 2560–2567. [Google Scholar] [CrossRef]
- Murali, H.S.; Mohan, M.S.; Manja, K.S.; Sankaran, R. Polar and nonpolar lipids and their fatty acid composition of a fewfusarium species. J. Am. Oil Chem. Soc. 1993, 70, 1039–1041. [Google Scholar] [CrossRef]
- Parcerisa, J.; Richardson, D.G.; Rafecas, M.; Codony, R.; Boatella, J. Fatty acid distribution in polar and nonpolar lipid classes of hazelnut oil (Corylus avellana L.). J. Agric. Food Chem. 1997, 45, 3887–3890. [Google Scholar] [CrossRef]
- Gopinath, A.; Puhan, S.; Nagarajan, G. Theoretical modeling of iodine value and saponification value of biodiesel fuels from their fatty acid composition. Renew. Energy 2009, 34, 1806–1811. [Google Scholar] [CrossRef]
- Rodrigues, J.D.A.; Cardoso, F.D.P.; Lachter, E.R.; Estevão, L.R.M.; Lima, E.; Nascimento, R.S.V. Correlating chemical structure and physical properties of vegetable oil esters. J. Am. Oil Chem. Soc. 2006, 83, 353–357. [Google Scholar] [CrossRef]
- Siddique, B.M.; Ahmad, A.; Ibrahim, M.H.; Hena, S.; Rafatullah, M. Physico-chemical properties of blends of palm olein with other vegetable oils. Grasas y Aceites 2010, 61, 423–429. [Google Scholar]
- Hong, I.K.; Jeon, G.S.; Lee, S.B. Prediction of biodiesel fuel properties from fatty acid alkyl ester. J. Ind. Eng. Chem. 2014, 20, 2348–2353. [Google Scholar] [CrossRef]
- Jena, P.C.; Raheman, H.; Kumar, G.V.P.; Machavaram, R. Biodiesel production from mixture of mahua and simarouba oils with high free fatty acids. Biomass Bioenergy 2010, 34, 1108–1116. [Google Scholar] [CrossRef]
- Agarwal, A.K. Experimental investigations of the effect of biodiesel utilization on lubricating oil tribology in diesel engines. Proc. Inst. Mech. Eng. Part D: J. Automob. Eng. 2005, 219, 703–713. [Google Scholar] [CrossRef]
- Mejía, J.D.; Salgado, N.; Orrego, C.E. Effect of blends of diesel and palm-castor biodiesels on viscosity, cloud point and flash point. Ind. Crops Prod. 2013, 43, 791–797. [Google Scholar] [CrossRef]

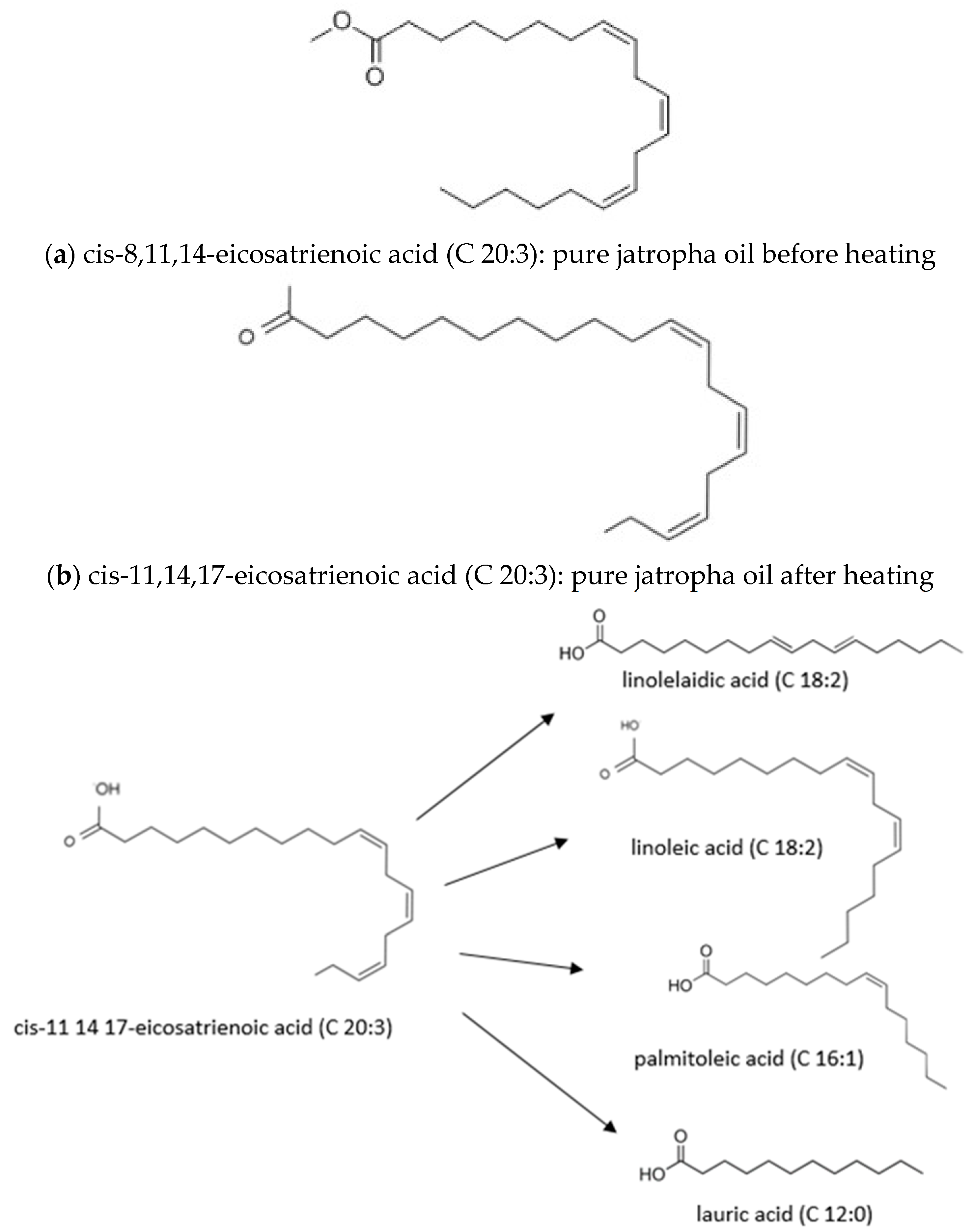
| Properties | Diesel Fuel | Coconut Oil | Jatropha Oil |
|---|---|---|---|
| Calorific Value (MJ/kg) | 46 a | 31.255 | 36.398 |
| Density at 40 °C (kg/m3) | 855.2 a,b | 885.95 | 941.28 |
| Kinematic Viscosity at 40 °C (cSt) | 4.27 a | 29.35 | 246.47 |
| Flash Point (°C) | 60 a | 249 | 224 |
| Fatty Acid | Molecular Formula | Abbreviation | Coconut Oils | Jatropha Oils |
|---|---|---|---|---|
| Caproic | C6H12O2 | C 6:0 | 0.35 | - |
| Caprilic | C8H16O2 | C 8:0 | 6.49 | - |
| Capric | C10H20O2 | C 10:0 | 5.79 | - |
| Lauric | C12H24O2 | C 12:0 | 47.22 | - |
| Tridecanoic | C13H26O2 | C 13:0 | 0.7 | - |
| Myristoleic | C14H26O2 | C 14:1 | 18.55 | - |
| Pentadecanoic | C15H30O2 | C 15:0 | 0.19 | - |
| Palmitic | C16H32O2 | C 16:0 | 1.79 | |
| Palmitoleic | C16H30O2 | C 16:1 | 9.41 | |
| Cis-9-Oleic | C18H34O2 | C 18:1 | 14.78 | |
| Linoleic | C18H32O2 | C 18:2 | 3.08 | 2.17 |
| Linolelaidic | C18H32O2 | C 18:2 | 8.23 | |
| Cis-11,14-Eicosadienoic | C20H36O2 | C 20:2 | 0.6 | |
| Cis-8,11,14-Eicosatrienoic | C20H34O2 | C 20:3 | 80.66 |
| Fatty Acid. | Formula | Percentage of Jatropha Oils | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% | ||
| Caproic | C 6:0 | 0.38 | - | - | - | - | - | - | - | - | - | - |
| Caprilic | C 8:0 | 6.80 | 6.53 | 6.56 | 6.41 | 5.97 | 5.85 | 5.71 | 5.22 | 3.9 | 2.82 | - |
| Capric | C 10:0 | 6.03 | 5.8 | 5.78 | 5.61 | 5.37 | 5.18 | 4.92 | 4.44 | 3.54 | 2.34 | - |
| Lauric | C 12:0 | 48.46 | 47.44 | 47.06 | 45.82 | 44.85 | 43.11 | 40.66 | 37.6 | 32.39 | 21.57 | - |
| Tridecanoic | C 13:0 | 0.32 | - | - | - | - | - | - | - | 0.23 | - | - |
| Myristoleic | C 14:1 | 18.35 | 18.55 | 18.33 | 17.83 | 17.55 | 16.87 | 15.82 | 14.43 | 12.75 | 7.99 | - |
| Pentadecanoic | C 15:0 | 0.11 | - | - | - | - | - | - | - | - | - | - |
| Palmitic | C 16:0 | - | - | - | - | - | - | - | - | - | - | 1.20 |
| Palmitoleic | C 16:1 | 8.90 | 9.46 | 9.44 | 9.44 | 9.57 | 9.65 | 9.6 | 9.46 | 9.73 | 9.45 | - |
| Cis-9 Oleic | C 18:1 | 7.72 | - | - | - | - | - | - | - | - | - | 9.82 |
| Linoleic | C 18:2 | 2.93 | 12.17 | 3.2 | 3.4 | 3.19 | 3.67 | 4.2 | 4.8 | 5.79 | 7.28 | - |
| Linolelaidic | C 18:2 | - | - | 9.56 | 11.32 | 13.17 | 15.33 | 17.9 | 23.06 | 30.63 | 43.17 | 1.42 |
| Linolenic | C 18:3 | - | - | - | - | 0.1 | - | 0.18 | - | - | 0.84 | - |
| Cis-11 Eicosenoic | C 20:1 | - | - | - | - | - | - | 0.23 | - | - | 0.92 | - |
| Cis-11,14 Eicosadienoic | C 20:2 | - | - | - | - | - | - | - | - | - | - | 0.37 |
| Cis-8,11,14 Eicosatrienoic | C 20:3 | - | - | - | - | - | - | 0.24 | 0.28 | - | 0.98 | - |
| Cis-11,14,17 Eicosatrienoic | C 20:3 | - | - | - | - | - | - | - | - | - | - | 87.19 |
| Docosanoic | C 22:0 | - | 0.07 | 0.1 | 0.18 | 0.25 | 0.36 | 0.56 | 0.73 | 1.05 | 2.26 | - |
| Molecular Structure | Percentage of Jatropha Oil | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% | |
| Saturated | 62.10 | 59.84 | 59.5 | 58.02 | 56.44 | 54.50 | 51.85 | 47.99 | 41.11 | 28.99 | 1.20 |
| Mono-unsaturated | 34.97 | 28.01 | 27.77 | 27.27 | 27.12 | 26.52 | 25.65 | 23.89 | 22.48 | 18.36 | 9.82 |
| Poly-unsaturated | 2.93 | 12.17 | 12.76 | 14.72 | 16.46 | 19.00 | 22.52 | 28.14 | 36.42 | 52.27 | 88.98 |
| Parameter | Percentage of Jatropha Oils | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% | |
| Average Chain Length | 12.95 | 13.11 | 13.15 | 13.27 | 13.40 | 13.56 | 13.81 | 14.14 | 14.71 | 15.79 | 19.73 |
| Degree of Unsaturation | 0.408 | 0.523 | 0.533 | 0.567 | 0.601 | 0.645 | 0.711 | 0.804 | 0.953 | 1.247 | 2.750 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahyudi; Wardana, I.N.G.; Widodo, A.; Wijayanti, W. Improving Vegetable Oil Properties by Transforming Fatty Acid Chain Length in Jatropha Oil and Coconut Oil Blends. Energies 2018, 11, 394. https://doi.org/10.3390/en11020394
Wahyudi, Wardana ING, Widodo A, Wijayanti W. Improving Vegetable Oil Properties by Transforming Fatty Acid Chain Length in Jatropha Oil and Coconut Oil Blends. Energies. 2018; 11(2):394. https://doi.org/10.3390/en11020394
Chicago/Turabian StyleWahyudi, I.N.G. Wardana, Agung Widodo, and Widya Wijayanti. 2018. "Improving Vegetable Oil Properties by Transforming Fatty Acid Chain Length in Jatropha Oil and Coconut Oil Blends" Energies 11, no. 2: 394. https://doi.org/10.3390/en11020394





