Sucrose Is a Promising Feedstock for the Synthesis of the Platform Chemical Hydroxymethylfurfural
Abstract
:1. Introduction
- The reactor type can influence the determination of kinetic data. Smith et al. [27], for example, investigated glucose decomposition in a batch and tubular reactor using sulfuric acid. Glucose decomposed 4.4 times faster in the continuous system. The activation energy was much lower (88 kJ/mol) compared with that of the continuous reactor (129 kJ/mol) [27].
- Either a linear or a power law dependence of acid concentration is included in the Arrhenius equation in some models. Depending on the study, the mass percentage of acid [27,28], hydronium concentration at ambient conditions [20], hydronium concentration at reaction conditions [23,25], an activity term [29], or multiple factors [30] may be used in modified Arrhenius equations.
- Kinetic rate constants are normally determined within small ranges for substrate concentration, catalyst concentration, or temperature [25]. An extrapolation to other reaction conditions is problematic.
- Only one reaction is assumed for hexose conversion in some studies. Other studies use networks of more than seven individual reactions [3].
2. Materials and Methods
2.1. Feedstock
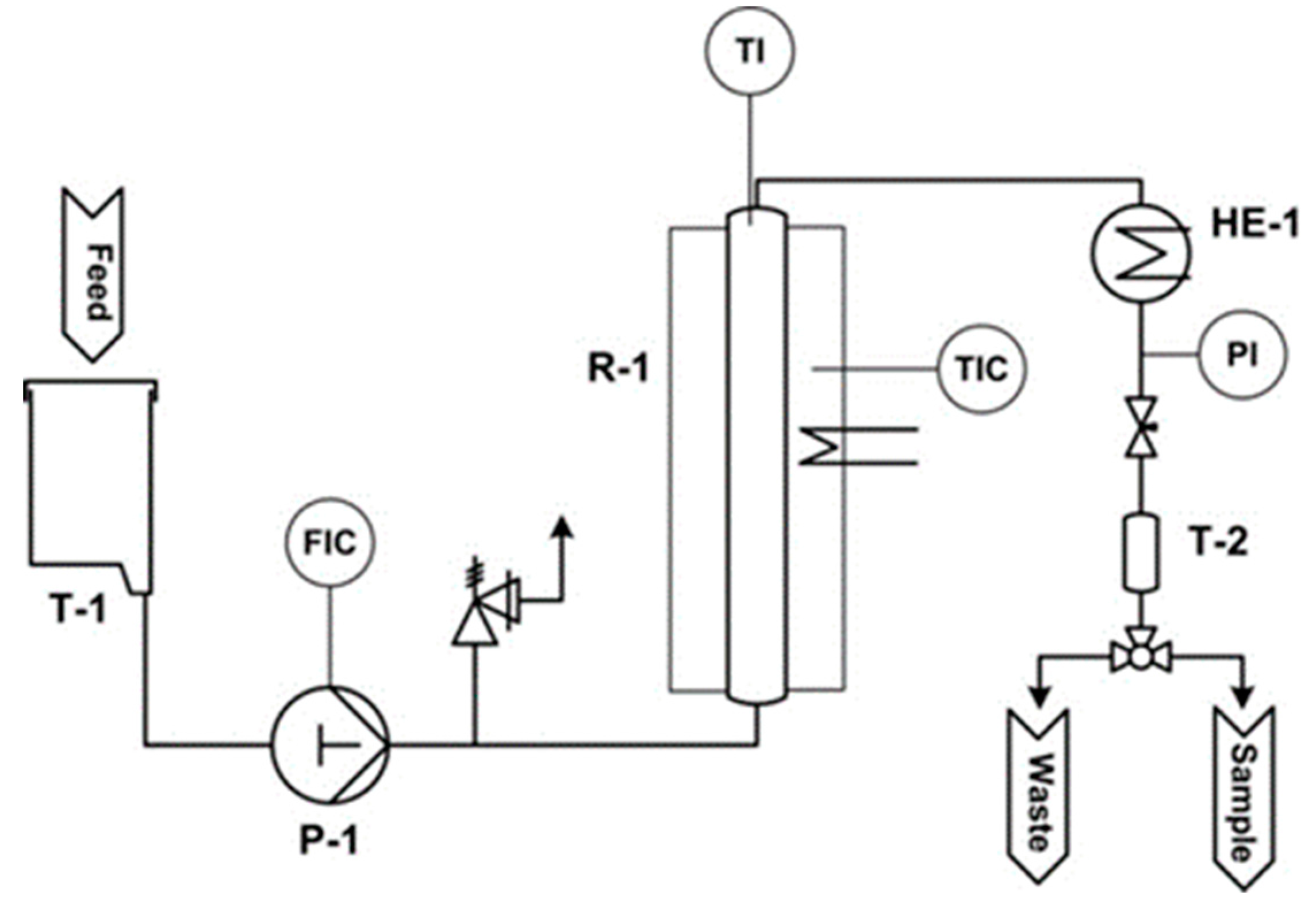
2.2. Acid-Catalyzed Conversion
2.3. Analytics
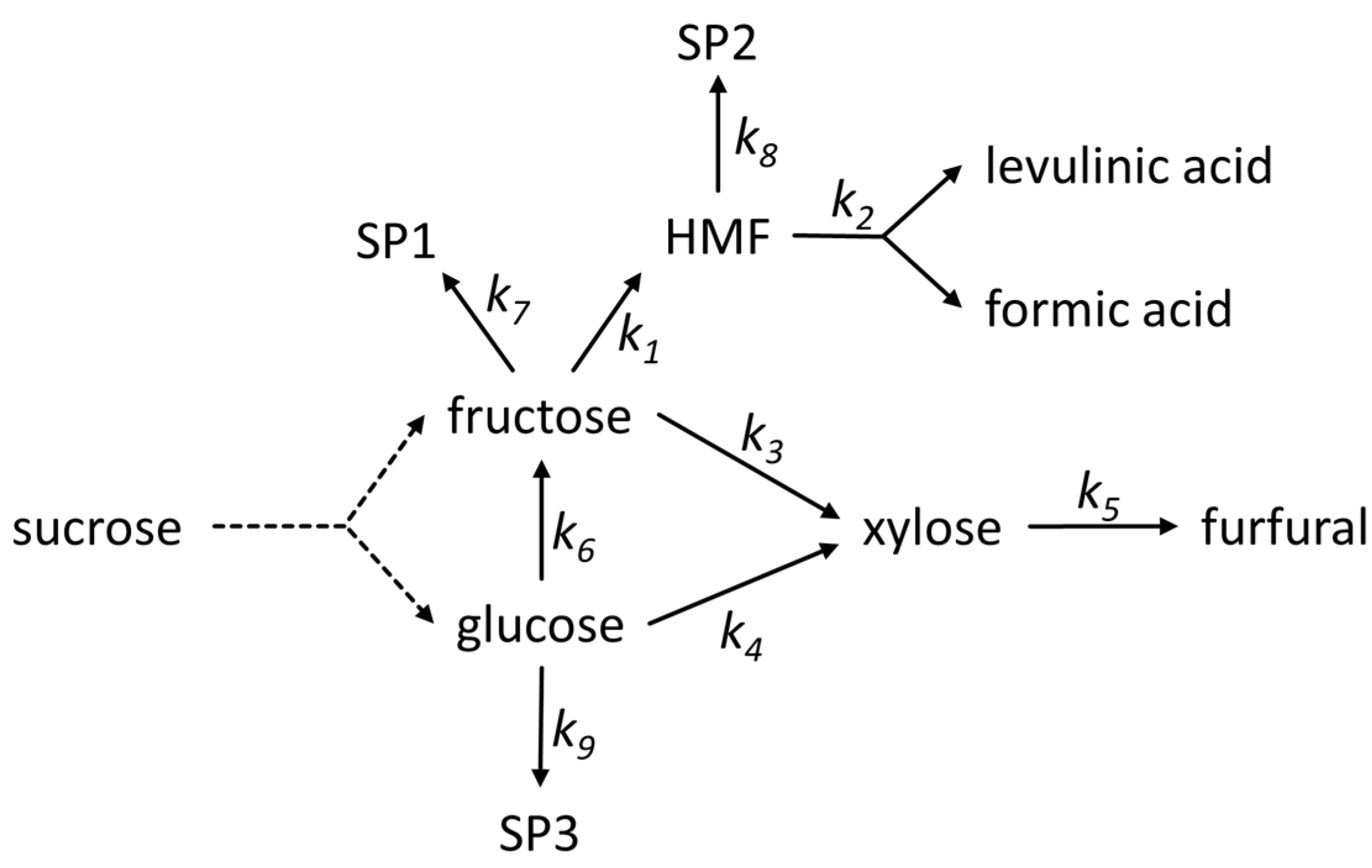
2.4. Reaction Model
2.5. Kinetic Modelling
3. Results and Discussion
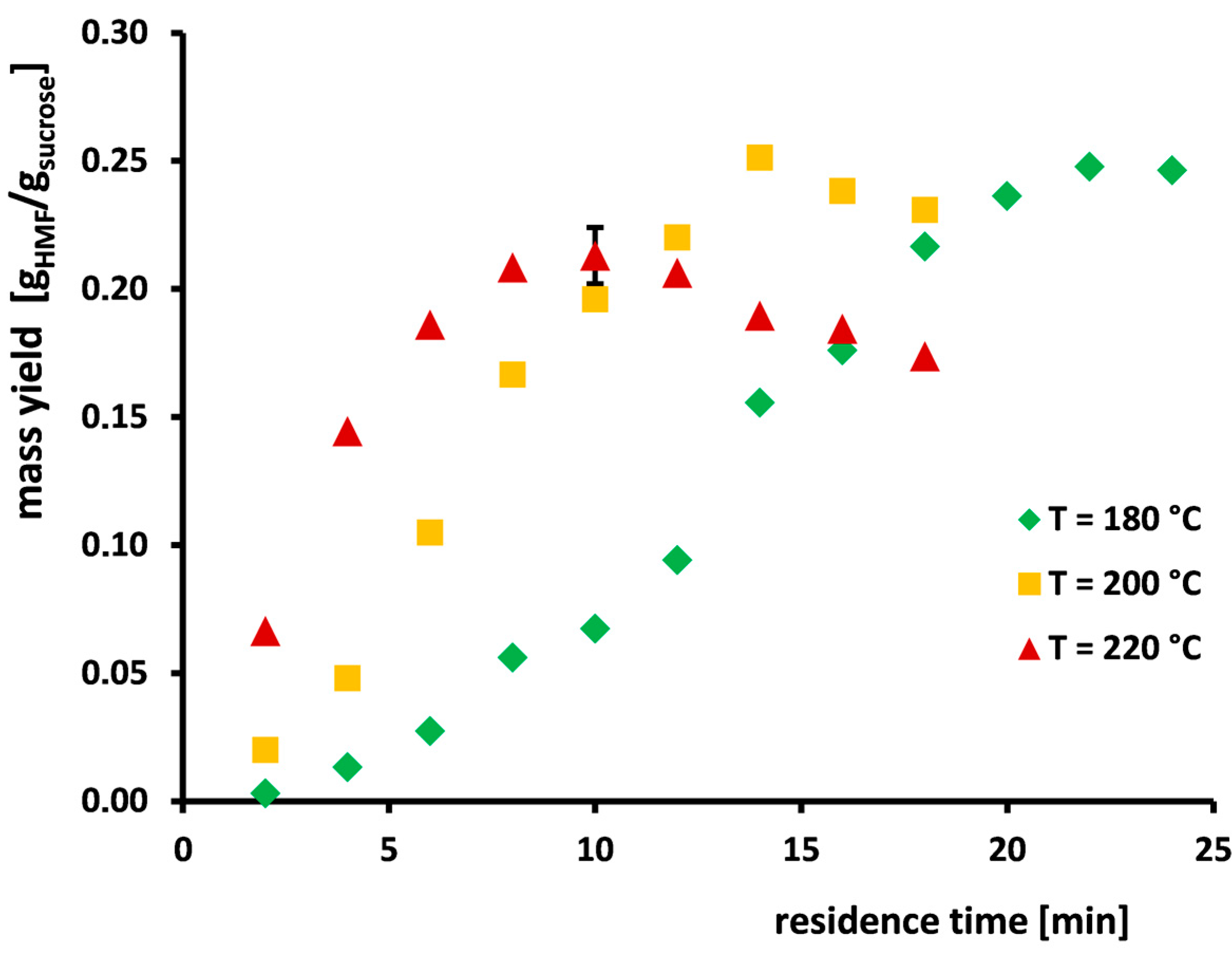
3.1. Maximum Mass Yield of HMF
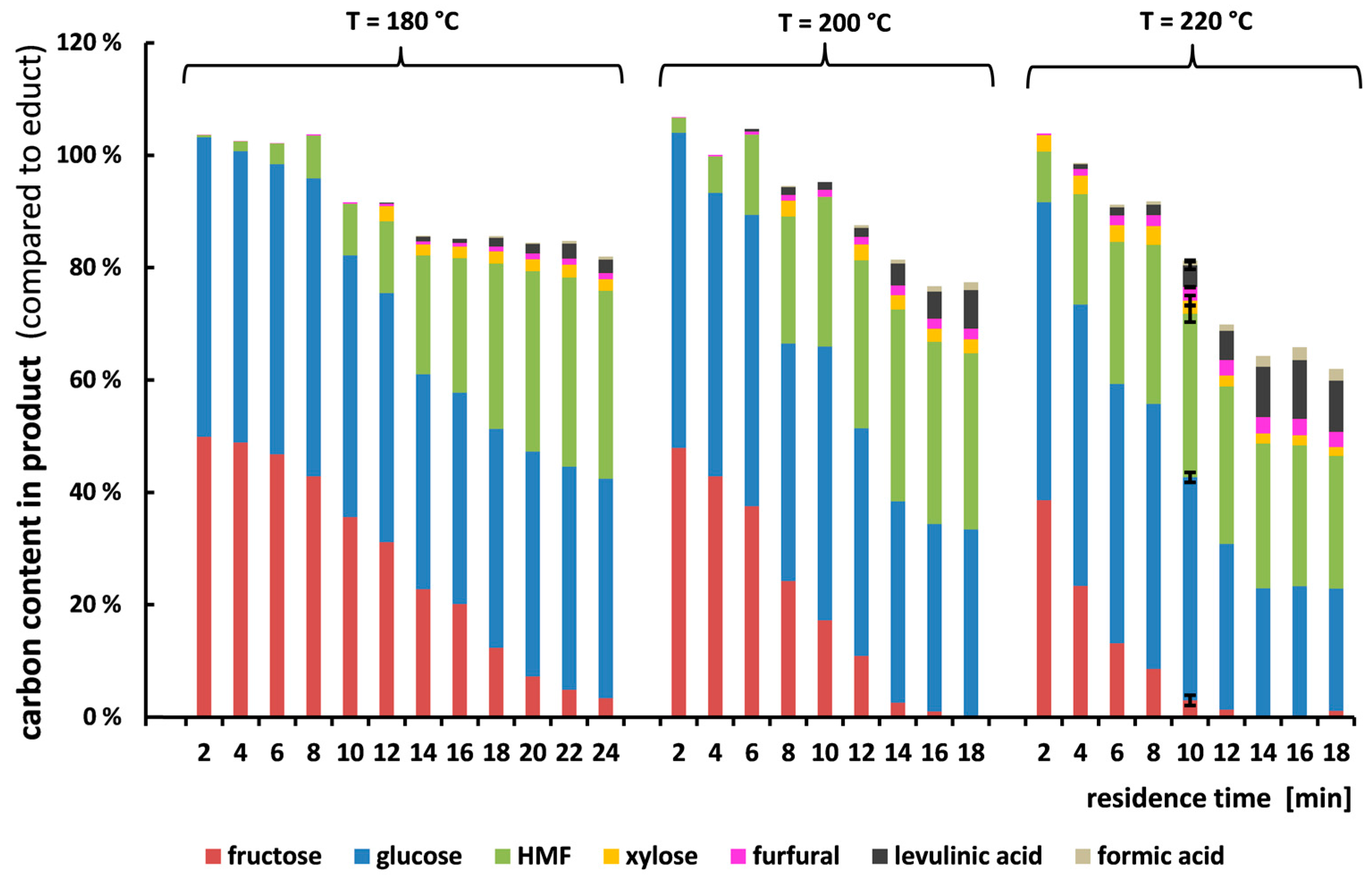
3.2. Carbon Balance of Sucrose Conversion
3.3. Carbon Balance of Glucose and Fructose Conversion
3.4. Modeling Results
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harmsen, P.; Hackmann, M.; Bos, H. Green building blocks for bio-based plastics. Biofuel Bioprod. Biorefin. 2014, 8, 306–324. [Google Scholar] [CrossRef]
- Aeschelmann, F.; Carus, M. Biobased building blocks and polymers in the world: Capacities, production, and applications—Status quo and trends towards 2020. Ind. Biotechnol. 2015, 11, 154–159. [Google Scholar] [CrossRef]
- Van Putten, R.J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Kläusli, T. AVA Biochem: Commercialising renewable platform chemical 5-HMF. Green Process. Synth. 2014, 3. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Steinbach, D.; Kruse, A.; Sauer, J. Pretreatment technologies of lignocellulosic biomass in water in view of furfural and 5-hydroxymethylfurfural production—A review. Biomass Convers. Bior. 2017, 7, 247–274. [Google Scholar] [CrossRef]
- Cottier, L.; Descotes, G. 5-Hydroxymethylfurfural syntheses and chemical transformations. Trends Heterocycl. Chem. 1991, 2, 233–248. [Google Scholar]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Gharpuray, M.M.; Lee, Y.H. Cellulose Hydrolysis; Springer: Berlin, Germany, 1987. [Google Scholar]
- Kruse, A.; Dinjus, E. Hot compressed water as reaction medium and reactant—Properties and synthesis reactions. J. Supercrit. Fluid 2007, 39, 362–380. [Google Scholar] [CrossRef]
- Kruse, A.; Dinjus, E. Hot compressed water as reaction medium and reactant—2. Degradation reactions. J. Supercrit. Fluid 2007, 41, 361–379. [Google Scholar] [CrossRef]
- Van Putten, R.J.; Soetedjo, J.N.M.; Pidko, E.A.; van der Waal, J.C.; Hensen, E.J.M.; de Jong, E.; Heeres, H.J. Dehydration of different ketoses and aldoses to 5-hydroxymethylfurfural. Chemsuschem 2013, 6, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.P. The chemistry involved in the steam treatment of lignocellulosic materials. Quim. Nova 2003, 26, 863–871. [Google Scholar] [CrossRef]
- Scallet, B.L.; Shieh, K.; Ehrenthal, I.; Slapshak, L. Studies in the isomerization of d-glucose. Starch Stärke 1974, 26, 405–408. [Google Scholar] [CrossRef]
- Yan, K.; Liu, Y.; Lu, Y.; Chai, J.; Sun, L. Catalytic application of layered double hydroxide-derived catalysts for the conversion of biomass-derived molecules. Catal. Sci. Technol. 2017, 7, 1622–1645. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Catalytic Isomerization of Biomass-Derived Aldoses: A Review. Chemsuschem 2016, 9, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Sugar: World Markets and Trade. (2016) United States Department of Agriculture. Available online: https://apps.fas.usda.gov/psdonline/circulars/sugar.pdf (accessed on 13 March 2018).
- Antal, M.J.; Mok, W.S.L.; Richards, G.N. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from d-fructose and sucrose. Carbohyd. Res. 1990, 199, 91–109. [Google Scholar] [CrossRef]
- Khajavi, S.H.; Kimura, Y.; Oomori, T.; Matsuno, R.; Adachi, S. Kinetics on sucrose decomposition in subcritical water. Lwt-Food Sci. Technol. 2005, 38, 297–302. [Google Scholar] [CrossRef]
- Bower, S.; Wickramasinghe, R.; Nagle, N.J.; Schell, D.J. Modeling sucrose hydrolysis in dilute sulfuric acid solutions at pretreatment conditions for lignocellulosic biomass. Bioresour. Technol. 2008, 99, 7354–7362. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Kobayashi, T.; Adachi, S. Kinetics of Sucrose Hydrolysis in a Subcritical Water-ethanol Mixture. J. Appl. Glycosci. 2014, 61, 9–13. [Google Scholar] [CrossRef]
- Tan-Soetedjo, J.N.M.; van de Bovenkamp, H.H.; Abdilla, R.M.; Rasrendra, C.B.; van Ginkel, J.; Heeres, H.J. Experimental and Kinetic Modeling Studies on the Conversion of Sucrose to Levulinic Acid and 5-Hydroxymethylfurfural Using Sulfuric Acid in Water. Ind. Eng. Chem. Res. 2017, 56, 13229–13240. [Google Scholar] [CrossRef] [PubMed]
- Fachri, B.A.; Abdilla, R.M.; van de Bovenkamp, H.H.; Rasrendra, C.B.; Heeres, H.J. Experimental and kinetic modeling studies on the sulfuric acid catalyzed conversion of d-fructose to 5-hydroxymethylfurfural and levulinic acid in water. ACS Sustain. Chem. Eng. 2015, 3, 3024–3034. [Google Scholar] [CrossRef]
- Girisuta, B.; Janssen, L.P.B.M.; Heeres, H.J. A kinetic study on the decomposition of 5-hydroxymethylfurfural into levulinic acid. Green Chem. 2006, 8, 701–709. [Google Scholar] [CrossRef]
- Girisuta, B.; Janssen, L.P.B.M.; Heeres, H.J. A kinetic study on the conversion of glucose to levulinic acid. Chem. Eng. Res. Des. 2006, 84, 339–349. [Google Scholar] [CrossRef]
- Torres, A.P.; Oliveira, F.A.R. Application of the acid hydrolysis of sucrose as a temperature indicator in continuous thermal processes. J. Food Eng. 1999, 40, 181–188. [Google Scholar] [CrossRef]
- Smith, P.C.; Grethlein, H.E.; Converse, A.O. Glucose decomposition at high-temperature, mild acid, and short residence times. Sol. Energy 1982, 28, 41–48. [Google Scholar] [CrossRef]
- Saeman, J.F. Kinetics of wood saccharification—Hydrolysis of cellulose and decomposition of sugars in dilute acid at high temperature. Ind. Eng. Chem. 1945, 37, 43–52. [Google Scholar] [CrossRef]
- McKibbins, S.W.; Harris, J.F.; Saeman, J.F.; Neill, W.K. Kinetics of the acid catalyzed conversion of glucose to 5-hydroxymethyl-2-furaldehyde and levulinic acid. For. Prod. J. 1962, 12, 17–23. [Google Scholar]
- Xiang, Q.; Lee, Y.Y.; Torget, R.W. Kinetics of glucose decomposition during dilute-acid hydrolysis of lignocellulosic biomass. Appl. Biochem. Biotechnol. 2004, 113, 1127–1138. [Google Scholar] [CrossRef]
- Asghari, F.S.; Yoshida, H. Acid-catalyzed production of 5-hydroxymethyl furfural from d-fructose in subcritical water. Ind. Eng. Chem. Res. 2006, 45, 2163–2173. [Google Scholar] [CrossRef]
- Asghari, F.S.; Yoshida, H. Kinetics of the decomposition of fructose catalyzed by hydrochloric acid in subcritical water: Formation of 5-hydroxymethylfurfural, levulinic, and formic acids. Ind. Eng. Chem. Res. 2007, 46, 7703–7710. [Google Scholar] [CrossRef]
- Forchheim, D.; Hornung, U.; Kruse, A.; Sutter, T. Kinetic modelling of hydrothermal lignin depolymerisation. Waste Biomass Valori. 2014, 5, 985–994. [Google Scholar] [CrossRef]
- Gasson, J.R.; Forchheim, D.; Sutter, T.; Hornung, U.; Kruse, A.; Barth, T. Modeling the lignin degradation kinetics in an ethanol/formic acid solvolysis approach. Part 1. Kinetic model development. Ind. Eng. Chem. Res. 2012, 51, 10595–10606. [Google Scholar] [CrossRef]
- Clarke, M.A.; Edye, L.A.; Eggleston, G. Sucrose decomposition in aqueous solution, and losses in sugar manufacture and refining. Adv. Carbohyd. Chem. Biochem. 1997, 52, 441–470. [Google Scholar] [CrossRef]
- Usuki, C.; Kimura, Y.; Adachi, S. Isomerization of Hexoses in Subcritical Water. Food Sci. Technol. Res. 2007, 13, 205–209. [Google Scholar] [CrossRef]
- Kabyemela, B.M.; Adschiri, T.; Malaluan, R.M.; Arai, K. Kinetics of glucose epimerization and decomposition in subcritical and supercritical water. Ind. Eng. Chem. Res. 1997, 36, 1552–1558. [Google Scholar] [CrossRef]
- Bonn, G.; Bobleter, O. Determination of the hydrothermal degradation products of d-(U-C14) glucose and d-(U-C-14) fructose by tlc. J. Radioanal. Chem. 1983, 79, 171–177. [Google Scholar] [CrossRef]
- Kabyemela, B.M.; Adschiri, T.; Malaluan, R.M.; Arai, K. Glucose and fructose decomposition in subcritical and supercritical water: Detailed reaction pathway, mechanisms, and kinetics. Ind. Eng. Chem. Res. 1999, 38, 2888–2895. [Google Scholar] [CrossRef]
- Chuntanapum, A.; Matsumura, Y. Formation of tarry material from 5-HMF in subcritical and supercritical water. Ind. Eng. Chem. Res. 2009, 48, 9837–9846. [Google Scholar] [CrossRef]
- Kruse, A.; Grandl, R. Hydrothermal carbonization: 3. Kinetic model. Chem. Ing. Tech. 2015, 87, 449–456. [Google Scholar] [CrossRef]
- Velebil, J.; Malaťák, J.; Bradna, J. Mass yield of biochar from hydrothermal carbonization of sucrose. Res. Agric. Eng. 2016, 62, 179–184. [Google Scholar] [CrossRef]

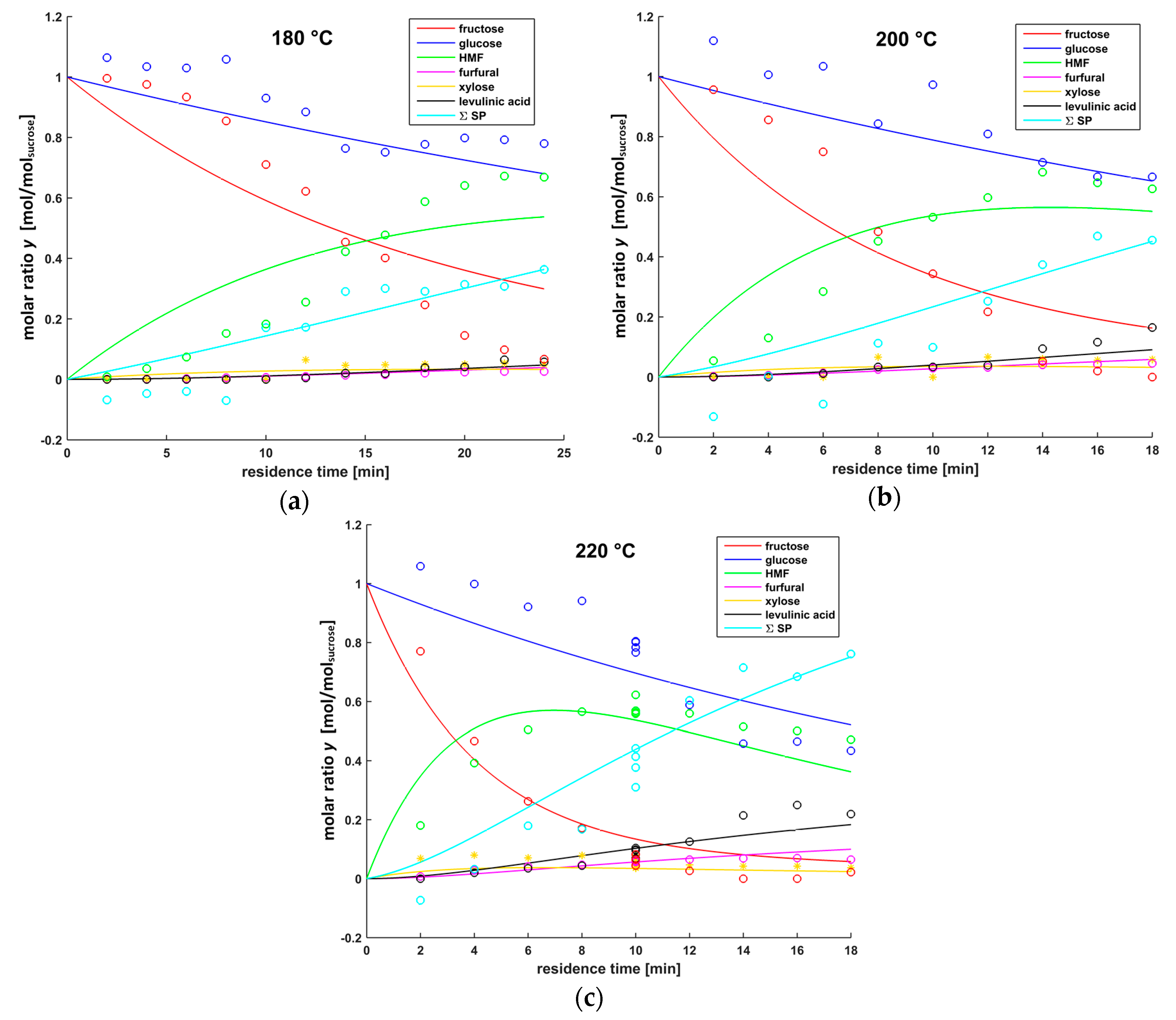

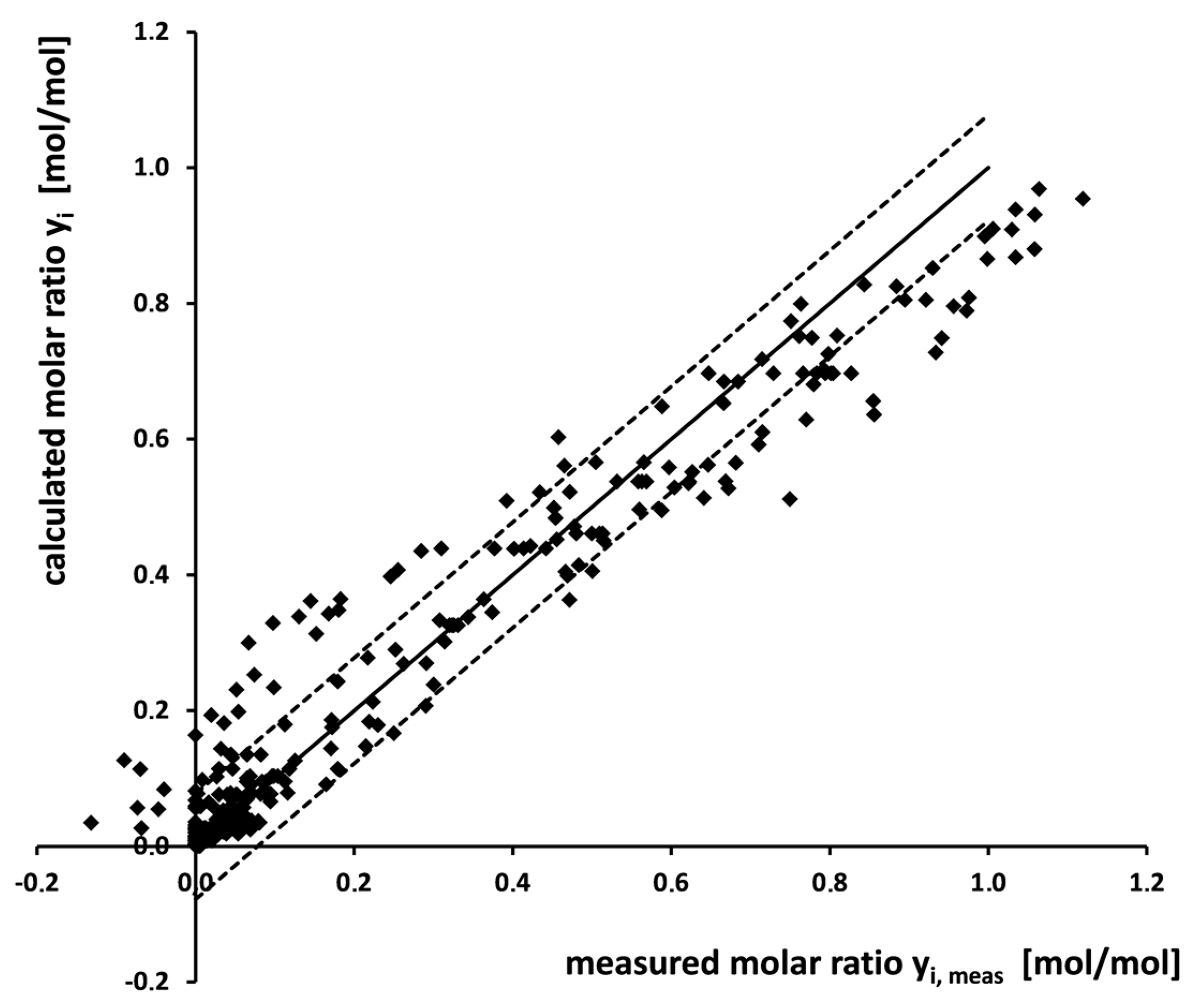
| Reaction | Rate Coefficient kn | at 180 °C [min−1] | at 200 °C [min−1] | at 220 °C [min−1] |
|---|---|---|---|---|
| fructose => HMF | k1 | 0.0525 | 0.116 | 0.241 |
| HMF => levulinic acid + formic acid | k2 | 0.0055 | 0.0114 | 0.0224 |
| fructose => xylose | k3 | 0.0033 | 0.0065 | 0.0123 |
| glucose => xylose | k4 | 0.0014 | 0.0027 | 0.0050 |
| xylose => furfural | k5 | 0.0642 | 0.111 | 0.185 |
| glucose => fructose | k6 | 0.0061 | 0.0116 | 0.0210 |
| fructose => SP1 | k7 | 0.0042 | 0.0047 | 0.0052 |
| HMF => SP2 | k8 | 0.0156 | 0.0347 | 0.0721 |
| glucose => SP3 | k9 | 0.0086 | 0.0094 | 0.0102 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinbach, D.; Kruse, A.; Sauer, J.; Vetter, P. Sucrose Is a Promising Feedstock for the Synthesis of the Platform Chemical Hydroxymethylfurfural. Energies 2018, 11, 645. https://doi.org/10.3390/en11030645
Steinbach D, Kruse A, Sauer J, Vetter P. Sucrose Is a Promising Feedstock for the Synthesis of the Platform Chemical Hydroxymethylfurfural. Energies. 2018; 11(3):645. https://doi.org/10.3390/en11030645
Chicago/Turabian StyleSteinbach, David, Andrea Kruse, Jörg Sauer, and Philipp Vetter. 2018. "Sucrose Is a Promising Feedstock for the Synthesis of the Platform Chemical Hydroxymethylfurfural" Energies 11, no. 3: 645. https://doi.org/10.3390/en11030645






