Experimental Study of Matrix Permeability of Gas Shale: An Application to CO2-Based Shale Fracturing
Abstract
:1. Introduction
2. Experimental Methodology
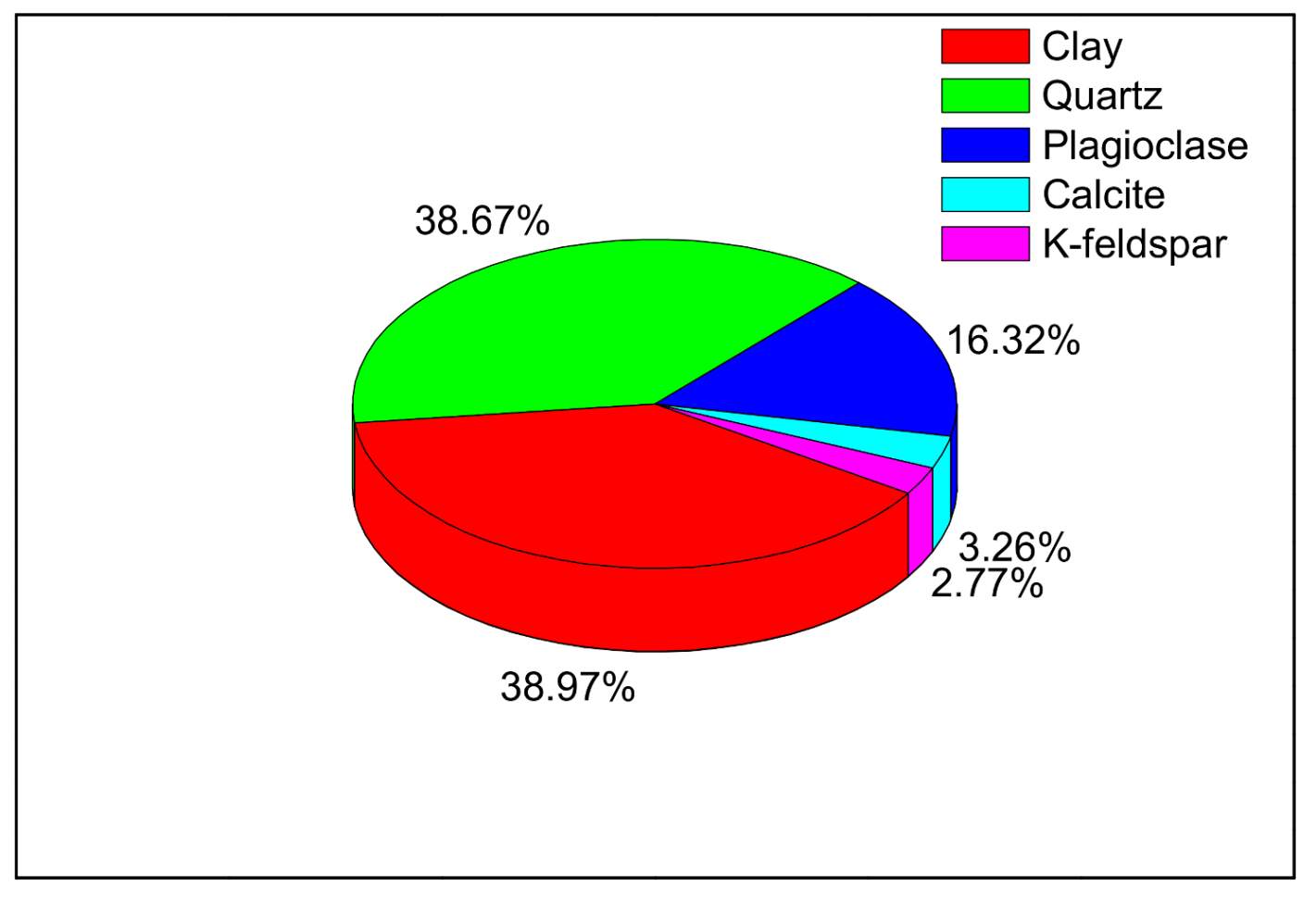
2.1. Samples Description
2.2. Experimental Set-Up
2.3. Experimental Procedure
2.3.1. Steady-State Conditions and Transient Conditions
2.3.2. Effect of Water Flooding
3. Comparison of Permeability between Steady-State and Transient Conditions
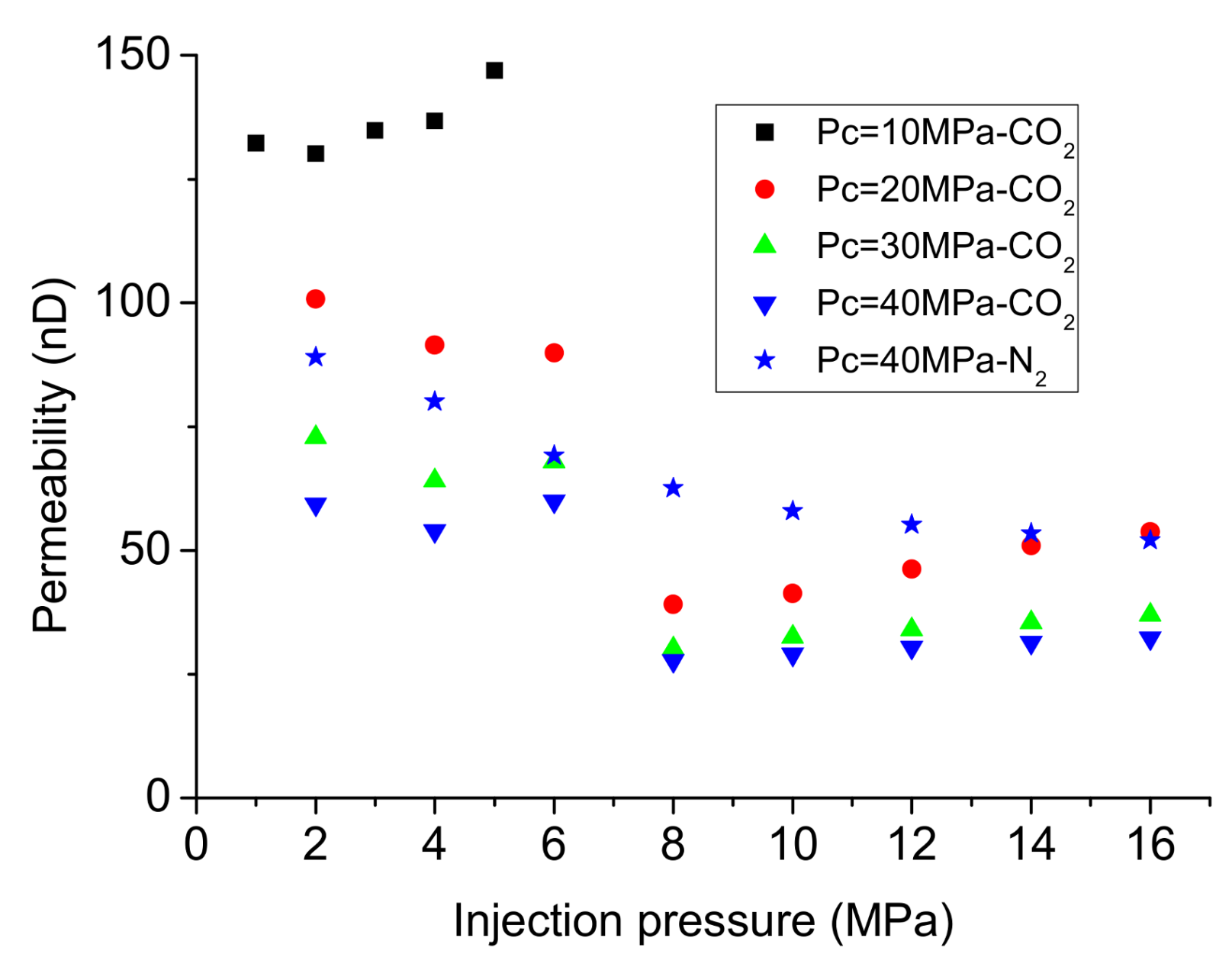
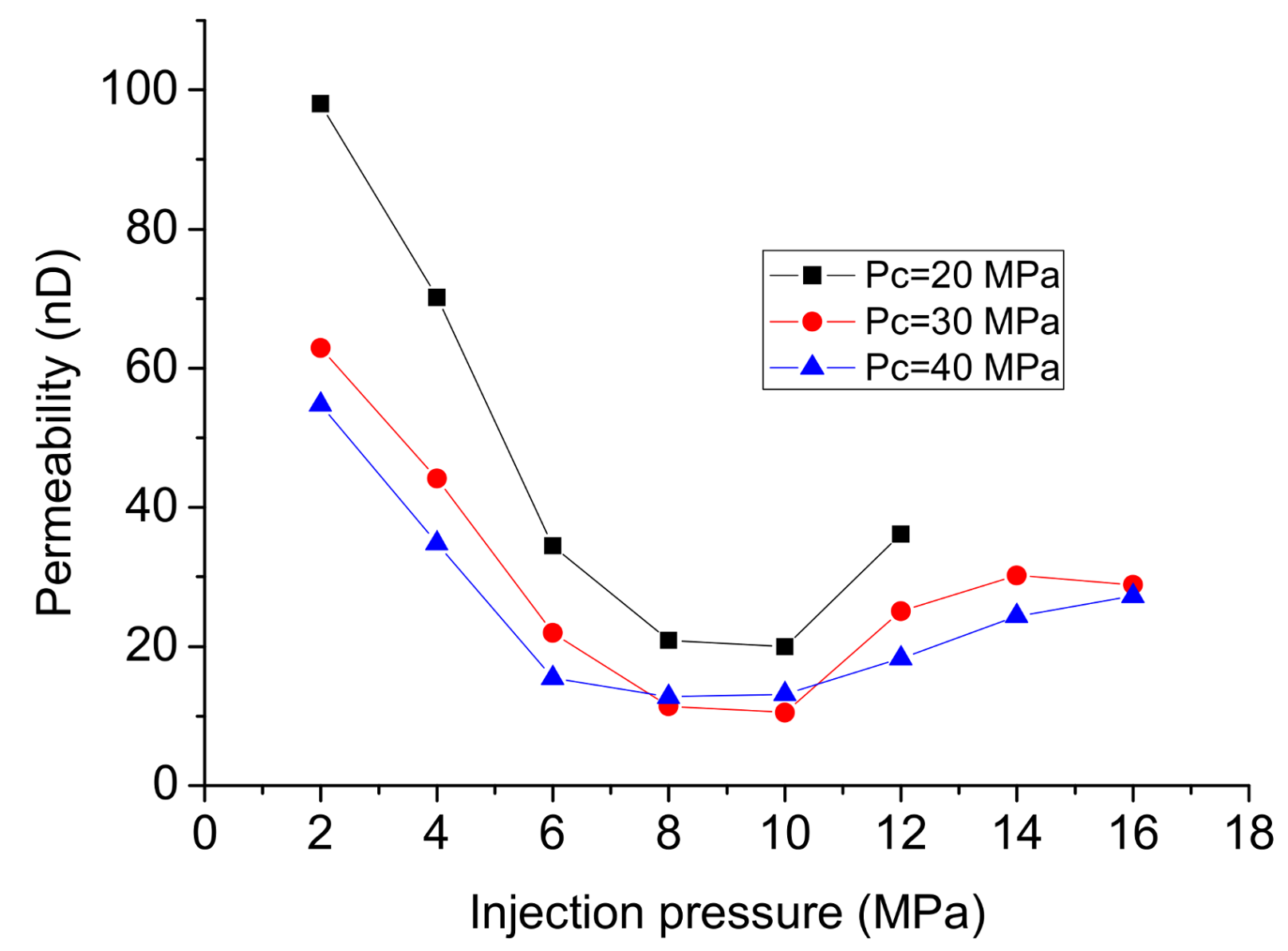
3.1. Permeability Testing under Steady-State Conditions
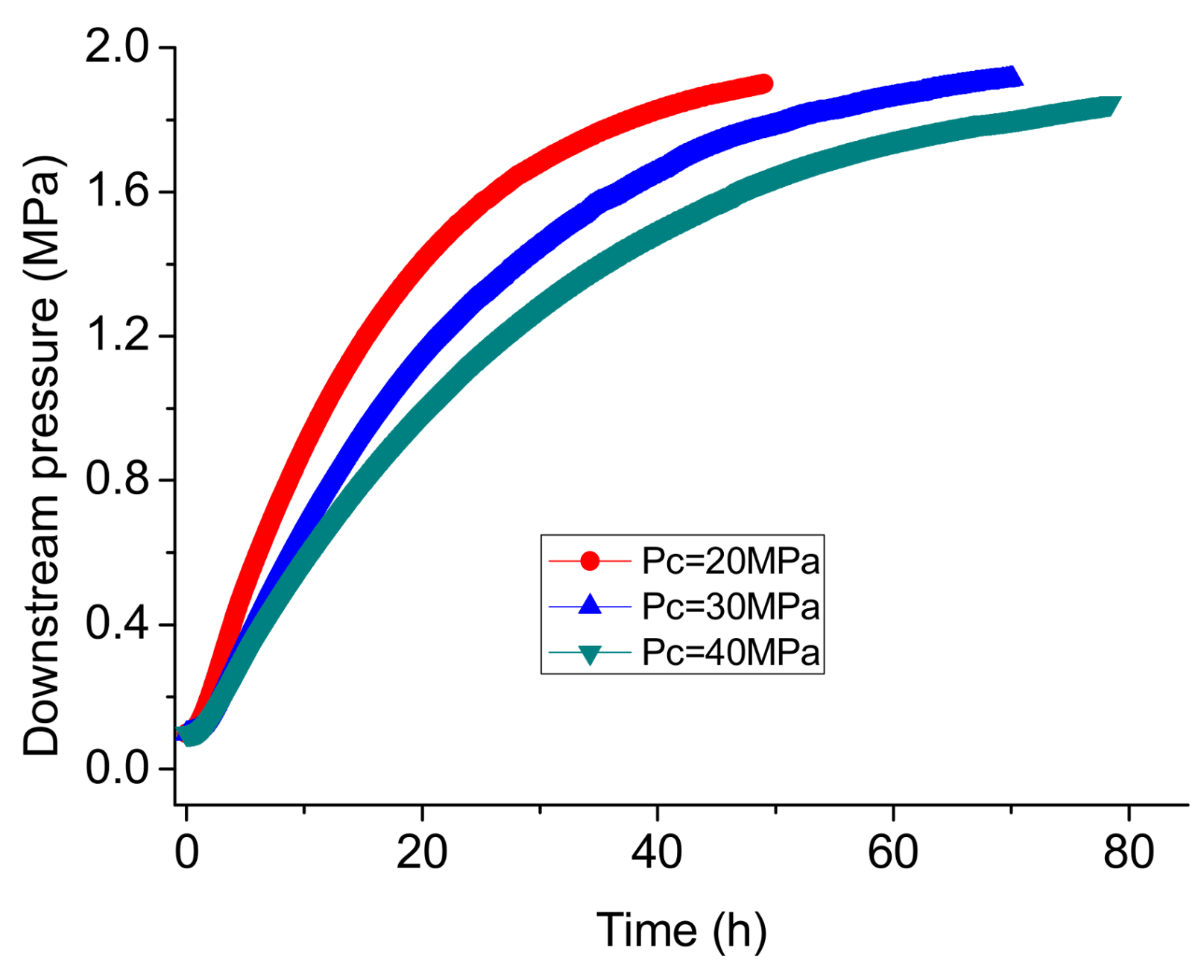
3.2. Permeability Testing under Transient Conditions
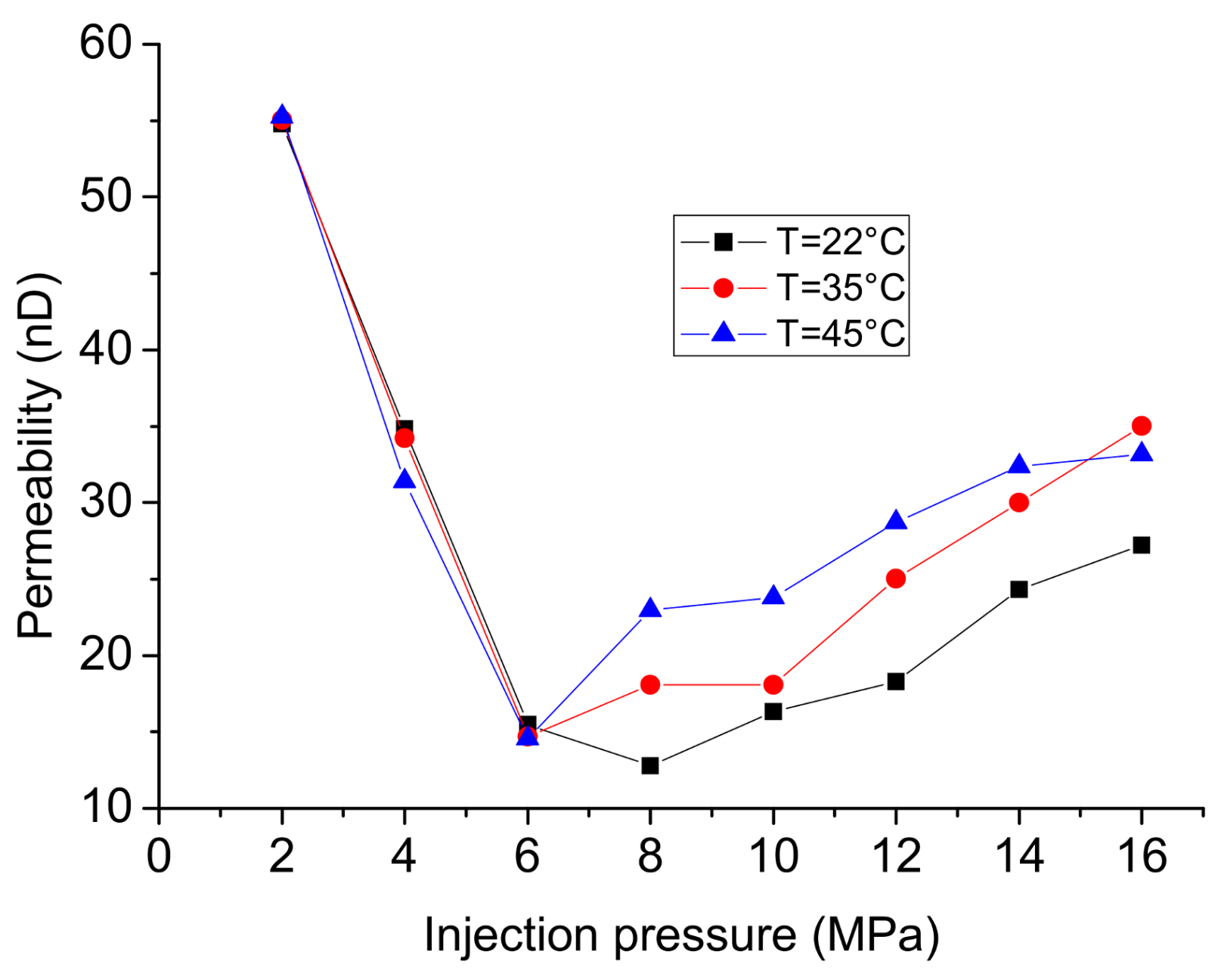
4. Effect of Temperature
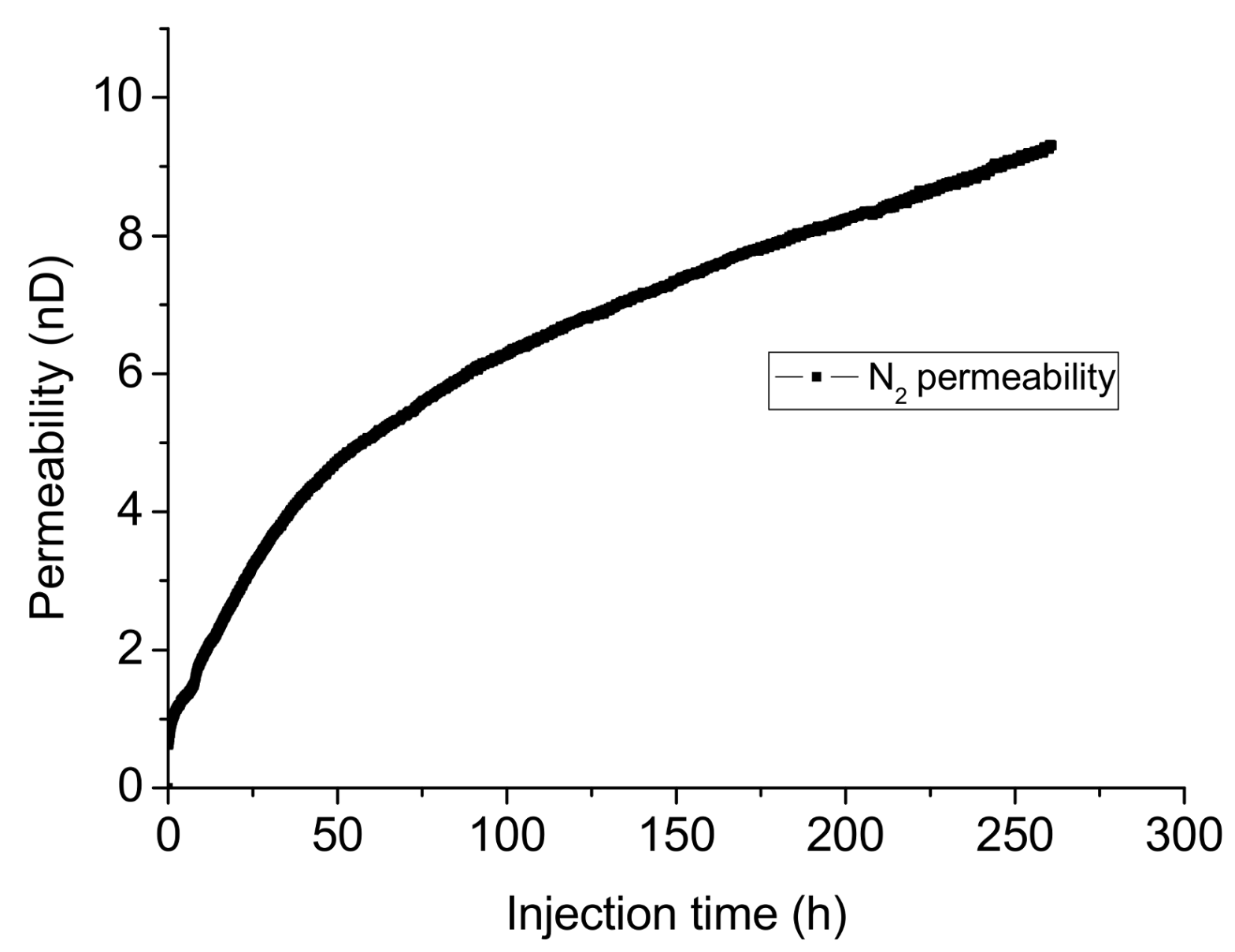
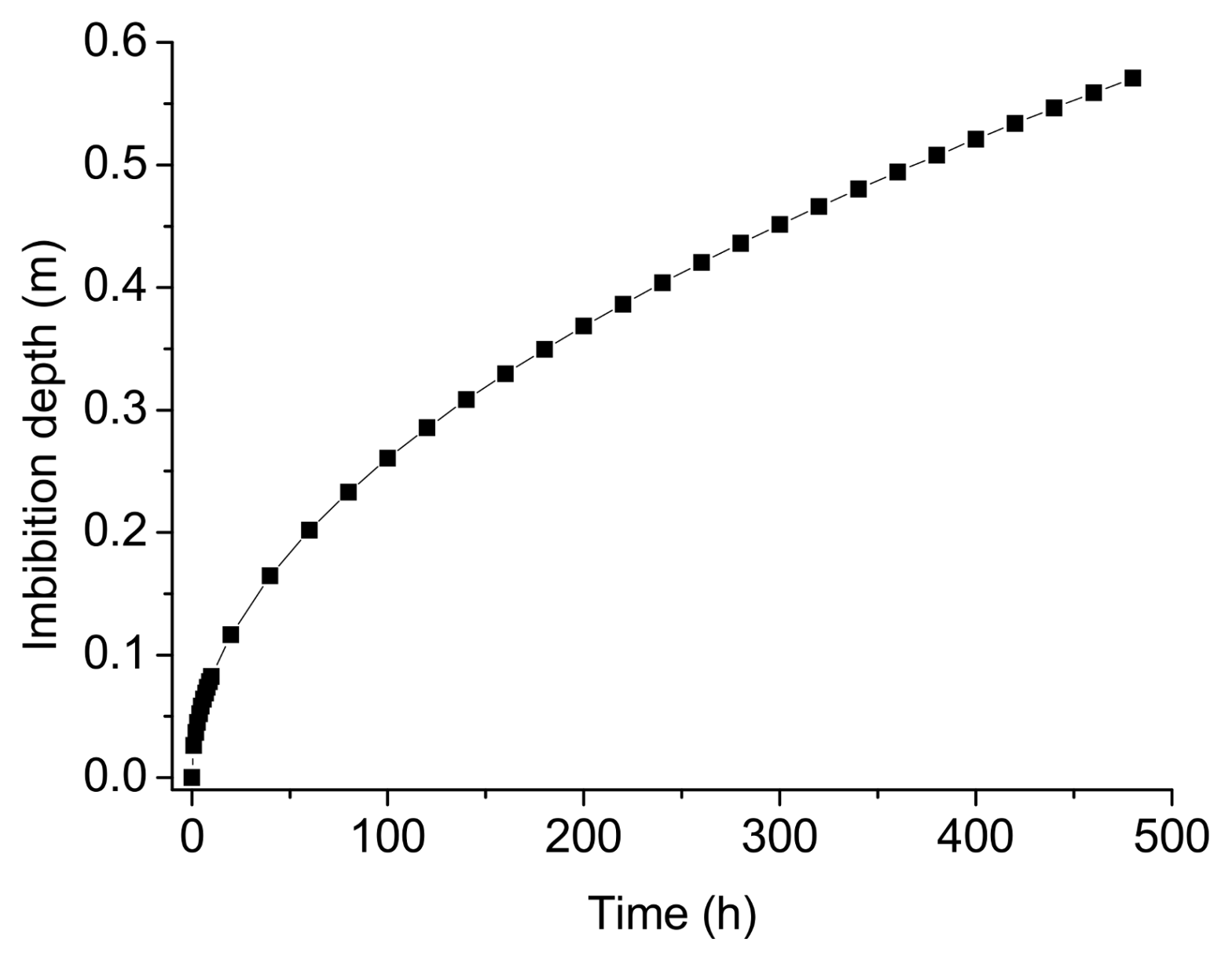
5. Effect of Water Flooding
6. Conclusions
- (1)
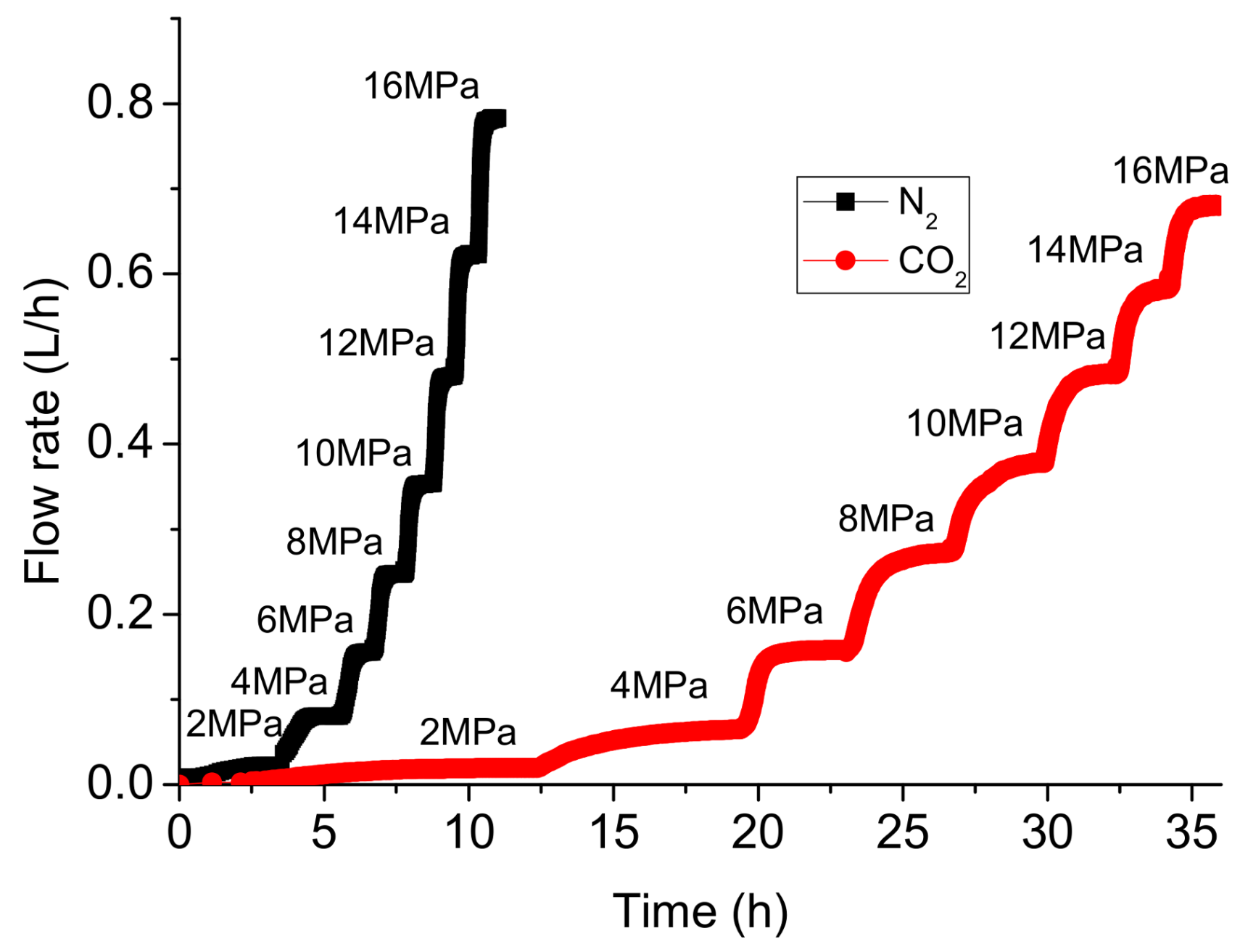
- Although the viscosity of N2 is slightly higher than that of gaseous CO2 when the injection pressure is lower than 6 MPa, the flow rates for N2 are higher than that for CO2 and the pressure equilibrium time for N2 is much shorter than CO2. This is possibly due to the higher adsorption capacity of CO2, because the flow channels for CO2 are squeezed by the thin adsorption layer, the shale permeability to gaseous CO2 measured using N2 is therefore over-valued.
- (2)
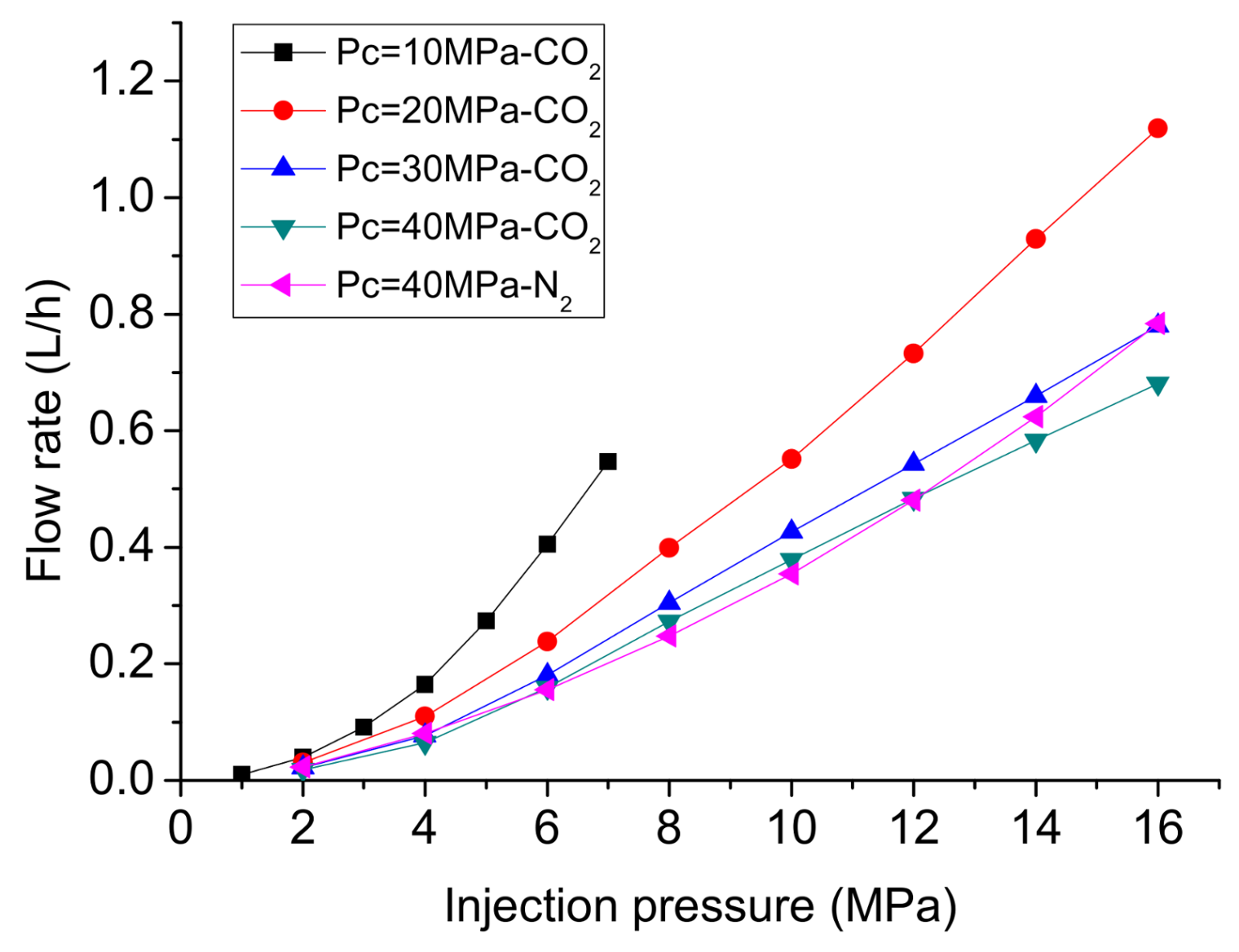
- In permeability tests using CO2 under steady-state conditions, phase transition can greatly influence the flow behavior of CO2, and the linear relationship between flow rate and injection pressure under high injection pressures indicates that the permeability to liquid CO2 is much lower than that to gaseous CO2. Permeability tests under transient conditions can successfully avoid the influence of the Klinkenberg effect at low pressure on higher injection pressures, and are more suitable for the hydraulic fracturing. The permeability for liquid CO2 under transient conditions is close to that for water, which can be regarded as the intrinsic permeability of the shale matrix.
- (3)
- The high temperatures of shale formations transform the injected CO2 into super-critical state, possibly due to the higher molecular energy, and the collisions between CO2 molecules and pore walls promote its flow rate, leading to a slightly higher permeability at higher temperatures. The lower density of super-critical CO2 can reduce the leak-off rate in terms of mass, while in contrast, its lower viscosity and higher permeability promote the leak-off rate in volume and dominate the effect of temperature on its leak-off. For example, leak-off in terms of mass increases around 40% at 16 MPa with the increase of temperature from 22 °C to 45 °C.
- (4)
- Due to the blocking effect of water, the much lower permeability of shale saturated by water can seriously reduce the gas production rate, and the very long time for recovery reduces the economic benefits of shale gas, which highlights the advantage of liquid CO2 as fracturing fluid. According to the Darcy’s law, the leak-off rate in mass of CO2 is around an order of magnitude higher than that of water, which indicates higher requirements for injection rate and total consumption. In contrast, the damaged zone formed by the injection of high viscosity fluid after CO2 injection in CO2 sequestration projects can significantly reduce the risk of CO2 leakage through the created fracture system.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Conti, J.; Holtberg, P.D.; Beamon, J.A.; Schaal, A.M.; Ayoub, J.C.; Turnure, J.T. Annual Energy Outlook 2014 with Projections to 2040; US Energy Information Administration: Washington, DC, USA, 2014.
- Rivard, C.; Lavoie, D.; Lefebvre, R.; Séjourné, S.; Lamontagne, C.; Duchesne, M. An overview of canadian shale gas production and environmental concerns. Int. J. Coal Geol. 2014, 126, 64–76. [Google Scholar] [CrossRef]
- Wanniarachchi, W.A.M.; Gamage, R.P.; Perera, M.S.A.; Rathnaweera, T.D.; Gao, M.; Padmanabhan, E. Investigation of depth and injection pressure effects on breakdown pressure and fracture permeability of shale reservoirs: An experimental study. Appl. Sci. 2017, 7, 664. [Google Scholar] [CrossRef]
- Zhang, C.; Ranjith, P.; Perera, M. Investigation of Flow Behavior through Hydraulic Fractures in Unconventional Gas Reservoirs Under Tri-Axial Drained Conditions. In Proceedings of the 51st US Rock Mechanics/Geomechanics Symposium, San Francisco, CA, USA, 25–28 June 2017; American Rock Mechanics Association (ARMA): Alexandria, VA, USA, 2017; pp. 17–99. [Google Scholar]
- Zhang, J.; Kamenov, A.; Zhu, D.; Hill, A. Development of new testing procedures to measure propped fracture conductivity considering water damage in clay-rich shale reservoirs: An example of the barnett shale. J. Pet. Sci. Eng. 2015, 135, 352–359. [Google Scholar] [CrossRef]
- Bostrom, N.; Chertov, M.; Pagels, M.; Willberg, D.; Chertova, A.; Davis, M.; Zagorski, W. The Time-Dependent Permeability Damage Caused by Fracture Fluid. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 26–28 February 2014; Society of Petroleum Engineers (SPE): Denver, CO, USA, 2014. [Google Scholar]
- Zhang, C.; Pathegama Gamage, R.; Perera, M.; Zhao, J. Characteristics of clay-abundant shale formations: Use of CO2 for production enhancement. Energies 2017, 10, 1887. [Google Scholar] [CrossRef]
- Johnson, E.G.; Johnson, L.A. Hydraulic fracture water usage in northeast british columbia: Locations, volumes and trends. Geosci. Rep. 2012, 41–63. [Google Scholar]
- Scholtens, B. Testing the Impact of the Anti-Fracking Movement in Changing Public Policy. Bachelor’s Thesis, Leiden University, Leiden, The Netherlands, 2017. [Google Scholar]
- Lillies, A.T.; King, S.R. Sand Fracturing with Liquid Carbon Dioxide. In Proceedings of the SPE Production Technology Symposium, Hobbs, NM, USA, 8–9 November 1982; Society of Petroleum Engineers (SPE): Denver, CO, USA, 1982. [Google Scholar]
- Bennour, Z.; Watanabe, S.; Chen, Y.; Ishida, T.; Akai, T. Evaluation of stimulated reservoir volume in laboratory hydraulic fracturing with oil, water and liquid carbon dioxide under microscopy using the fluorescence method. Geomech. Geophys. Geo-Energy Geo-Resour. 2017, 4, 39–50. [Google Scholar] [CrossRef]
- Li, X.; Feng, Z.; Han, G.; Elsworth, D.; Marone, C.; Saffer, D.; Cheon, D.-S. Breakdown pressure and fracture surface morphology of hydraulic fracturing in shale with H2O, CO2 and N2. Geomech. Geophys. Geo-Energy Geo-Resour. 2016, 2, 63–76. [Google Scholar] [CrossRef]
- Middleton, R.S.; Carey, J.W.; Currier, R.P.; Hyman, J.D.; Kang, Q.; Karra, S.; Jiménez-Martínez, J.; Porter, M.L.; Viswanathan, H.S. Shale gas and non-aqueous fracturing fluids: Opportunities and challenges for supercritical CO2. Appl. Energy 2015, 147, 500–509. [Google Scholar] [CrossRef]
- Nicot, J.-P.; Scanlon, B.R. Water use for shale-gas production in Texas, US. Environ. Sci. Technol. 2012, 46, 3580–3586. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Lee, H.; Li, J. Modeling of Land Movement Due to Groundwater Pumping from an Aquifer System with Stress-Dependent Storage. In Proceedings of the Shale Energy Engineering 2014: Technical Challenges, Environmental Issues, and Public Policy, Pittsburgh, PA, USA, 21–23 July 2014; pp. 1–10. [Google Scholar]
- Vengosh, A.; Warner, N.; Jackson, R.; Darrah, T. The effects of shale gas exploration and hydraulic fracturing on the quality of water resources in the United States. Procedia Earth Planet. Sci. 2013, 7, 863–866. [Google Scholar] [CrossRef]
- Ziemkiewicz, P.F.; He, Y.; Quaranta, J.D. Characterization of Waste Waters from Hydraulic Fracturing. In Proceedings of the Shale Energy Engineering 2014: Technical Challenges, Environmental Issues, and Public Policy, Pittsburgh, PA, USA, 21–23 July 2014; pp. 63–73. [Google Scholar]
- Chareonsuppanimit, P.; Mohammad, S.A.; Robinson, R.L., Jr.; Gasem, K.A. High-pressure adsorption of gases on shales: Measurements and modeling. Int.J. Coal Geol. 2012, 95, 34–46. [Google Scholar] [CrossRef]
- Godec, M.; Koperna, G.; Petrusak, R.; Oudinot, A. Potential for enhanced gas recovery and CO2 storage in the Marcellus Shale in the Eastern United States. Int. J. Coal Geol. 2013, 118, 95–104. [Google Scholar] [CrossRef]
- Arnold, D. Liquid CO2 and sand: An alternative to water-based stimulation fluids. Pet. Eng. Int. 1998, 71, 89–92. [Google Scholar]
- Fathi, E.; Akkutlu, I.Y. Multi-component gas transport and adsorption effects during CO2 injection and enhanced shale gas recovery. Int. J. Coal Geol. 2014, 123, 52–61. [Google Scholar] [CrossRef]
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Reinicke, A.; Rybacki, E.; Stanchits, S.; Huenges, E.; Dresen, G. Hydraulic fracturing stimulation techniques and formation damage mechanisms—Implications from laboratory testing of tight sandstone–proppant systems. Chem. der Erde-Geochem. 2010, 70, 107–117. [Google Scholar] [CrossRef]
- Ghanizadeh, A.; Bhowmik, S.; Haeri-Ardakani, O.; Sanei, H.; Clarkson, C.R. A comparison of shale permeability coefficients derived using multiple non-steady-state measurement techniques: Examples from the Duvernay Formation, Alberta (Canada). Fuel 2015, 140, 371–387. [Google Scholar] [CrossRef]
- Zhang, C.; Ranjith, P.; Perera, M.; Zhao, J.; Zhang, D.; Wanniarachchi, W. A novel approach to precise evaluation of carbon dioxide flow behaviour in siltstone under tri-axial drained conditions. J. Nat. Gas Sci. Eng. 2016, 34, 331–340. [Google Scholar] [CrossRef]
- Tanikawa, W.; Shimamoto, T. Klinkenberg effect for gas permeability and its comparison to water permeability for porous sedimentary rocks. Hydrol. Earth Syst. Sci. Discuss. 2006, 3, 1315–1338. [Google Scholar] [CrossRef]
- Sander, R.; Pan, Z.; Connell, L.D. Laboratory measurement of low permeability unconventional gas reservoir rocks: A review of experimental methods. J. Nat. Gas Sci. Eng. 2017, 37, 248–279. [Google Scholar] [CrossRef]
- Xiao, Z.; Yang, R.; Feng, T.; Cao, Y.; Wang, Q.; Yang, T.; Wang, Z.; Deng, Y. Reservoir-forming conditions and exploration potential of shale gas in Lower Cambrian Niutitang Formation, Northwestern Hunan. J. Hunan Univ. Sci. Technol. 2012, 27, 50–54. [Google Scholar]
- Xing, L.; Xi, Y.; Jiehui, Z.; Honglin, S. Reservoir forming conditions and favorable exploration zones of shale gas in the Weixin sag, Dianqianbei Depression. Pet. Explor. Dev. 2011, 38, 693–699. [Google Scholar] [CrossRef]
- Bhandari, A.R.; Flemings, P.B.; Polito, P.J.; Cronin, M.B.; Bryant, S.L. Anisotropy and stress dependence of permeability in the Barnett shale. Transp. Porous Media 2015, 108, 393–411. [Google Scholar] [CrossRef]
- Zhou, Z.; Abass, H.; Li, X.; Teklu, T. Experimental investigation of the effect of imbibition on shale permeability during hydraulic fracturing. J. Nat. Gas Sci. Eng. 2016, 29, 413–430. [Google Scholar] [CrossRef]
- De Silva, G.; Ranjith, P.; Perera, M.; Chen, B. Effect of bedding planes, their orientation and clay depositions on effective re-injection of produced brine into clay rich deep sandstone formations: Implications for deep earth energy extraction. Appl. Energy 2016, 161, 24–40. [Google Scholar] [CrossRef]
- Kang, S.M.; Fathi, E.; Ambrose, R.J.; Akkutlu, I.Y.; Sigal, R.F. Carbon dioxide storage capacity of organic-rich shales. SPE J. 2011, 16, 842–855. [Google Scholar] [CrossRef]
- Kollek, J. The determination of the permeability of concrete to oxygen by the Cembureau method—A recommendation. Mater. Struct. 1989, 22, 225–230. [Google Scholar] [CrossRef]
- Heller, R.; Vermylen, J.; Zoback, M. Experimental investigation of matrix permeability of gas shales. AAPG Bull. 2014, 98, 975–995. [Google Scholar] [CrossRef]
- Fridleifsson, I.B.; Bertani, R.; Huenges, E.; Lund, J.W.; Ragnarsson, A.; Rybach, L. The Possible Role and Contribution of Geothermal Energy to the Mitigation of Climate Change. In Proceedings of the IPCC Scoping Meeting on Renewable Energy Sources, Luebeck, Germany, 20–25 January 2008; pp. 59–80. [Google Scholar]
- Niezgoda, T.; Miedzińska, D.; Małek, E.; Kędzierski, P.; Sławiński, G. Study on carbon dioxide thermodynamic behavior for the purpose of shale rock fracturing. Bull. Pol. Acad. Sci. 2013, 61, 605–612. [Google Scholar] [CrossRef]
- Lemmon, E.; Huber, M.; McLinden, M. NIST Standard Reference Database 23, NIST Reference Fluid Thermodynamic and Transport Properties, REFPROP, Version 9.0, Standard Reference Data Program; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2010.
- Klinkenberg, L. The Permeability of Porous Media to Liquids and Gases; Drilling and Production Practice; American Petroleum Institute: Tulsa, OK, USA, 1941; pp. 200–213. [Google Scholar]
- Javadpour, F.; Fisher, D.; Unsworth, M. Nanoscale gas flow in shale gas sediments. J. Can. Pet. Technol. 2007, 46, 55–61. [Google Scholar] [CrossRef]
- Kondash, A.J.; Albright, E.; Vengosh, A. Quantity of flowback and produced waters from unconventional oil and gas exploration. Sci. Total Environ. 2017, 574, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, C.R.; Jensen, J.L.; Pedersen, P.K.; Freeman, M. Innovative methods for flow-unit and pore-structure analyses in a tight siltstone and shale gas reservoir. AAPG Bull. 2012, 96, 355–374. [Google Scholar] [CrossRef]
- Gu, H.; Lecerf, B.; Weng, X.; Kresse, O. Effect of Fracture Breakdown Pressure on Multicluster Hydraulic Fracturing Treatments. In Proceedings of the 49th US Rock Mechanics/Geomechanics Symposium, San Francisco, CA, USA, 28 June–1 July 2015; American Rock Mechanics Association (ARMA): Alexandria, VA, USA, 2015. [Google Scholar]
- Campbell, S.; Fairchild, N., Jr.; Arnold, D. Liquid CO2 and Sand Stimulations in the Lewis Shale San Juan Basin New Mexico: A Case Study. In Proceedings of the SPE Rocky Mountain Regional/Low-Permeability Reservoirs Symposium and Exhibition, Denver, CO, USA, 12–15 March 2000; Society of Petroleum Engineers: Denver, CO, USA, 2000. [Google Scholar]
- Nuttall, B.C.; Eble, C.F.; Drahovzal, J.A.; Bustin, R.M. Analysis of Devonian Black Shales in Kentucky for Potential Carbon Dioxide Sequestration and Enhanced Natural Gas Production; Kentucky Geological Survey Report DE-FC26-02NT41442; University of Kentucky: Lexington, KY, USA, 2005. [Google Scholar]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Ranjith, P.G. Experimental Study of Matrix Permeability of Gas Shale: An Application to CO2-Based Shale Fracturing. Energies 2018, 11, 702. https://doi.org/10.3390/en11040702
Zhang C, Ranjith PG. Experimental Study of Matrix Permeability of Gas Shale: An Application to CO2-Based Shale Fracturing. Energies. 2018; 11(4):702. https://doi.org/10.3390/en11040702
Chicago/Turabian StyleZhang, Chengpeng, and Pathegama Gamage Ranjith. 2018. "Experimental Study of Matrix Permeability of Gas Shale: An Application to CO2-Based Shale Fracturing" Energies 11, no. 4: 702. https://doi.org/10.3390/en11040702




