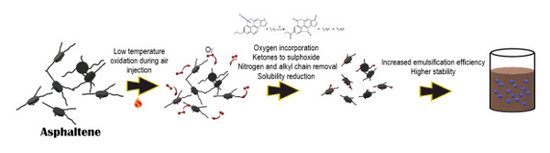
Effect of the Asphaltene Oxidation Process on the Formation of Emulsions of Water in Oil (W/O) Model Solutions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Asphaltenes
2.1.1. Thermogravimetric Analysis
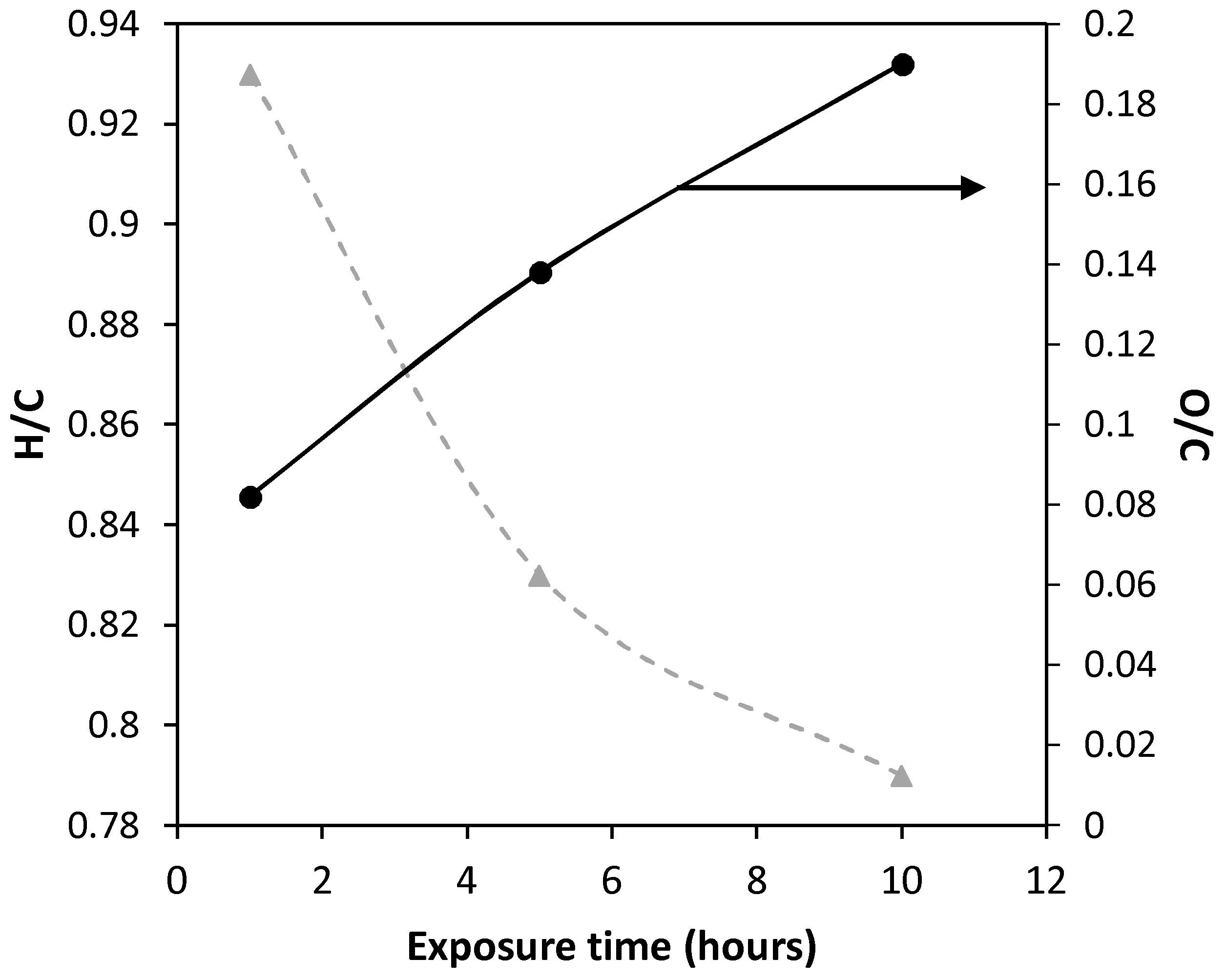
2.1.2. Elemental Analysis
2.1.3. Solubility of Thermally Modified Asphaltene
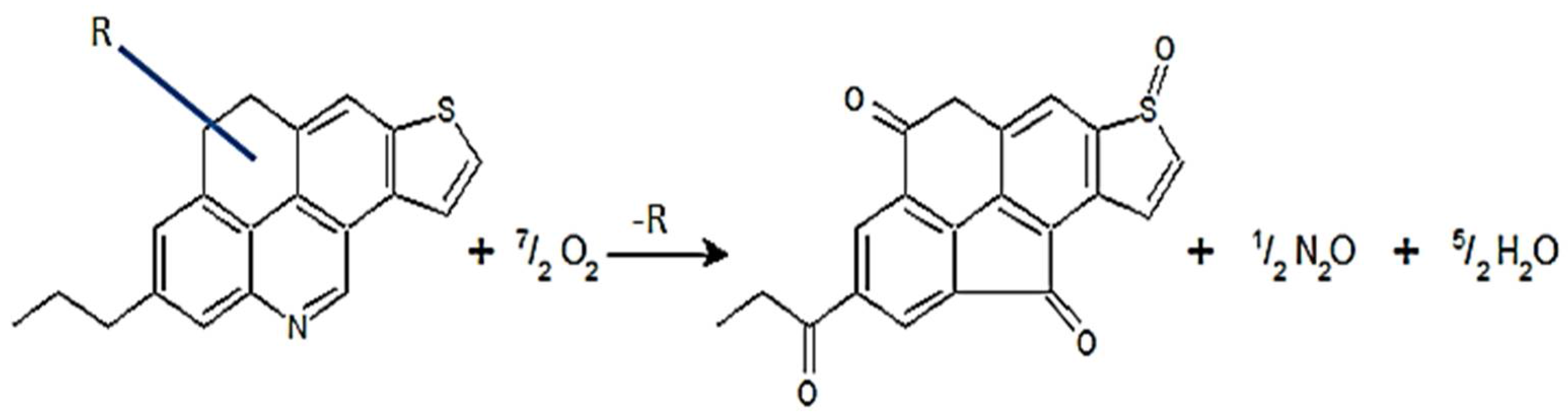
2.1.4. FTIR Analysis of Solid Asphaltenes
2.2. Emulsions
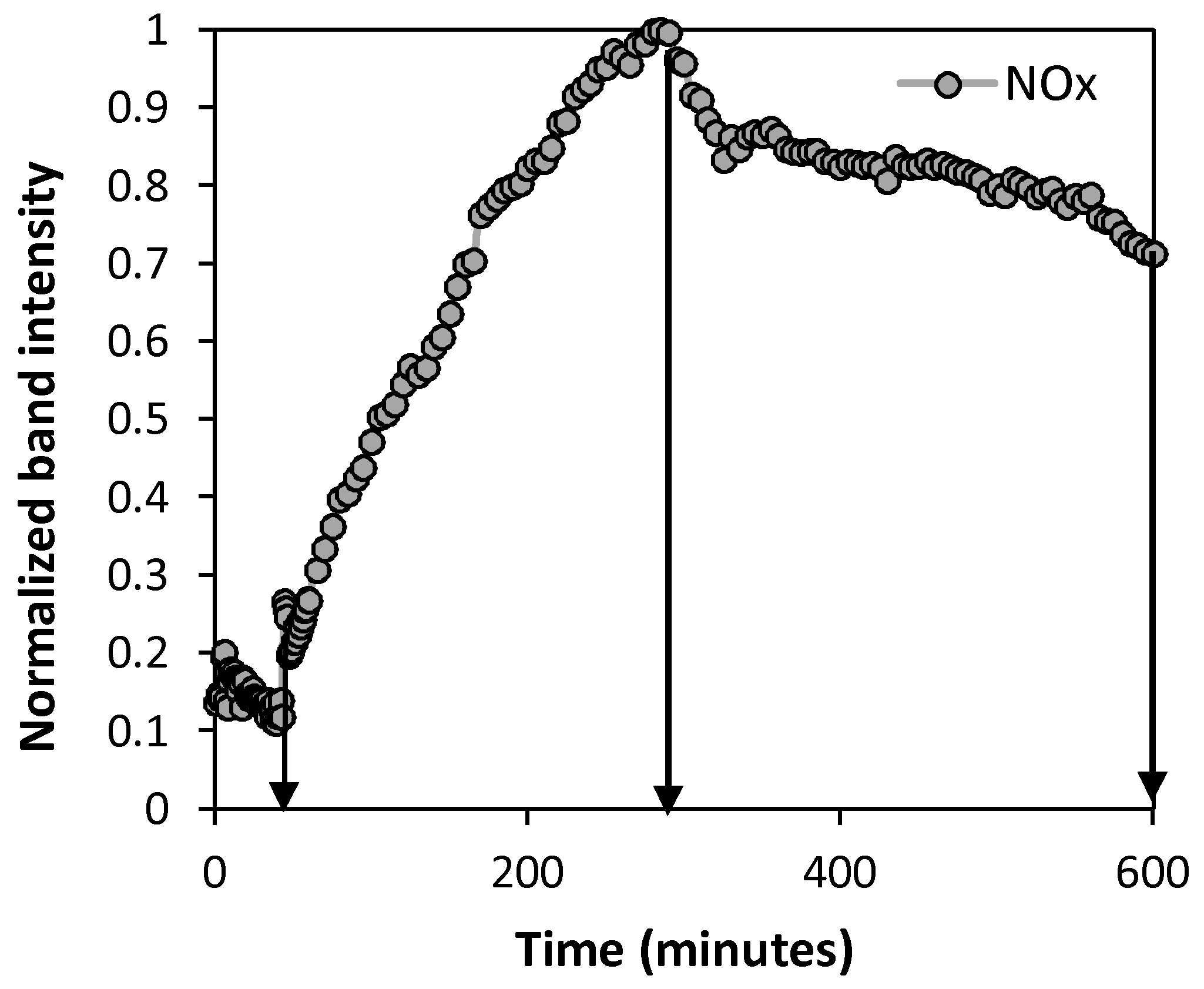
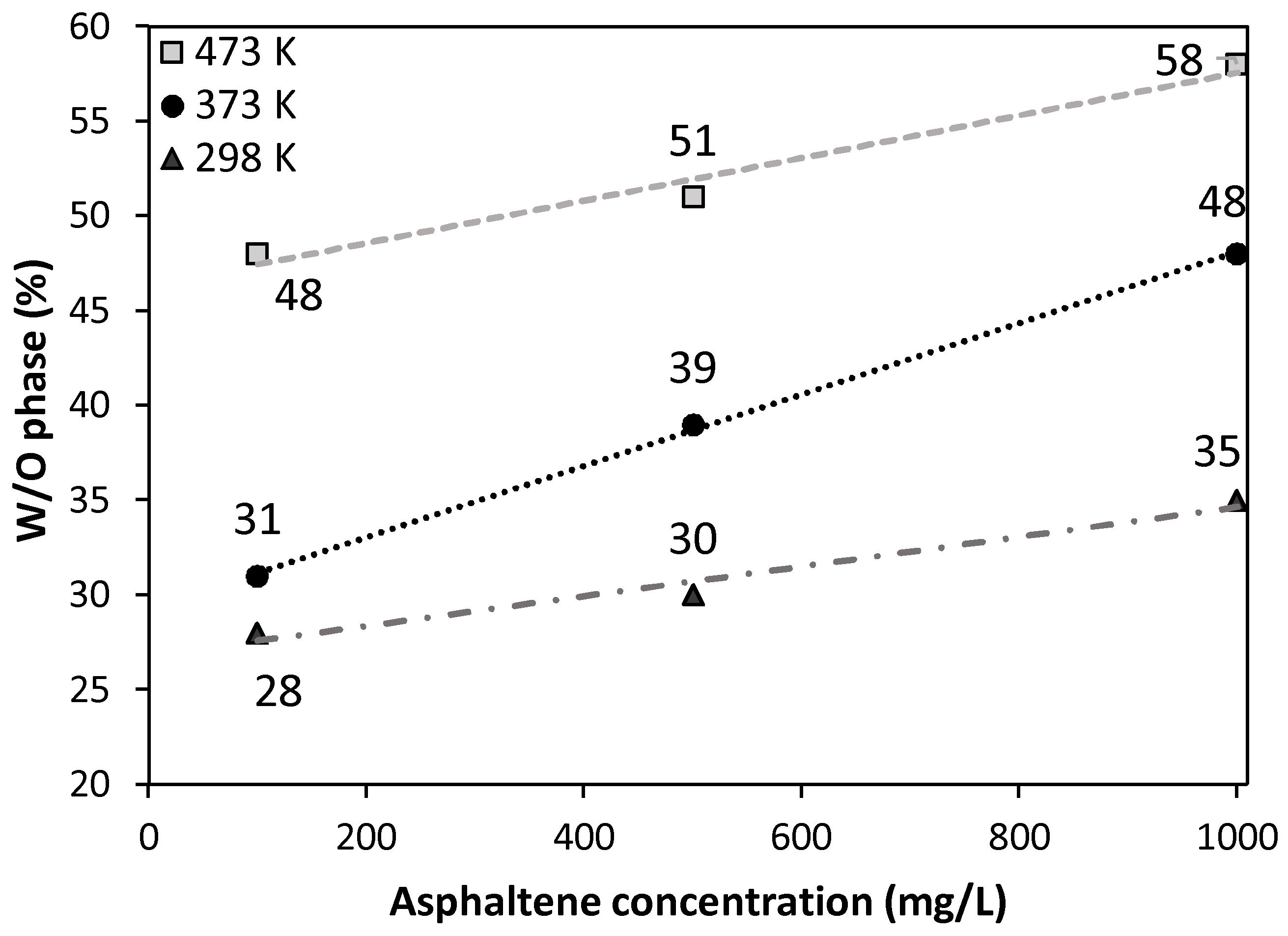
2.2.1. Oxidation Temperature Effect
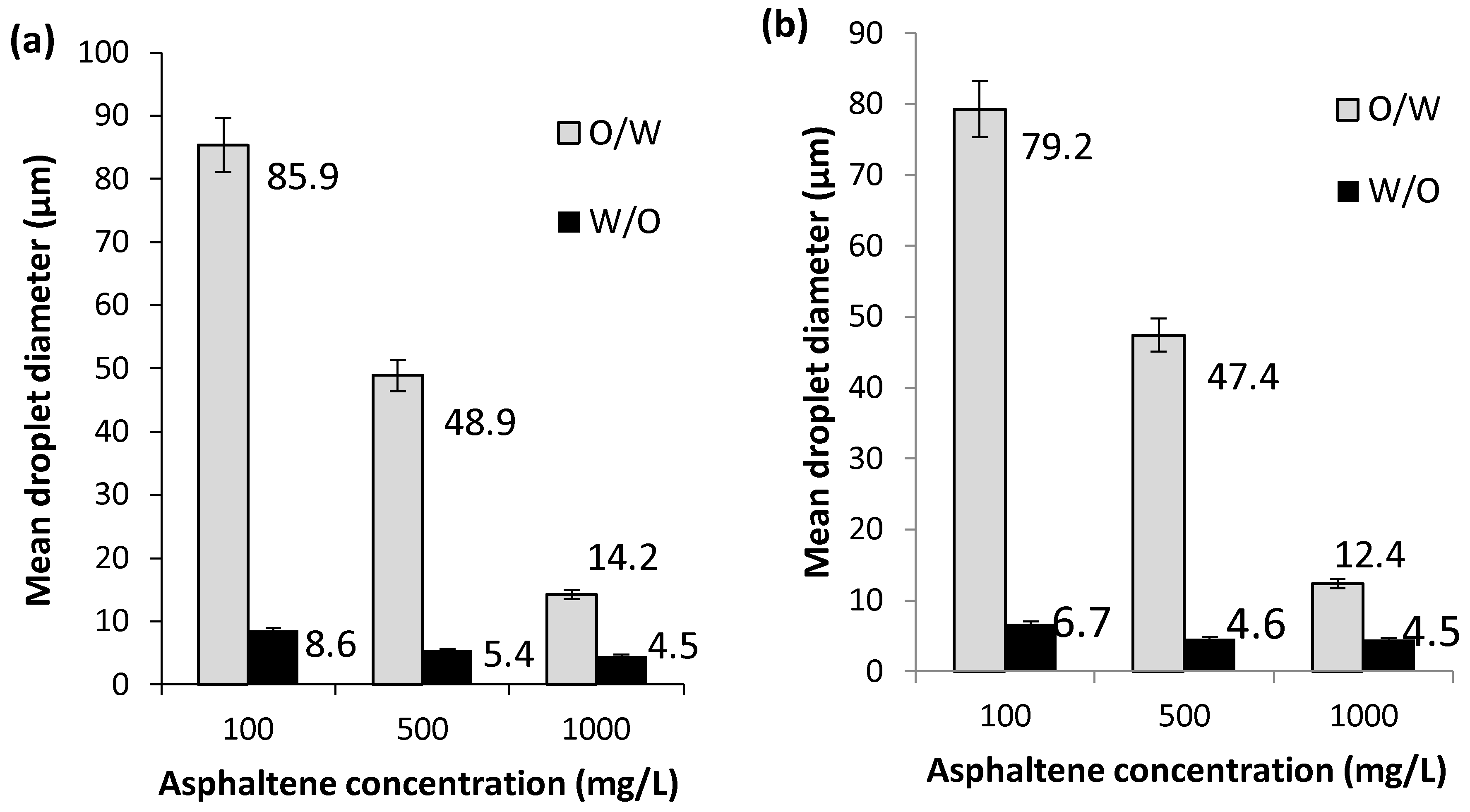
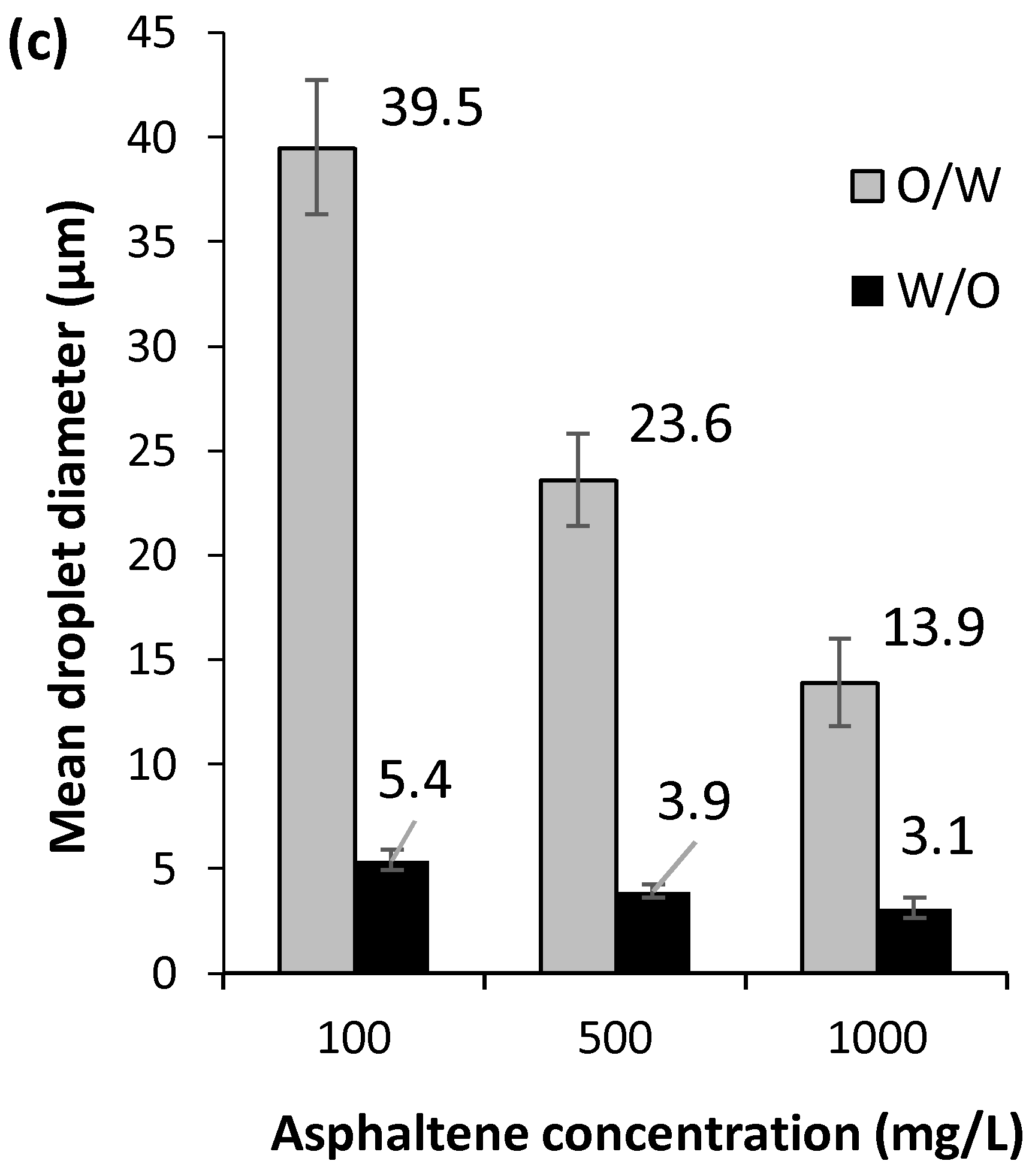
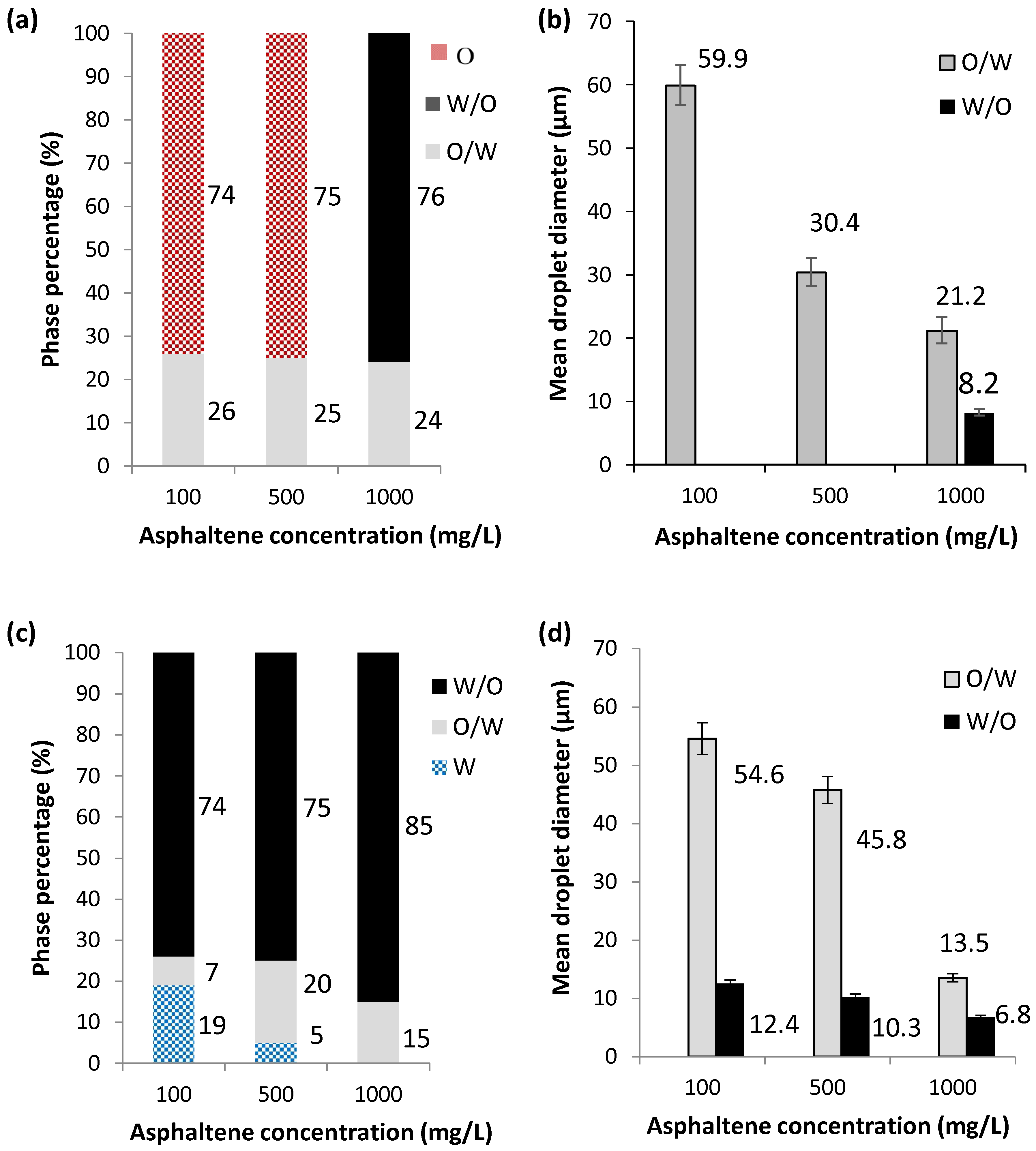
2.2.2. Effect of Model Solution to Water Ratio
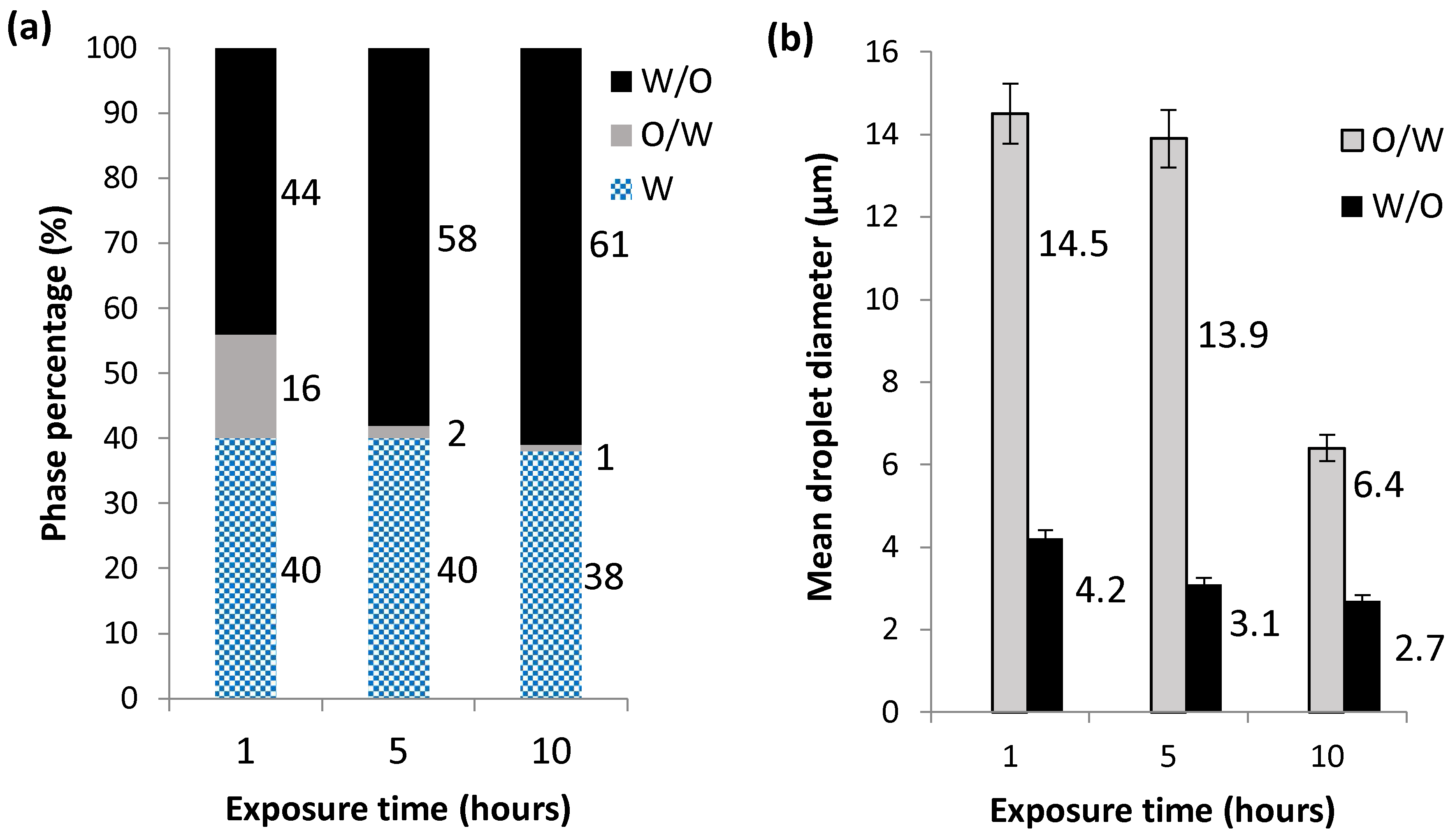
2.2.3. Effect of Exposure Time on Oxidative Atmosphere
2.2.4. Interfacial Tension Measurements
3. Materials and Methods
3.1. Materials
3.2. Asphaltene Isolation and Air Exposure
3.3. Asphaltene Characterization
3.3.1. Elemental Analyses
3.3.2. Solubility Tests
3.3.3. Thermogravimetric Analysis
3.3.4. FTIR of Solid Asphaltenes
3.4. Preparation of Emulsions
3.5. Emulsions Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alboudwarej, H.; Felix, J.; Taylor, S.; Badry, R.; Bremner, C.; Brough, B.; Skeates, C.; Baker, A.; Palmer, D.; Pattison, K. Highlighting heavy oil. Oilfield Rev. 2006, 18, 34–53. [Google Scholar]
- Mullins, O.C. The asphaltenes. Annu. Rev. Anal. Chem. 2011, 4, 393–418. [Google Scholar] [CrossRef] [PubMed]
- Clark, H.P.; Ascanio, F.A.; Van Kruijsdijk, C.P.; Chavarria, J.L.; Zatka, M.J.; Williams, W.; Yahyai, A.H.; Shaw, J.D.; Bedry, M. Method to Improve Thermal EOR Performance Using Intelligent Well Technology: Orion SAGD Field Trial. In Proceedings of the Canadian Unconventional Resources and International Petroleum Conference, Calgary, AB, Canada, 19–21 October 2010; Society of Petroleum Engineers: Richardson, TX, USA, 2010. [Google Scholar]
- Al Adasani, A.; Bai, B. Analysis of EOR projects and updated screening criteria. J. Pet. Sci. Eng 2011, 79, 10–24. [Google Scholar] [CrossRef]
- Wei, L.; Zhu, J.-H.; Qi, J.-H. Application of nano-nickel catalyst in the viscosity reduction of Liaohe extra-heavy oil by aqua-thermolysis. J. Fuel Chem. Technol. 2007, 35, 176–180. [Google Scholar]
- Zabala, R.; Franco, C.; Cortés, F. Application of Nanofluids for Improving Oil Mobility in Heavy Oil and Extra-Heavy Oil: A Field Test. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016; Society of Petroleum Engineers: Richardson, TX, USA, 2016. [Google Scholar]
- Pereira, J.S.; Mello, P.A.; Moraes, D.P.; Duarte, F.A.; Dressler, V.L.; Knapp, G.; Flores, É.M. Chlorine and sulfur determination in extra-heavy crude oil by inductively coupled plasma optical emission spectrometry after microwave-induced combustion. Spectrochim. Acta Part B 2009, 64, 554–558. [Google Scholar] [CrossRef]
- Hong, C.; Kishita, A.; Watanabe, T.; Enomoto, H. Formation mechanism of emulsion in in-situ combustion and physical model for in-situ combustion. J. Jpn. Assoc. Pet. Technol. 1998, 63, 251–257. [Google Scholar] [CrossRef]
- Bennion, D.B. An overview of formation damage mechanisms causing a reduction in the productivity and injectivity of oil and gas producing formations. J. Can. Pet. Technol. 2002, 41. [Google Scholar] [CrossRef]
- Mandal, A.; Samanta, A.; Bera, A.; Ojha, K. Characterization of Oil–Water Emulsion and Its Use in Enhanced Oil Recovery. Ind. Eng. Chem. Res. 2010, 49, 12756–12761. [Google Scholar] [CrossRef]
- Uzoigwe, A.; Marsden, S., Jr. Emulsion Rheology and Flow through Unconsolidated Synthetic Porous Media. In Proceedings of the Fall Meeting of the Society of Petroleum Engineers of AIME, Houston, TX, USA, 4–7 October 1970; Society of Petroleum Engineers: Richardson, TX, USA, 1970. [Google Scholar]
- Moore, R.; Laureshen, C.; Mehta, S.; Ursenbach, M. Observations and design considerations for in situ combustion projects. J. Can. Pet. Technol. 1999, 38. [Google Scholar] [CrossRef]
- Cheih, C. State-of-the-art review of fireflood field projects (includes associated papers 10901 and 10918). J. Pet. Technol. 1982, 34, 19–36. [Google Scholar] [CrossRef]
- Burger, J.G. Chemical aspects of in-situ combustion-heat of combustion and kinetics. Soc. Pet. Eng. J. 1972, 12, 410–422. [Google Scholar] [CrossRef]
- Kang, W.; Xu, B.; Wang, Y.; Li, Y.; Shan, X.; An, F.; Liu, J. Stability mechanism of W/O crude oil emulsion stabilized by polymer and surfactant. Colloids Surf. A 2011, 384, 555–560. [Google Scholar] [CrossRef]
- Bobra, M. A Study of the Formation of Water-in-Oil Emulsions. In Proceedings of the Thirteenth Arctic and Marine Oilspill Program Technical Seminar, Edmonton, AB, Canada, 6–8 June 1990; pp. 6–8. [Google Scholar]
- Kokal, S.L. Crude oil emulsions: A state-of-the-art review. SPE Prod. Facil. 2005, 20, 5–13. [Google Scholar] [CrossRef]
- Mandal, A.; Bera, A. Modeling of flow of oil-in-water emulsions through porous media. Pet. Sci. 2015, 12, 273–281. [Google Scholar] [CrossRef]
- Ortiz, D.; Baydak, E.; Yarranton, H. Effect of surfactants on interfacial films and stability of water-in-oil emulsions stabilized by asphaltenes. J. Colloid Interface Sci. 2010, 351, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Morales Corozo, J.R. Estudio del comportamiento reológico de emulsiones de crudos pesados. Tesis de Grado para la obtención del Título de Ingeniero Químico. 2014. Carrera de Ingeniería Química. Quito: UCE. 75 p. Available online: http://www.dspace.uce.edu.ec/bitstream/25000/2776/1/T-UCE-0017-65.pdf (accessed on 3 February 2018).
- Kokal, S.; Al-Juraid, J. Quantification of Various Factors Affecting Emulsion Stability: Watercut, Temperature, Shear, Asphaltene Content, Demulsifier Dosage and Mixing Different Crudes. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 3–6 October 1999; Society of Petroleum Engineers: Richardson, TX, USA, 1999. [Google Scholar]
- Spiecker, P.M.; Gawrys, K.L.; Kilpatrick, P.K. Aggregation and solubility behavior of asphaltenes and their subfractions. J. Colloid Interface Sci. 2003, 267, 178–193. [Google Scholar] [CrossRef]
- Kilpatrick, P.K.; Spiecker, P.M. Asphaltene Emulsions; Marcel Dekker: New York, NY, USA, 2001; Volume 3, pp. 707–730. [Google Scholar]
- Tambe, D.E.; Sharma, M.M. The effect of colloidal particles on fluid-fluid interfacial properties and emulsion stability. Adv. Colloid Interface Sci. 1994, 52, 1–63. [Google Scholar] [CrossRef]
- Rane, J.P.; Harbottle, D.; Pauchard, V.; Couzis, A.; Banerjee, S. Adsorption kinetics of asphaltenes at the oil–water interface and nanoaggregation in the bulk. Langmuir 2012, 28, 9986–9995. [Google Scholar] [CrossRef] [PubMed]
- Rane, J.P.; Pauchard, V.; Couzis, A.; Banerjee, S. Interfacial rheology of asphaltenes at oil–water interfaces and interpretation of the equation of state. Langmuir 2013, 29, 4750–4759. [Google Scholar] [CrossRef] [PubMed]
- Rane, J.P.; Zarkar, S.; Pauchard, V.; Mullins, O.C.; Christie, D.; Andrews, A.B.; Pomerantz, A.E.; Banerjee, S. Applicability of the Langmuir Equation of State for asphaltene adsorption at the oil-water interface: Coal-derived, petroleum and synthetic asphaltenes. Energy Fuels 2015, 29, 3584–3590. [Google Scholar] [CrossRef]
- Pauchard, V.; Rane, J.P.; Zarkar, S.; Couzis, A.; Banerjee, S. Long-Term Adsorption Kinetics of Asphaltenes at the Oil–Water Interface: A Random Sequential Adsorption Perspective. Langmuir 2014, 30, 8381–8390. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.D.; Kilpatrick, P.K. Effects of asphaltene solvency on stability of water-in-crude-oil emulsions. J. Colloid Interface Sci. 1997, 189, 242–253. [Google Scholar] [CrossRef]
- Yarranton, H.W.; Hussein, H.; Masliyah, J.H. Water-in-hydrocarbon emulsions stabilized by asphaltenes at low concentrations. J. Colloid Interface Sci. 2000, 228, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Lu, S.; Cao, G. Stability and demulsification of emulsions stabilized by asphaltenes or resins. J. Colloid Interface Sci. 2004, 271, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Ancheyta, J.; Centeno, G.; Trejo, F.; Marroquin, G. Changes in asphaltene properties during hydrotreating of heavy crudes. Energy Fuels 2003, 17, 1233–1238. [Google Scholar] [CrossRef]
- AlHumaidan, F.S.; Hauser, A.; Rana, M.S.; Lababidi, H.M. Impact of Thermal Treatment on Asphaltene Functional Groups. Energy Fuels 2016, 30, 2892–2903. [Google Scholar] [CrossRef]
- Moschopedis, S.; Speight, J. Oxidative degradation of Athabasca asphaltenes. Fuel 1971, 50, 211–217. [Google Scholar] [CrossRef]
- Gonzalez, L.; Ferrer, J.; Fuenmayor, M.; Castillo, N.; Gil, E.; Farouq Ali, S. Use of Reservoir Simulation to Estimate Drainage Area and Recovery Factor of an In-Situ Combustion Project in a Complex Water-Drive Heavy Oil Reservoir. In Proceedings of the SPE Heavy Oil Conference-Canada, Calgary, AB, Canada, 10–12 June 2014; Society of Petroleum Engineers: Richardson, TX, USA, 2014. [Google Scholar]
- Orea, M.; Bruzual, J.; Diaz, A.; Árraga, T.; Benítez, N.; Castillo, J. Formation of stable emulsions under in-situ combustion conditions: An assessment of the injected gas−crude oil−water−rock interplay in Quifa oilfield, Los Llanos Basin, Colombia. J. Pet. Sci. Eng. 2017, 159, 103–114. [Google Scholar] [CrossRef]
- Prieto, H.; Lima, E.; Gaviria, M.; Gil, E.; Benitez, N.; Fuenmayor, M. Design and Operation of Production Facilities of the Quifa Field In-situ Combustion Project. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 28–30 September 2015; Society of Petroleum Engineers: Richardson, TX, USA, 2015. [Google Scholar]
- Franco, C.A.; Montoya, T.; Nassar, N.N.; Pereira-Almao, P.; Cortés, F.B. Adsorption and subsequent oxidation of colombian asphaltenes onto Nickel and/or Palladium oxide supported on fumed silica nanoparticles. Energy Fuels 2013, 27, 7336–7347. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Comparative oxidation of adsorbed asphaltenes onto transition metal oxide nanoparticles. Colloids Surfaces A 2011, 384, 145–149. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Metal oxide nanoparticles for asphaltene adsorption and oxidation. Energy Fuels 2011, 25, 1017–1023. [Google Scholar] [CrossRef]
- Habib, F.K.; Diner, C.; Stryker, J.M.; Semagina, N.; Gray, M.R. Suppression of addition reactions during thermal cracking using hydrogen and sulfided iron catalyst. Energy Fuels 2013, 27, 6637–6645. [Google Scholar] [CrossRef]
- Alshareef, A.H.; Scherer, A.; Tan, X.; Azyat, K.; Stryker, J.M.; Tykwinski, R.R.; Gray, M.R. Formation of archipelago structures during thermal cracking implicates a chemical mechanism for the formation of petroleum asphaltenes. Energy Fuels 2011, 25, 2130–2136. [Google Scholar] [CrossRef]
- Curtis, C.W.; Jeon, Y.W.; Clapp, D.J. Adsorption of asphalt functionalities and oxidized asphalts on aggregate surfaces. Fuel Sci. Technol. Int. 1989, 7, 1225–1268. [Google Scholar] [CrossRef]
- Moreno, L.S.; Babadagli, T. Quantitative and Visual Characterization of Asphaltenic Components of Heavy-Oil and Bitumen Samples after Solvent Interaction at Different Temperatures and Pressures. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 8–10 April 2013; Society of Petroleum Engineers: Richardson, TX, USA, 2013. [Google Scholar]
- Rojas-González, A.F.; Barraza-Burgos, J.M. Efecto de la relación atómica oxígeno/carbono del carbón sobre la reactividad en la combustión de carbonizados. Ing. Univ. 2013, 17, 41–57. [Google Scholar]
- Hosseinpour, N.; Khodadadi, A.A.; Bahramian, A.; Mortazavi, Y. Asphaltene adsorption onto acidic/basic metal oxide nanoparticles toward in situ upgrading of reservoir oils by nanotechnology. Langmuir 2013, 29, 14135–14146. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour, N.; Mortazavi, Y.; Bahramian, A.; Khodatars, L.; Khodadadi, A.A. Enhanced pyrolysis and oxidation of asphaltenes adsorbed onto transition metal oxides nanoparticles towards advanced in-situ combustion EOR processes by nanotechnology. Appl. Catal. A Gen. 2014, 477, 159–171. [Google Scholar] [CrossRef]
- Yan, C.; Yang, Y.; Liu, M.; Nie, M.; Zhou, J.L. Phenanthrene sorption to Chinese coal: Importance of coal’s geochemical properties. J. Hazard. Mater. 2011, 192, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, S.; Castro, A.; Vásquez, E.; Marcano, F.; Ranaudo, M.A. Investigation of physical chemistry properties of asphaltenes using solubility parameters of asphaltenes and their fractions A1 and A2. Energy Fuels 2010, 24, 5921–5933. [Google Scholar] [CrossRef]
- Acevedo, S.; Escobar, O.; Echevarria, L.; Gutiérrez, L.B.; Méndez, B. Structural analysis of soluble and insoluble fractions of asphaltenes isolated using the PNP method. Relation between asphaltene structure and solubility. Energy Fuels 2004, 18, 305–311. [Google Scholar] [CrossRef]
- Permanyer, A.; Douifi, L.; Lahcini, A.; Lamontagne, J.; Kister, J. FTIR and SUVF spectroscopy applied to reservoir compartmentalization: A comparative study with gas chromatography fingerprints results. Fuel 2002, 81, 861–866. [Google Scholar] [CrossRef]
- Calemma, V.; Iwanski, P.; Nali, M.; Scotti, R.; Montanari, L. Structural characterization of asphaltenes of different origins. Energy Fuels 1995, 9, 225–230. [Google Scholar] [CrossRef]
- Mojelsky, T.; Ignasiak, T.; Frakman, Z.; McIntyre, D.; Lown, E.; Montgomery, D.; Strausz, O. Structural features of Alberta oil sand bitumen and heavy oil asphaltenes. Energy Fuels 1992, 6, 83–96. [Google Scholar] [CrossRef]
- Siddiqui, M.N. Alkylation and oxidation reactions of Arabian asphaltenes. Fuel 2003, 82, 1323–1329. [Google Scholar] [CrossRef]
- Callejas, M.; Martınez, M.; Blasco, T.; Sastre, E. Coke characterisation in aged residue hydrotreating catalysts by solid-state 13C-NMR spectroscopy and temperature-programmed oxidation. Appl. Catal. A Gen. 2001, 218, 181–188. [Google Scholar] [CrossRef]
- Muhammad, A.B. Thermal evolution of aliphatic and aromatic moieties of asphaltenes from coals of different rank: Possible implication to the molecular architecture of asphaltenes. Chin. J. Geochem. 2015, 34, 422–430. [Google Scholar] [CrossRef]
- Quintero, K.; López, L.; De Lima, L. Espectroscopía infrarroja con transformadas de Fourier-Reflectancia total atenuada (IRTF/RTA) aplicada a la caracterización de crudos y su relación con la gravedad API. Rev. Fac. Ing. Univ. Cent. Venezuela 2014, 29, 93–102. [Google Scholar]
- Chang, C.-L.; Fogler, H.S. Stabilization of asphaltenes in aliphatic solvents using alkylbenzene-derived amphiphiles. 1. Effect of the chemical structure of amphiphiles on asphaltene stabilization. Langmuir 1994, 10, 1749–1757. [Google Scholar] [CrossRef]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta 2006, 573, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Brangule, A.; Gross, K.A. Importance of FTIR spectra deconvolution for the analysis of amorphous calcium phosphates. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2015. [Google Scholar]
- Lee, D.G.; Noureldin, N.A. Effect of water on the low-temperature oxidation of heavy oil. Energy Fuels 1989, 3, 713–715. [Google Scholar] [CrossRef]
- Adams, J.J. Asphaltene adsorption, a literature review. Energy Fuels 2014, 28, 2831–2856. [Google Scholar] [CrossRef]
- Zarkar, S.; Pauchard, V.; Farooq, U.; Couzis, A.; Banerjee, S. Interfacial properties of asphaltenes at toluene–water interfaces. Langmuir 2015, 31, 4878–4886. [Google Scholar] [CrossRef] [PubMed]
- Sjöblom, J.; Aske, N.; Auflem, I.H.; Brandal, Ø.; Havre, T.E.; Sæther, Ø.; Westvik, A.; Johnsen, E.E.; Kallevik, H. Our current understanding of water-in-crude oil emulsions: Recent characterization techniques and high-pressure performance. Adv. Colloid Interface Sci. 2003, 100, 399–473. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Lopetinsky, R.; Xu, Z.; Masliyah, J.H. Asphaltene monolayers at a toluene/water interface. Energy Fuels 2005, 19, 1330–1336. [Google Scholar] [CrossRef]
- Lobato, M.; Pedrosa, J.; Hortal, A.; Martinez-Haya, B.; Lebron-Aguilar, R.; Lago, S. Characterization and Langmuir film properties of asphaltenes extracted from Arabian light crude oil. Colloids Surfaces A 2007, 298, 72–79. [Google Scholar] [CrossRef]
- Fingas, M.; Fieldhouse, B. A Review of Knowledge on Water-in-oil Emulsions. In Proceedings of the 29. Arctic and Marine Oilspill Program (AMOP) Technical Seminar, Ottawa, ON, Canada, 6–8 June 2006. [Google Scholar]
- Sztukowski, D.M.; Jafari, M.; Alboudwarej, H.; Yarranton, H.W. Asphaltene self-association and water-in-hydrocarbon emulsions. J. Colloid Interface Sci. 2003, 265, 179–186. [Google Scholar] [CrossRef]
- Gafonova, O.V.; Yarranton, H.W. The stabilization of water-in-hydrocarbon emulsions by asphaltenes and resins. J. Colloid Interface Sci. 2001, 241, 469–478. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, L.; Xie, L.; Lu, X.; Liu, Q.; Mantilla, C.A.; van den Berg, F.G.; Zeng, H. Interaction mechanism of oil-in-water emulsions with asphaltenes determined using droplet probe AFM. Langmuir 2016, 32, 2302–2310. [Google Scholar] [CrossRef] [PubMed]
- Andrews, A.B.; McClelland, A.; Korkeila, O.; Demidov, A.; Krummel, A.; Mullins, O.C.; Chen, Z. Molecular orientation of asphaltenes and PAH model compounds in Langmuir−Blodgett films using sum frequency generation spectroscopy. Langmuir 2011, 27, 6049–6058. [Google Scholar] [CrossRef] [PubMed]
- Yarranton, H.W. Asphaltene Solubility and Asphaltene Stabilized Water-in-Oil Emulsions. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 1997. [Google Scholar]
- Fan, Y.; Simon, S.; Sjöblom, J. Influence of nonionic surfactants on the surface and interfacial film properties of asphaltenes investigated by Langmuir balance and Brewster angle microscopy. Langmuir 2010, 26, 10497–10505. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Abreu, C.; Delgado-Linares, J.G.; Bullon, J. Properties of Venezuelan asphaltenes in the bulk and dispersed state. J. Oleo Sci. 2006, 55, 563–571. [Google Scholar] [CrossRef]
- Delgado, J. Asfaltenos. Composición, Agregación, Precipitación. Laboratorio FIRP: Cuaderno FIRP 369. Universidad de los Andes: Mérida-Venezuela, Mexico, Unpublished work. 2006. [Google Scholar]
- Khadim, M.A.; Sarbar, M.A. Role of asphaltene and resin in oil field emulsions. J. Pet. Sci. Eng. 1999, 23, 213–221. [Google Scholar] [CrossRef]
- Harbottle, D.; Chen, Q.; Moorthy, K.; Wang, L.; Xu, S.; Liu, Q.; Sjoblom, J.; Xu, Z. Problematic stabilizing films in petroleum emulsions: Shear rheological response of viscoelastic asphaltene films and the effect on drop coalescence. Langmuir 2014, 30, 6730–6738. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.C. Asphalt Oxidation—AH Overview Including a New Modelfor Oxidation Proposing that Physicochemical Factors Dominate the Oxidation Kinetics. Fuel Sci. Technol. Int. 1993, 11, 57–87. [Google Scholar] [CrossRef]
- Shi, H.; Xu, T.; Zhou, P.; Jiang, R. Combustion properties of saturates, aromatics, resins, and asphaltenes in asphalt binder. Constr. Build. Mater. 2017, 136, 515–523. [Google Scholar] [CrossRef]
- Mendez, Z.; Anton, R.E.; Salager, J.-L. Surfactant-oil-water systems near the affinity inversion. Part XI. pH sensitive emulsions containing carboxylic acids. J. Dispers. Sci. Technol. 1999, 20, 883–892. [Google Scholar] [CrossRef]
- Saien, J.; Akbari, S. Interfacial tension of toluene+ water+ sodium dodecyl sulfate from (20 to 50) C and pH between 4 and 9. J. Chem. Eng. Data 2006, 51, 1832–1835. [Google Scholar] [CrossRef]
- Nassar, N.N. Asphaltene adsorption onto alumina nanoparticles: Kinetics and thermodynamic studies. Energy Fuels 2010, 24, 4116–4122. [Google Scholar] [CrossRef]
- Franco, C.A.; Nassar, N.N.; Ruiz, M.A.; Pereira-Almao, P.; Cortés, F.B. Nanoparticles for inhibition of asphaltenes damage: Adsorption study and displacement test on porous media. Energy Fuels 2013, 27, 2899–2907. [Google Scholar] [CrossRef]
- Nadkarni, R. Guide to ASTM Test Methods for the Analysis of Petroleum Products and Lubricants; ASTM International West Conshohocken: Conshohocken, PA, USA, 2007. [Google Scholar]
- Franco, C.A.; Lozano, M.M.; Acevedo, S.; Nassar, N.N.; Cortés, F.B. Effects of Resin I on Asphaltene Adsorption onto Nanoparticles: A Novel Method for Obtaining Asphaltenes/Resin Isotherms. Energy Fuels 2015, 30, 264–272. [Google Scholar] [CrossRef]
- Franco, C.; Martínez, M.; Benjumea, P.; Patiño, E.; Cortés, F. Water Remediation Based on Oil Adsorption Using Nanosilicates Functionalized with a Petroleum Vacuum Residue. Adsorpt. Sci. Technol. 2014, 32, 197–208. [Google Scholar] [CrossRef]
- Peeri, N.; Kister, J.; Quoniam, L.; Planche, J.; Germanaud, L. Assessment of Asphalt Rheological Behaviors by Studying Their Aromatic Structures. Polycycl. Aromat. Compd. 1996, 9, 29–36. [Google Scholar] [CrossRef]
- Lamontagne, J.; Dumas, P.; Mouillet, V.; Kister, J. Comparison by Fourier transform infrared (FTIR) spectroscopy of different ageing techniques: Application to road bitumens. Fuel 2001, 80, 483–488. [Google Scholar] [CrossRef]
- Rohlf, F. tpsDig 2.17, digitize landmarks and outlines, Morphometric software distributed via the Stony Brook Morphometrics. State University of New York at Stony Brook: New York, NY, USA, Unpublished work. 2013. [Google Scholar]
- Aveyard, R.; Binks, B.; Fletcher, P.; Ye, X.; Lu, J. The resolution of emulsions, including crude oil emulsions, in relation to HLB behaviour. In Emulsions—A Fundamental and Practical Approach; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1992; pp. 97–110. [Google Scholar]

| Exposure Time (h) | Mass Increase (%) |
|---|---|
| 1 | 0.20 |
| 5 | 0.57 |
| 10 | 2.70 |
| Sample | Exposure Time (h) | Parameter | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C (wt %) | H (wt %) | N (wt %) | S (wt %) | O * (wt %) | H/C | O/C | N/C | S/C | ||
| Asf12 | - | 82.52 | 7.291 | 2.252 | 5.291 | 2.646 | 1.061 | 0.025 | 0.023 | 0.024 |
| Asf12/373 | 5 | 80.11 | 7.154 | 1.941 | 5.113 | 5.682 | 1.056 | 0.054 | 0.020 | 0.024 |
| Asf12/473 | 1 | 77.83 | 6.038 | 1.932 | 4.956 | 9.244 | 0.931 | 0.082 | 0.021 | 0.024 |
| 5 | 75.42 | 5.221 | 0.685 | 4.813 | 13.861 | 0.831 | 0.138 | 0.008 | 0.024 | |
| 10 | 72.17 | 4.816 | 0.618 | 4.219 | 18.177 | 0.801 | 0.190 | 0.007 | 0.022 | |
| Sample | Exposure Time (h) | Concentration (mg/L) |
|---|---|---|
| Asf12 | - | 20,000 |
| Asf12/373 | 5 | 15,000 |
| Asf12/473 | 1 | 2500 |
| 5 | 2100 | |
| 10 | 1500 |
| Concentration (mg/L) | W/O Emulsion (%) | O/W Emulsion (%) | ||||
|---|---|---|---|---|---|---|
| Asf12 | Asf12/373 | Asf12/473 | Asf12 | Asf12/373 | Asf12/473 | |
| 100 | 28 | 31 | 48 | 52 | 25 | 3 |
| 500 | 30 | 39 | 51 | 70 | 61 | 3 |
| 1000 | 35 | 48 | 58 | 65 | 52 | 2 |
| Oily Medium | Aqueous Medium | Asphaltene Concentration (mg/L) | Interfacial Tension ± 1 mN/m |
|---|---|---|---|
| Asf12 model solution | Water | 100 | 28 |
| 500 | 26 | ||
| 1000 | 22 | ||
| Alkaline water | 1000 | 9 | |
| Asf12/473 model solution | Water | 100 | 25 |
| 500 | 23 | ||
| 1000 | 19 | ||
| Alkaline water | 1000 | 10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llanos, S.; Acevedo, S.; Cortés, F.B.; Franco, C.A. Effect of the Asphaltene Oxidation Process on the Formation of Emulsions of Water in Oil (W/O) Model Solutions. Energies 2018, 11, 722. https://doi.org/10.3390/en11040722
Llanos S, Acevedo S, Cortés FB, Franco CA. Effect of the Asphaltene Oxidation Process on the Formation of Emulsions of Water in Oil (W/O) Model Solutions. Energies. 2018; 11(4):722. https://doi.org/10.3390/en11040722
Chicago/Turabian StyleLlanos, Sebastián, Sócrates Acevedo, Farid B. Cortés, and Camilo A. Franco. 2018. "Effect of the Asphaltene Oxidation Process on the Formation of Emulsions of Water in Oil (W/O) Model Solutions" Energies 11, no. 4: 722. https://doi.org/10.3390/en11040722