Influence of In Situ Pyrolysis on the Evolution of Pore Structure of Oil Shale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Oil Shale Samples
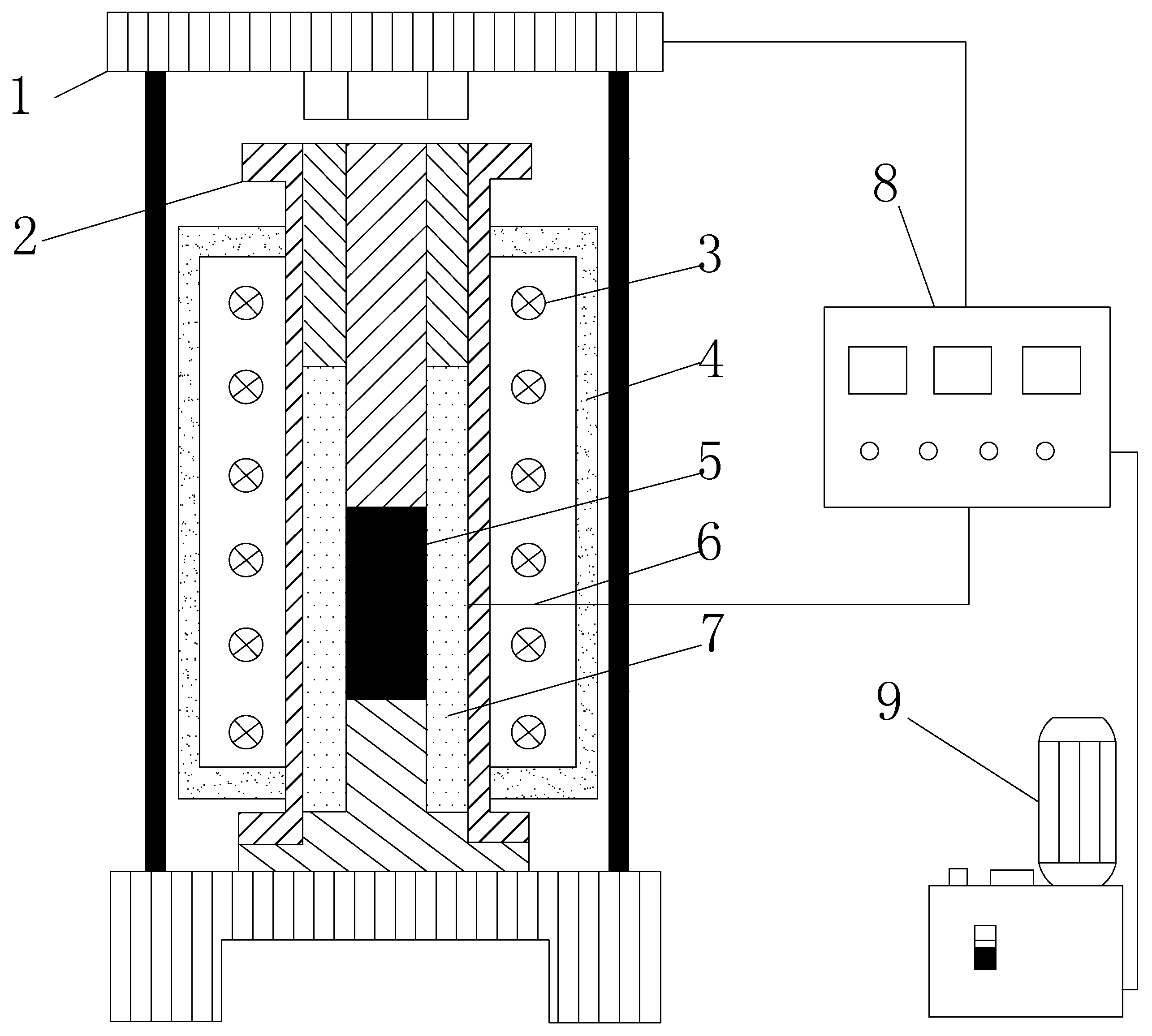
2.2. In Situ Pyrolysis Experiment
2.3. Pore Structure Tests at Different Scales
3. Results and Analysis
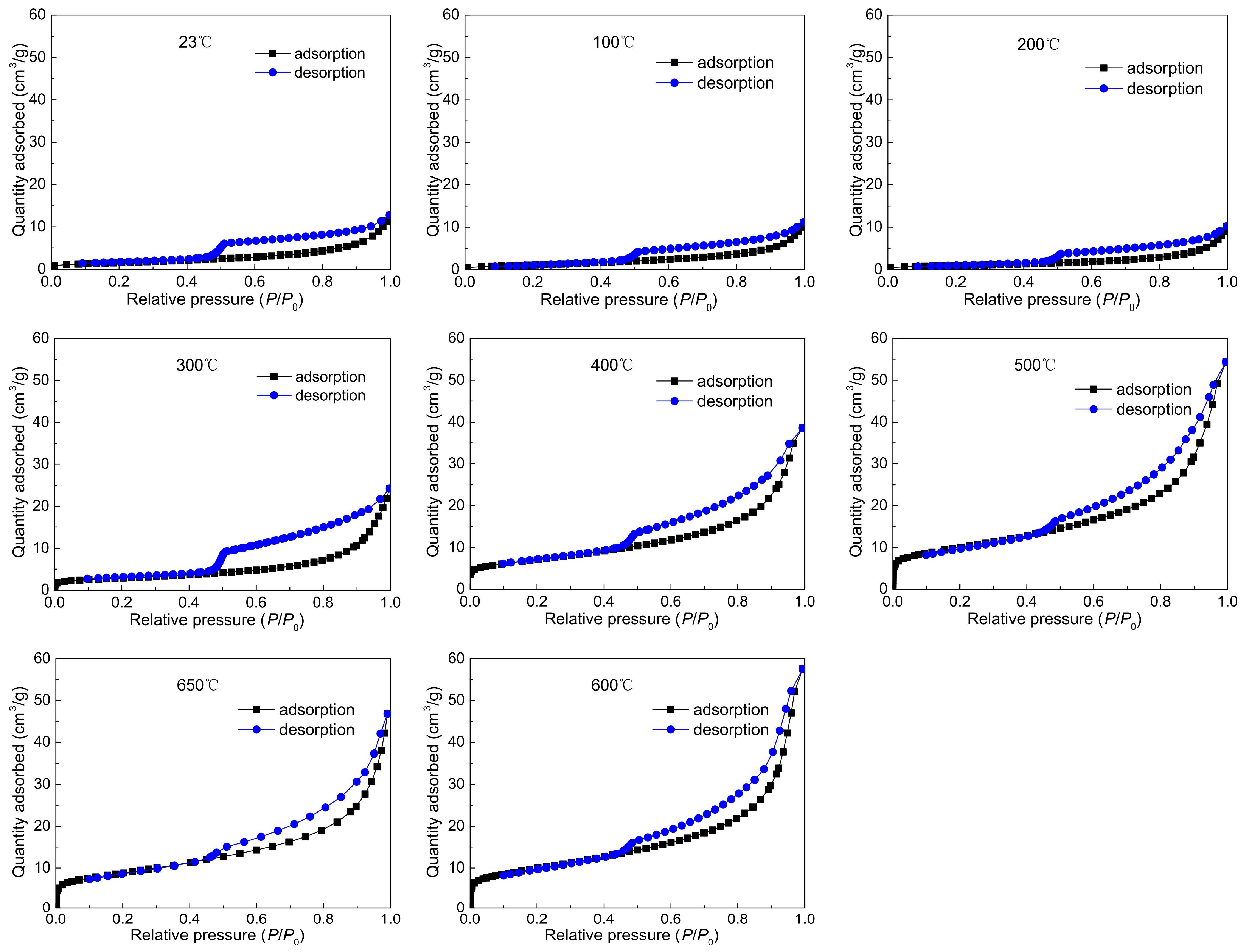
3.1. Experimental Results and Analysis Based on LPNA
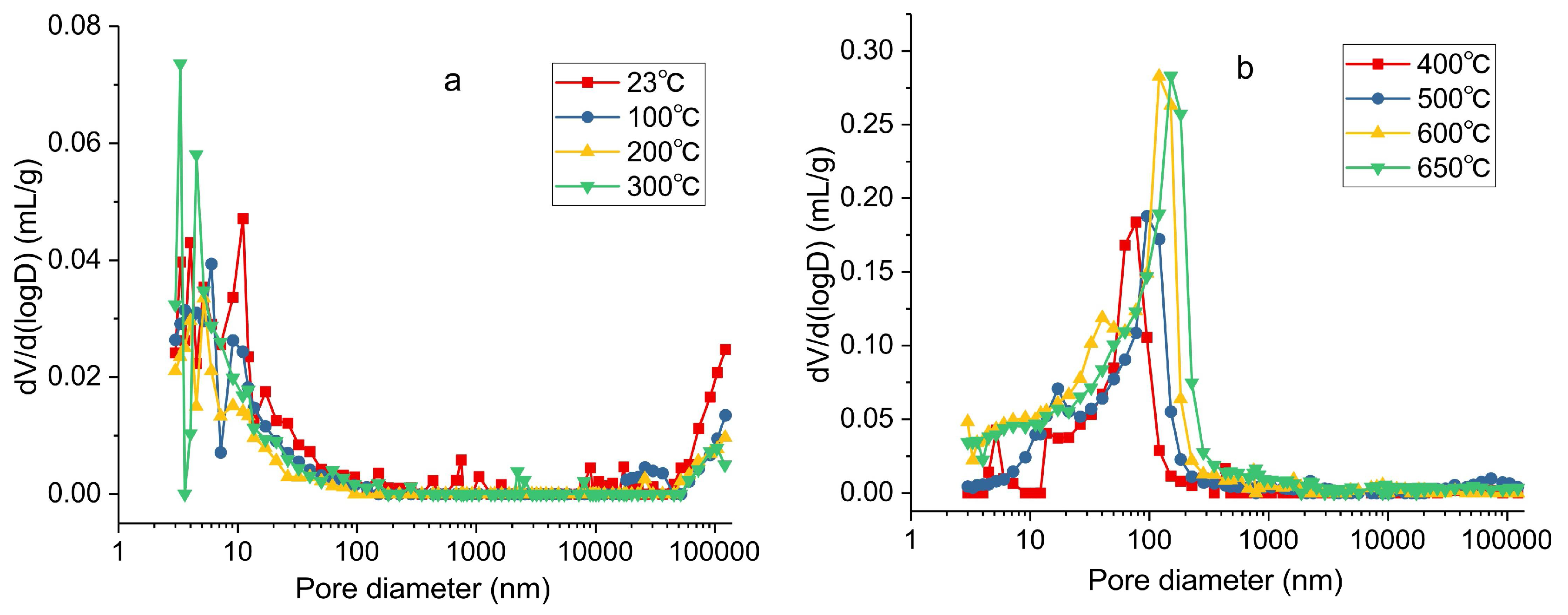
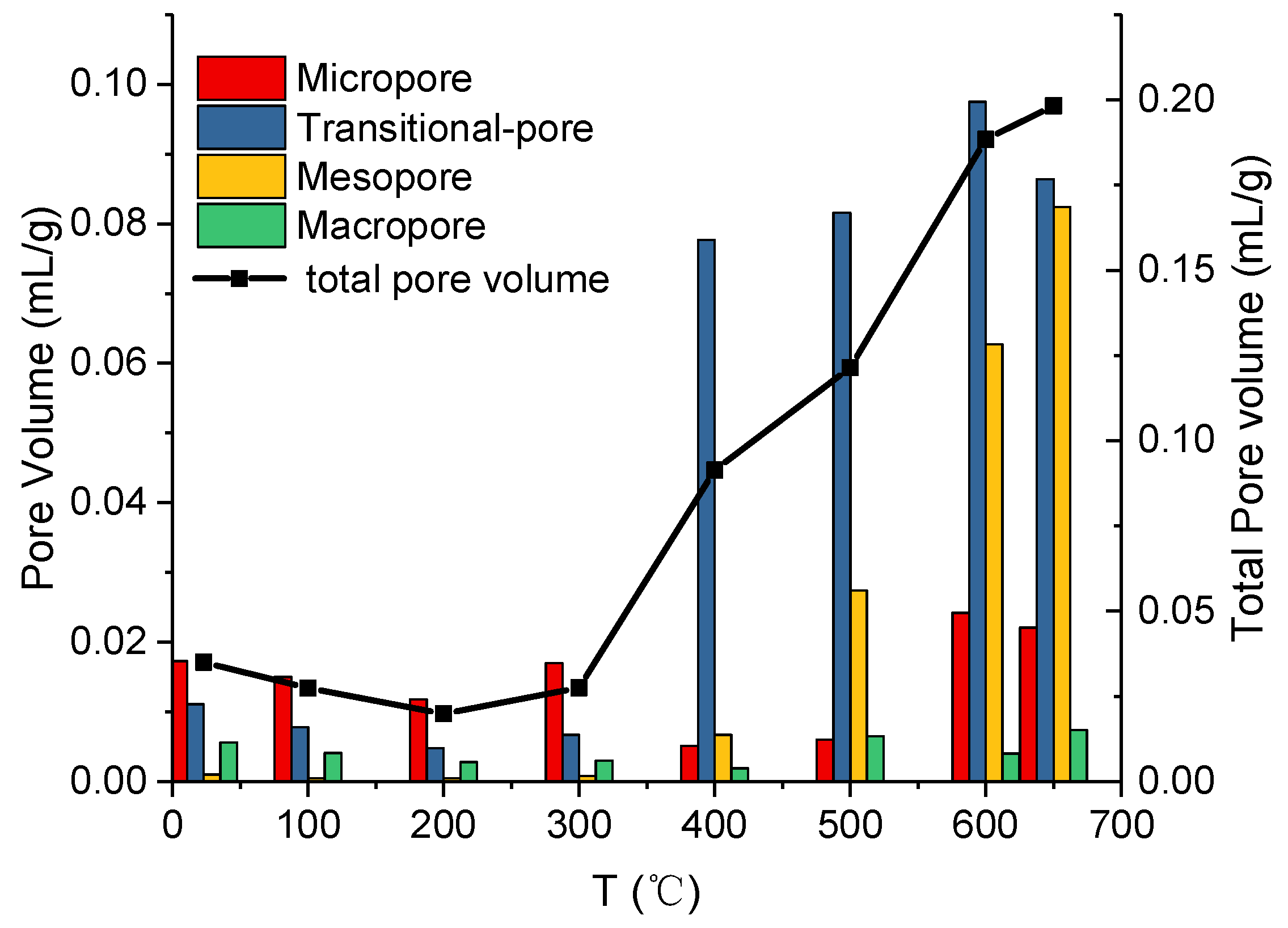
3.2. Experimental Results and Analysis Based on MIP
4. Discussion
5. Conclusions
- (1)
- In situ pyrolysis is the process of heat injection into an oil shale reservoir under lithostatic pressure. The lithostatic pressure does not cause change in the evolution discipline of the pore structure, but the inhibitory effect on the development of pores is significant and cannot be neglected.
- (2)
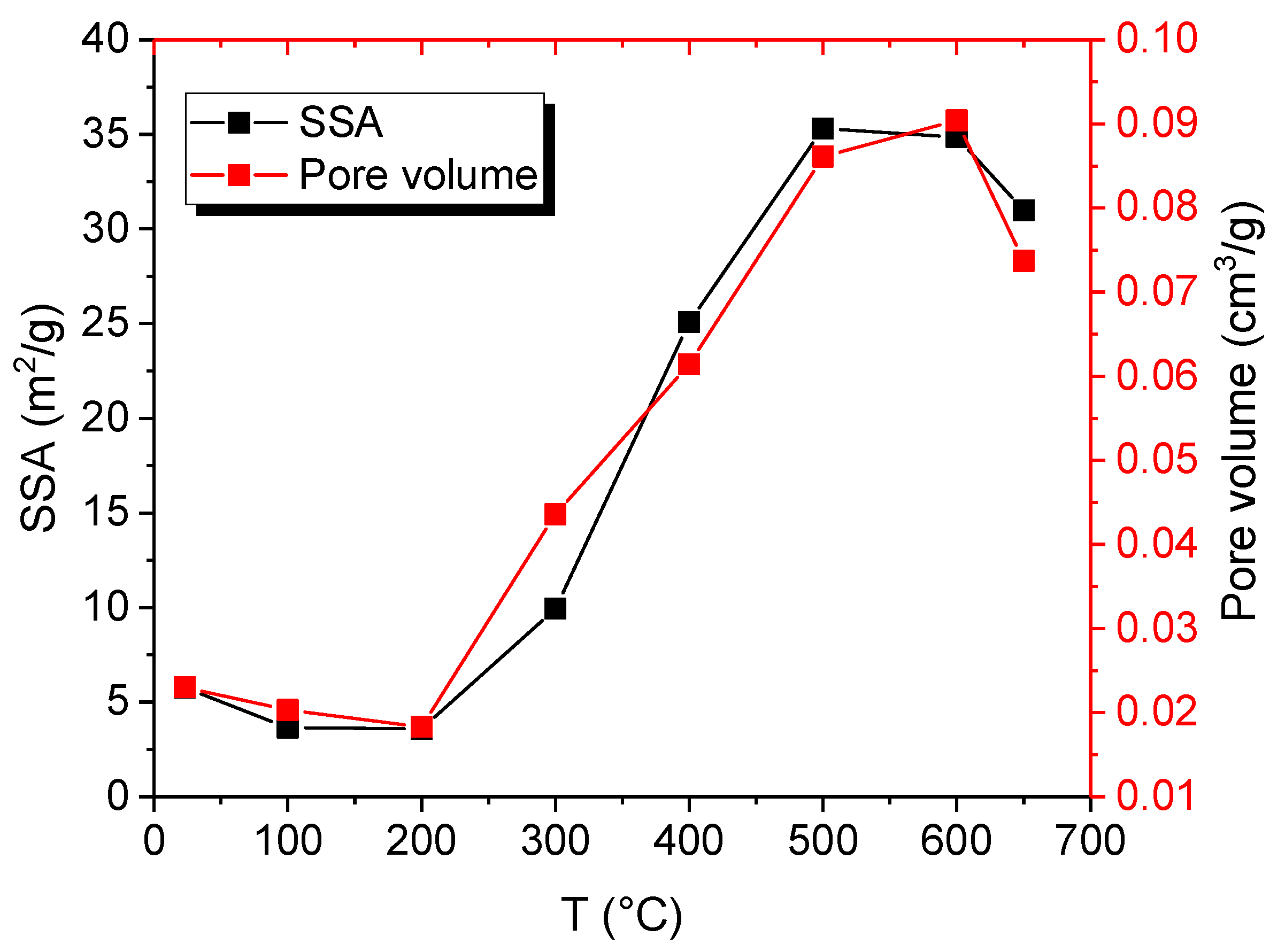
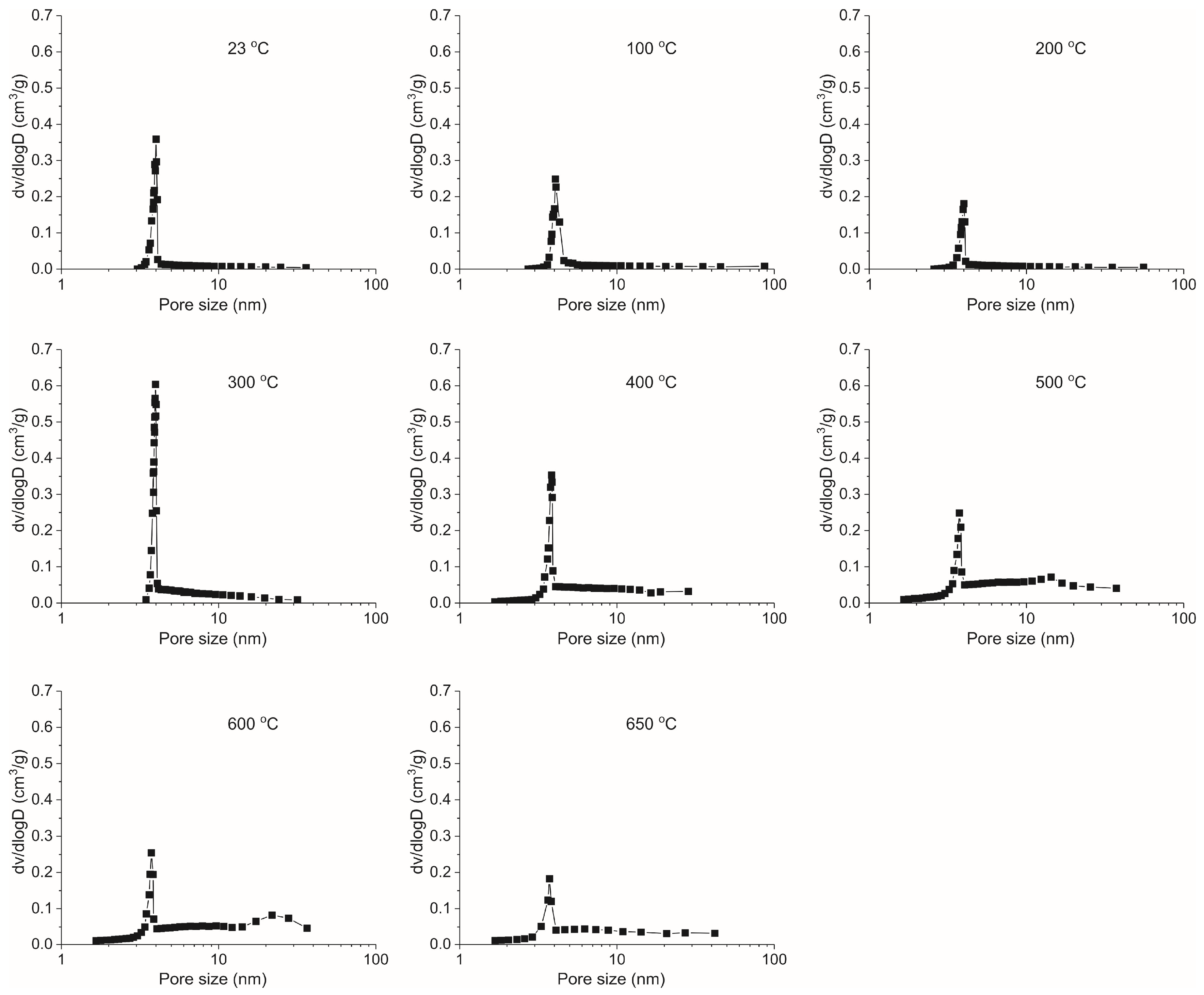
- The LPNA results display that pores are primarily ink-bottle shaped, and the effect of heating on the pore structure of oil shale is insignificant at 23–300 °C. However, at 400–650 °C, pores are mainly slit pores, which is closely related to the pyrolysis of oil shale organic matter at 350 °C. This means that pyrolysis plays a controlling role in the evolution of oil shale pore structure. The SSA shows a three-stage characteristic with increasing temperature: it decreases slowly from 23 to 200 °C, significantly increases from 300 to 500 °C—reaching a maximum at 500 °C—and slightly decreases from 600 to 650 °C.
- (3)
- The results from the MIP show that micropores, transitional pores, and macropores are developed from 23 to 300 °C, and the connectivity among pores is poor. While the transitional pores and mesopores are developed from 400 to 650 °C, the PSD becomes concentrated, and pore connectivity is improved. The porosity changes insignificantly from 23 to 300 °C, and increases steadily from 300 to 600 °C, while the development of porosity is limited at 600–650 °C. It can be concluded that temperature increase is beneficial for increasing porosity; however, porosity remains stable when the temperature is above 600 °C.
- (4)
- The evolution of oil shale pore structure involves complex physicochemical processes which are the result of the interaction between organic matter, pyrolysis products, and inorganic minerals under the actions of pressure and temperature. The change in pore structure before 350 °C is small and primarily caused by the deformation and fine adjustment of the components of oil shale under temperature and pressure. The pore structure changes greatly from 350 to 540 °C, mainly caused by the influence of the pyrolysis of organic matter and the escape of the product, followed by the decomposition and thermal cracking of inorganic minerals. At 600 °C, the further expansion of pores is attributed to the decomposition and thermal cracking of inorganic minerals. Above 600 °C, as a result of pressure, the mineral skeleton cannot support the pore space, resulting in the collapse and blockage of pores, complicating the pore structure. Therefore, the in situ pyrolysis final temperature should be selected on the premise of complete organic matter decomposition and optimization of pore structure parameters.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Energy Council. World Energy Focus Annual 2017. Available online: https://www.worldenergy.org/publications/2017/world-energy-focus-2017/ (accessed on 10 January 2018).
- Sun, Y.; Bai, F.; Liu, B.; Liu, Y.; Guo, M.; Guo, W.; Wang, Q.; Lü, X.; Yang, F.; Yang, Y. Characterization of the oil shale products derived via topochemical reaction method. Fuel 2014, 115, 338–346. [Google Scholar] [CrossRef]
- World Energy Council. World Energy Resources 2016. Available online: https://www.worldenergy.org/publications/2016/world-energy-resources-2016/ (accessed on 10 January 2018).
- Ishii, S.; Seta, M.; Nagasaki, T.; Nakai, N.; Nagai, M.; Miyamoto, Y.; Imada, H.; Doihata, K.; Saito, K.; Sekimoto, Y. Oil Shales of the World; Pergamon Press: Oxford, UK, 1990; pp. 1285–1291. [Google Scholar]
- Dyni, J.R. Geology and resources of some world oil-shale deposits. Oil Shale 2003, 20, 193–252. [Google Scholar]
- Mohr, S.H.; Wang, J.; Ellem, G.; Ward, J.; Giurco, D. Projection of world fossil fuels by country. Fuel 2015, 141, 120–135. [Google Scholar] [CrossRef]
- Kang, Z.; Yang, D.; Zhao, Y.; Hu, Y. Thermal cracking and corresponding permeability of Fushun oil shale. Oil Shale 2011, 28, 273–283. [Google Scholar] [CrossRef]
- Symington, W.A.; Olgaard, D.L.; Otten, G.A.; Phillips, T.C.; Thomas, M.M.; Yeakel, J.D. ExxonMobil’s Electrofrac™ Process for In Situ Oil Shale Conversion. In Oil Shale: A Solution to the Liquid Fuel Dilemma; ACS Symposium Series; ACS Publication: Washington, DC, USA, 2008; Volume 1032, pp. 185–216. [Google Scholar]
- Beer, G.; Zhang, E.; Wellington, S.; Ryan, R.; Vinegar, H. Shell’s In situ Conversion Process—Factors Affecting the Properties of Produced Shale Oil. In Proceedings of the 28th Oil Shale Symposium US, Golden, CO, USA, 13–15 October 2008. [Google Scholar]
- Dwyer, A.S.; Kasevich, R.S.; Kolker, M. In Situ Radio Frequency Selective Heating Process. U.S. Patent 4,140,179, 20 February 1979. [Google Scholar]
- Yang, D.; Elsworth, D.; Kang, Z.Q.; Zhao, Y.S.; Zheng, B. Experiments on permeability evolution with temperature of oil shale. In Proceedings of the 28th 46th US Rock Mechanics/Geomechanics Symposium, Chicago, IL, USA, 24–27 June 2012; Volume 3, pp. 1831–1835. [Google Scholar]
- Sresty, G.C.; Snow, R.H.; Bridges, J.E. Recovery of Liquid Hydrocarbons from Oil Shale by Electromagnetic Heating In Situ. U.S. Patent 4,485,869, 4 December 1984. [Google Scholar]
- Tham, M.J. Serially Burning and Pyrolyzing to Produce Shale Oil from a Subterranean Oil Shale. U.S. Patent 3,987,851, 26 October 1976. [Google Scholar]
- Braun, R.L. Mathematical Modeling of Modified In Situ and Aboveground Oil Shale Retorting; Lawrence Livermore National Laboratory: Livermore, CA, USA, 1981.
- Eseme, E.; Urai, J.L.; Krooss, B.M.; Littke, R. Review of mechanical properties of oil shales: Implications for exploitation and basin modelling. Oil Shale 2007, 24, 159–174. [Google Scholar]
- Saif, T.; Lin, Q.; Bijeljic, B.; Blunt, M.J. Microstructural imaging and characterization of oil shale before and after pyrolysis. Fuel 2017, 197, 562–574. [Google Scholar] [CrossRef]
- Bai, F.; Sun, Y.; Liu, Y.; Guo, M. Evaluation of the porous structure of Huadian oil shale during pyrolysis using multiple approaches. Fuel 2017, 187, 1–8. [Google Scholar] [CrossRef]
- Tong, J.; Han, X.; Wang, S.; Jiang, X. Evaluation of Structural Characteristics of Huadian Oil Shale Kerogen Using Direct Techniques (Solid-State13C NMR, XPS, FT-IR, and XRD). Energy Fuels 2011, 25, 4006–4013. [Google Scholar] [CrossRef]
- Remusat, L.; Derenne, S.; Robert, F.; Knicker, H. New pyrolytic and spectroscopic data on Orgueil and Murchison insoluble organic matter: A different origin than soluble? Geochim. Cosmochim. Acta 2005, 69, 3919–3932. [Google Scholar] [CrossRef]
- Mao, J.; Fang, X.; Lan, Y.; Schimmelmann, A.; Mastalerz, M.; Xu, L.; Schmidt-Rohr, K. Chemical and nanometer-scale structure of kerogen and its change during thermal maturation investigated by advanced solid-state 13C NMR spectroscopy. Geochim. Cosmochim. Acta 2010, 74, 2110–2127. [Google Scholar] [CrossRef]
- Bhargava, S.; Awaja, F.; Subasinghe, N. Characterisation of some Australian oil shale using thermal, X-ray and IR techniques. Fuel 2005, 84, 707–715. [Google Scholar] [CrossRef]
- Fertl, W.H. Kerogen, Insoluble Organic Matter from Sedimentary Rocks. Earth-Sci. Rev. 1982, 18, 87–88. [Google Scholar] [CrossRef]
- Burnham, A.K.; Happe, J.A. On the mechanism of kerogen pyrolysis. Fuel 1984, 63, 1353–1356. [Google Scholar] [CrossRef]
- Na, J.G.; Im, C.H.; Chung, S.H.; Lee, K.B. Effect of oil shale retorting temperature on shale oil yield and properties. Fuel 2012, 95, 131–135. [Google Scholar] [CrossRef]
- Tiwari, P.; Deo, M. Compositional and kinetic analysis of oil shale pyrolysis using TGA–MS. Fuel 2012, 94, 333–341. [Google Scholar] [CrossRef]
- Kok, M.V. Heating rate effect on the DSC kinetics of oil shales. J. Therm. Anal. Calorim. 2007, 90, 817–821. [Google Scholar] [CrossRef]
- Bai, F.; Guo, W.; Lü, X.; Liu, Y.; Guo, M.; Li, Q.; Sun, Y. Kinetic study on the pyrolysis behavior of Huadian oil shale via non-isothermal thermogravimetric data. Fuel 2015, 146, 111–118. [Google Scholar] [CrossRef]
- Maier, C.G.; Zimmerly, S.R. Chemical dynamics of the transformation of the organic matter to bitumen in oil shale. Utah Univ. Res. Invest. Bull. 1924, 14, 62–81. [Google Scholar]
- Lewan, M.D.; Spiro, B.; Illich, H.; Raiswell, R.; Mackenzie, A.S.; Durand, B.; Manning, D.A.C.; Comet, P.A.; Berner, R.A.; Leeuw, J.W.D. Evaluation of Petroleum Generation by Hydrous Pyrolysis Experimentation. Philos. Trans. R. Soc. Lond. 1985, 315, 123–134. [Google Scholar] [CrossRef]
- Hubbard, A.B.; Robinson, W.E. A Thermal Decomposition Study of Colorado Oil Shale; US Department of the Interior, Bureau of Mines: Washington, DC, USA, 1950.
- Allred, V.D. Kinetics of oil shale pyrolysis. Chem. Eng. Prog. 1966, 62, 55–60. [Google Scholar]
- Rothman, A.J. Recent Experimental Developments in Retorting Oil Shale at the Lawrence Livermore Laboratory; Lawrence Livermore Lab.: Livermore, CA, USA, 1978. [Google Scholar]
- Jiang, Z.; Zhang, W.; Chao, L.; Wang, Y.; Liu, H.; Xiang, C. Characteristics and evaluation elements of shale oil reservoir. Acta Petrolei Sin. 2014, 35, 184–196. [Google Scholar]
- Bernard, S.; Wirth, R.; Schreiber, A.; Schulz, H.-M.; Horsfield, B. Formation of nanoporous pyrobitumen residues during maturation of the Barnett Shale (Fort Worth Basin). Int. J. Coal Geol. 2012, 103, 3–11. [Google Scholar] [CrossRef]
- Curtis, M.E.; Cardott, B.J.; Sondergeld, C.H.; Rai, C.S. Development of organic porosity in the Woodford Shale with increasing thermal maturity. Int. J. Coal Geol. 2012, 103, 26–31. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, X. Evolution of nanoporosity in organic-rich shales during thermal maturation. Fuel 2014, 129, 173–181. [Google Scholar] [CrossRef]
- Modica, C.J.; Lapierre, S.G. Estimation of kerogen porosity in source rocks as a function of thermal transformation: Example from the Mowry Shale in the Powder River Basin of Wyoming. AAPG Bull. 2012, 96, 87–108. [Google Scholar] [CrossRef]
- Kang, Z.; Zhao, J.; Yang, D.; Zhao, Y.; Hu, Y. Study of the evolution of micron-scale pore structure in oil shale at different temperatures. Oil Shale 2017, 34, 42. [Google Scholar] [CrossRef]
- Saif, T.; Lin, Q.; Singh, K.; Bijeljic, B.; Blunt, M.J. Dynamic imaging of oil shale pyrolysis using synchrotron X-ray microtomography. Geophys. Res. Lett. 2016, 43, 6799–6807. [Google Scholar] [CrossRef]
- Tiwari, P.; Deo, M.; Lin, C.L.; Miller, J.D. Characterization of oil shale pore structure before and after pyrolysis by using X-ray micro CT. Fuel 2013, 107, 547–554. [Google Scholar] [CrossRef]
- Yang, L.; Yang, D.; Zhao, J.; Liu, Z.; Kang, Z. Changes of oil shale pore structure and permeability at different temperatures. Oil Shale 2016, 33, 101–110. [Google Scholar] [CrossRef]
- Han, X.; Jiang, X.; Yan, J.; Liu, J. Effects of Retorting Factors on Combustion Properties of Shale Char. 2. Pore Structure. Energy Fuels 2011, 25, 97–102. [Google Scholar] [CrossRef]
- Sun, L.; Tuo, J.; Zhang, M.; Wu, C.; Wang, Z.; Zheng, Y. Formation and development of the pore structure in Chang 7 member oil-shale from Ordos Basin during organic matter evolution induced by hydrous pyrolysis. Fuel 2015, 158, 549–557. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, G.; Liu, H.; Bai, J.; Li, S. Variation of the pore structure during microwave pyrolysis of oil shale. Oil Shale 2010, 27, 135–146. [Google Scholar]
- Geng, Y.; Liang, W.; Liu, J.; Cao, M.; Kang, Z. Evolution of Pore and Fracture Structure of Oil Shale under High Temperature and High Pressure. Energy Fuels 2017, 31, 10404–10413. [Google Scholar] [CrossRef]
- Nottenburg, R.N.; Rajeshwar, K.; Rosenvold, R.J.; Dubow, J.B. Temperature and stress dependence of electrical and mechanical properties of Green River oil shale. Fuel 1979, 58, 144–148. [Google Scholar] [CrossRef]
- Allan, A.M.; Clark, A.C.; Vanorio, T.; Kanitpanyacharoen, W.; Wenk, H.R. On the evolution of the elastic properties of organic-rich shale upon pyrolysis-induced thermal maturation. Geophysics 2015, 81, D271–D289. [Google Scholar] [CrossRef]
- General Administration of Quality Supervision, Inspection and Quarantine of China. Methods for Determining the Physical and Mechanical Properties of Coal and Rock—Part 2: Methods for Determining the True Density of Coal and Rock; GB/T 23561.2-2009; China Standards Press: Beijing, China, 2009.
- Oil and Gas Development Professional Standardization Committee. Practices for Core Analysis; SY/T 5336-2006; Petroleum Industry Press: Beijing, China, 2007. [Google Scholar]
- Shi, J.; Ma, Y.; Li, S.; Zhang, L. Characteristics of Thermal Bitumen Structure as the Pyrolysis Intermediate of Longkou Oil Shale. Energy Fuels 2017, 31, 10535–10544. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, X.; Han, X. Study on the Characteristics of the Oil Shale and Shale Char Mixture Pyrolysis. Energy Fuels 2009, 23, 5792–5797. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Washburn, E.W. The Dynamics of Capillary Flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Han, X.; Jiang, X.; Cui, Z. Change of Pore Structure of Oil Shale Particles during Combustion. 2. Pore Structure of Oil-Shale Ash. Chem. Phys. Lett. 2008, 198, 367–372. [Google Scholar] [CrossRef]
- Xi, Z.; Tang, S.; Zhang, S.; Sun, K. Pore structure characteristics of marine–continental transitional shale: A case study in the Qinshui Basin, China. Energy Fuels 2017, 31, 7854–7866. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Everett, D.H.; Fairbridge, C.; Haynes, M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Guidelines for the Characterization of Porous Solids. Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area, and Porosity; Academic Press: Cambridge, MA, USA, 1982; pp. 220–221. [Google Scholar]
- Rigby, S.P.; Edler, K.J. The influence of mercury contact angle, surface tension, and retraction mechanism on the interpretation of mercury porosimetry data. J. Colloid Interface Sci. 2002, 250, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, N.C.; Mckellar, M. Mercury porosimetry and the interpretation of pore geometry in sedimentary rocks and artificial models. Powder Technol. 1981, 29, 127–143. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D. Comparison of low-field NMR and mercury intrusion porosimetry in characterizing pore size distributions of coals. Fuel 2012, 95, 152–158. [Google Scholar] [CrossRef]
- Kibodeaux, K.R. Evolution of porosity, permeability, and fluid saturations during thermal conversion of oil shale. In Proceedings of the SPE Annual Technical Conference and Exhibition, Amsterdam, The Netherlands, 27–29 October 2014. [Google Scholar]
- Fan, Z.; Parker, J.C. An Efficient Modeling Approach to Simulate Heat Transfer Rate between Fracture and Matrix Regions for Oil Shale Retorting. Transp. Porous Media 2010, 84, 229–240. [Google Scholar]
- Williams, P.F.V. Thermogravimetry and decomposition kinetics of British Kimmeridge Clay oil shale. Fuel 1985, 64, 540–545. [Google Scholar] [CrossRef]
- Mckee, R.H.; Lyder, E.E. The Thermal Decomposition of Shales. I—Heat Effects. Ind. Eng. Chem. 2002, 13, 613–618. [Google Scholar] [CrossRef]
- Li, M.; Zhan, J.H.; Lai, D.; Tian, Y.; Liu, X.; Xu, G. Study on the evolution characteristic of intermediate during the pyrolysis of oil shale. J. Therm. Anal. Calorim. 2017, 130, 2227–2238. [Google Scholar] [CrossRef]
- Sun, K.; Zhao, Y.; Yang, D. Thermoelastoplastic damage model of heterogeneous medium and its application to thermal cracking analysis of oil shale in underground mining. Chin. J. Rock Mech. Eng. 2008, 27, 42–52. [Google Scholar]
- Burnham, A.K.; Singleton, M.F. High-Pressure Pyrolysis of Colorado Oil Shale; American Chemical Society Division of Fuel Chemistry: DuPage County, IL, USA, 1983; Volume 28. [Google Scholar]
- Meurer, W.P.; Symington, W.A.; Braun, A.L.; Kaminsky, R.D.; Olgaard, D.L.; Otten, G.A.; Phillips, T.C.; Thomas, M.M.; Wenger, L.M.; Yeakel, J.D. Parameteric Controls on the Composition of Oil Generated by In Situ Pyrolysis of Oil Shale. In Proceedings of the 28th Oil Shale Symposium, Golden, CO, USA, 13–15 October 2008. [Google Scholar]



| Physical Parameters (23 °C) | Mineral Composition (%) | ||||||
|---|---|---|---|---|---|---|---|
| Density (g/cm3) | Porosity (%) | Quartz | Microcline | Albite | Pyrite | Ankerite | Clay |
| 2.19 | 7.02 | 45.2 | 5.1 | 4.2 | 1.0 | 1.8 | 42.7 |
| Sample | Specific Surface Area | Average Pore Size | Pore Volume | Volume of Different-Sized Pores cm3/g | ||
|---|---|---|---|---|---|---|
| °C | m2/g | nm | cm3/g | Micropores | Transitional-Pores | Mesopores |
| 23 | 5.758 | 4.31 | 0.023 | 0.0151 | 0.0079 | 0 |
| 100 | 3.6486 | 5.27 | 0.0203 | 0.0123 | 0.008 | 0 |
| 200 | 3.5883 | 6.53 | 0.0183 | 0.0105 | 0.0078 | 0 |
| 300 | 9.9257 | 6.14 | 0.0436 | 0.0276 | 0.016 | 0 |
| 400 | 25.0759 | 8.62 | 0.0614 | 0.0324 | 0.029 | 0 |
| 500 | 35.3099 | 10.85 | 0.0861 | 0.0401 | 0.046 | 0 |
| 600 | 34.8532 | 12.17 | 0.0904 | 0.0368 | 0.0536 | 0 |
| 650 | 30.9812 | 10.9 | 0.0737 | 0.0336 | 0.0401 | 0 |
| Sample | Average Pore Size | Porosity | Pore Volume | Volume of Different-Sized Pores cm3/g | |||
|---|---|---|---|---|---|---|---|
| °C | nm | % | cm3/g | Micropores | Transitional-Pores | Mesopores | Macropores |
| 23 | 12.9 | 7.0189 | 0.035 | 0.0173 | 0.0111 | 0.001 | 0.0056 |
| 100 | 12.4 | 5.5175 | 0.0274 | 0.015 | 0.0078 | 5 × 10−4 | 0.0041 |
| 200 | 8.4 | 4.2829 | 0.0199 | 0.0118 | 0.0048 | 5 × 10−4 | 0.0028 |
| 300 | 10.2 | 5.6020 | 0.0275 | 0.017 | 0.0067 | 8 × 10−4 | 0.003 |
| 400 | 35.8 | 18.2599 | 0.0914 | 0.0051 | 0.0777 | 0.0067 | 0.0019 |
| 500 | 38.6 | 22.7861 | 0.1215 | 0.006 | 0.0816 | 0.0274 | 0.0065 |
| 600 | 25.8 | 32.4670 | 0.1884 | 0.0242 | 0.0975 | 0.0627 | 0.004 |
| 650 | 30 | 32.3090 | 0.1983 | 0.0221 | 0.0864 | 0.0824 | 0.0074 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Yang, D.; Hu, Y.; Zhang, J.; Shao, J.; Song, S.; Kang, Z. Influence of In Situ Pyrolysis on the Evolution of Pore Structure of Oil Shale. Energies 2018, 11, 755. https://doi.org/10.3390/en11040755
Liu Z, Yang D, Hu Y, Zhang J, Shao J, Song S, Kang Z. Influence of In Situ Pyrolysis on the Evolution of Pore Structure of Oil Shale. Energies. 2018; 11(4):755. https://doi.org/10.3390/en11040755
Chicago/Turabian StyleLiu, Zhijun, Dong Yang, Yaoqing Hu, Junwen Zhang, Jixi Shao, Su Song, and Zhiqin Kang. 2018. "Influence of In Situ Pyrolysis on the Evolution of Pore Structure of Oil Shale" Energies 11, no. 4: 755. https://doi.org/10.3390/en11040755





