Opportunities and Barriers to Bioenergy Conversion Techniques and Their Potential Implementation on Swine Manure
Abstract
:1. Introduction
2. Swine Manure Composition and Characteristics
3. Swine Manure Pretreatments
3.1. Manure Drying
3.2. Manure Solid Separation
3.3. Challenges Associated with Swine Manure Pretreatments
4. Biological Treatments of the Swine Manure
4.1. Swine Manure Composting
4.2. Swine Manure Anaerobic Digestion
4.3. Swine Manure Biodrying (Partial Composting)
4.4. Challenges Associated with Swine Manure Biological Conversion
- Poor design and equipment selection: Design and equipment selection for biological conversion systems can include decisions related to the manure pumping and conveyance systems, the gas cleaning and electrical generation equipment. Therefore, it is essential that the proper technology and equipment be selected. A failure of any one of these system components can result in operation failure, reduced revenue generation or added capital costs to replace faulty equipment.
- Lack of appropriate technical expertise: There is a lack of technical expertise in managing biological conversion systems in many cases due to the complexity of manure treatment systems. Although animal farmers routinely manage other complex systems, and training programs on biological conversion systems are available, there is a need to enhance the manure management skills to be able to sustain steady-state operations.
- Lack of system maintenance: Biological conversion systems need to be well maintained. Accordingly, producers need to dedicate some time and costs for maintaining these systems to avoid downtime.
- Lack of commitment by the operator: Given the seasonal nature of farming, there can be times of the year when the biological conversion systems may not receive the required attention and the careful maintenance. Again, this can be associated with the view that these systems are not an essential business function of the animal farm.
5. Thermochemical Conversion of Swine Manure
5.1. Swine Manure Combustion
- C, H, S, O, N, and Ash represent the elemental analysis of the feedstock.
- HHV is the higher heating value, MJ·kg−1.
- H is the hydrogen weight fraction in the sample.
- LHV is the lower heating value, MJ·kg−1.
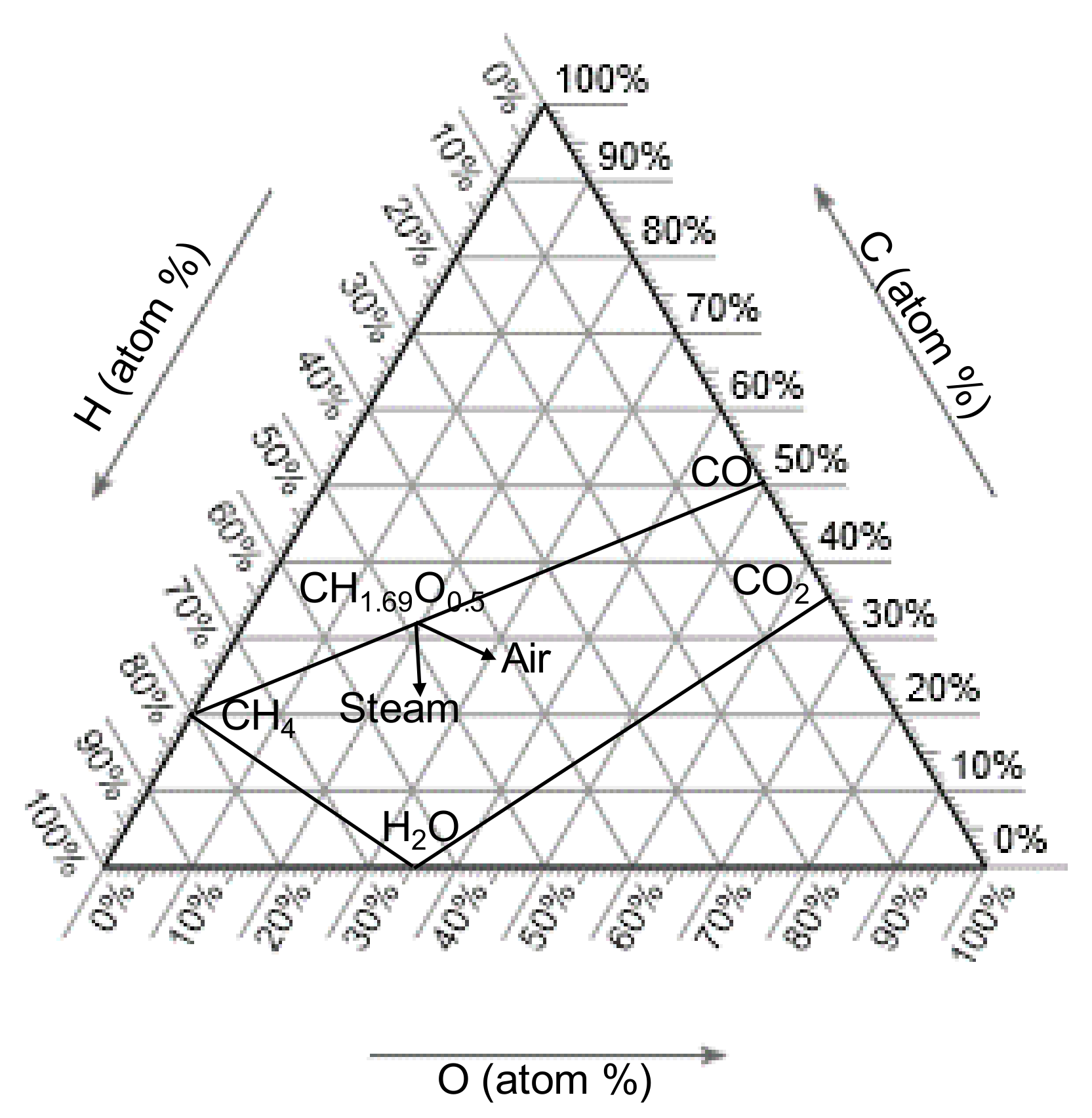
5.2. Swine Manure Gasification
5.3. Swine Manure Pyrolysis
5.4. Swine Manure Hydrothermal Liquefaction
5.5. Swine Manure Carbonization
5.6. Challenges Associated with Swine Manure Thermochemical Conversion
6. Conclusions
- Increases in scale and aggregation of swine production farms have resulted in manure accumulation problems in high production regions.
- Various manure management technologies, i.e., biological, and thermochemical, could be utilized to convert swine manure to value-added products while mitigating its negative impacts on surrounding ecosystems.
- Thermochemical conversion technologies are mature, stable and modular but, so far, underutilized in swine manure management.
- Gasification of swine manure solids, although under-investigated, can overcome the challenges associated with high-ash feedstock, and also generate a biochar stream.
- There is a need for integrating the swine manure biological and thermochemical conversion technologies to maximize the benefit of such a feedstock.
- There is a lack of research studies that investigate the kinetics of swine manure solids decomposition. These solids could be produced from biological conversion or solid separation technologies.
- It is crucial to develop comprehensive assessments of environmental impacts of thermochemical conversion as a manure management strategy.
Acknowledgments
Conflicts of Interest
References
- USDA-NASS. Quarterly Hogs and Pigs December 2017. ISSN 1949-1921. Available online: http://usda.mannlib.cornell.edu/usda/current/HogsPigs/HogsPigs-12-22-2017.pdf (accessed on 26 February 2018).
- American Society of Agricultural and Biological Engineers (ASABE). Standard D3843.2. In Manure Production and Characteristics 2005; ASABE: St. Joseph, MI, USA, 2005. [Google Scholar]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- USDA-NASS. Overview of the U.S. Hog Industry; NASS: Washington, DC, USA, 2009.
- Fantozzi, F.; Bartocci, P.; D’Alessandro, B.; Arampatzis, S.; Manos, B. Public–private partnerships value in bioenergy projects: Economic feasibility analysis based on two case studies. Biomass Bioenergy 2014, 66, 387–397. [Google Scholar] [CrossRef]
- Manos, B.; Partalidou, M.; Fantozzi, F.; Arampatzis, S.; Papadopoulou, O. Agro-energy districts contributing to environmental and social sustainability in rural areas: Evaluation of a local public–private partnership scheme in Greece. Renew. Sustain. Energy Rev. 2014, 29, 85–95. [Google Scholar] [CrossRef]
- Manos, B.; Bartocci, P.; Partalidou, M.; Fantozzi, F.; Arampatzis, S. Review of public–private partnerships in agro-energy districts in Southern Europe: The cases of Greece and Italy. Renew. Sustain. Energy Rev. 2014, 39, 667–678. [Google Scholar] [CrossRef]
- Hodgkinson, R.A.; Chambers, B.J.; Withers, P.J.A.; Cross, R. Phosphorus losses to surface waters following organic manure applications to a drained clay soil. Agric. Water Manag. 2002, 57, 155–173. [Google Scholar] [CrossRef]
- Novak, J.M.; Watts, D.W.; Hunt, P.G.; Stone, K.C. Phosphorus movement through a coastal plain soil after a decade of intensive swine manure application. J. Environ. Qual. 2000, 29, 1310–1315. [Google Scholar] [CrossRef]
- Aguirre-Villegas, H.A.; Larson, R.; Reinemann, D.J. From waste-to-worth: Energy, emissions, and nutrient implications of manure processing pathways. Biofuels Bioprod. Biorefin. 2014, 8, 770–793. [Google Scholar] [CrossRef]
- Miller, J.J.; Chanasyk, D.S.; Curtis, T.W.; Olson, B.M. Phosphorus and nitrogen in runoff after phosphorus- or nitrogen-based manure applications. J. Environ. Qual. 2011, 40, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Salvano, E.; Flaten, D.N.; Rousseau, A.N.; Quilbe, R. Are current phosphorus risk indicators useful to predict the quality of surface waters in southern Manitoba, Canada? J. Environ. Qual. 2009, 38, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- OSU. Ohio Livestock Manure and Wastewater Management Guide; Bulletin 604; Ohio State University Extension: Columbus, OH, USA, 2000; Available online: https://agcrops.osu.edu/sites/agcrops/files/imce/fertility/bulletin_604.pdf (accessed on 10 January 2018).
- Prince Edward Island. Guidelines for Manure Management for Prince Edward Island; Government of Prince Edward Island: Charlottetown, PE, Canada, 2000. Available online: http://www.gov.pe.ca/agric/index.php3?number=70588&lang=F (accessed on 20 December 2017).
- Day, D.; Funk, T.; Hatfield, J.; Stewart, B. Processing Manure: Physical, Chemical and Biological Treatment, in Animal Waste Utilization: Effective Use of Manure as a Soil Resource; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Sadaka, S.; Van Devender, K. Evaluation of Chemically Coagulated Swine Manure Solids as Value-Added Products. J. Sustain. Bioenergy Syst. 2015, 5, 136–150. [Google Scholar] [CrossRef]
- Zhang, R.H.; Westerman, P.W. Solid-liquid separation of animal manure for odor control and nutrient management. Appl. Eng. Agric. 1997, 13, 657–664. [Google Scholar] [CrossRef]
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. Solid-liquid separation of animal slurry in theory and practice—A review. Agron. Sustain. Dev. 2009, 30, 153–180. [Google Scholar] [CrossRef]
- Ford, M.; Fleming, R. Mechanical Solid-Liquid Separation of Livestock Manure—Literature Review; Prepared for Ontario Pork; Ridgetown College, University of Guelph: Ridgetown, ON, Canada, 2002; Available online: http://mie.esab.upc.es/ms/informacio/residus_ramaders/Separator%20manure.pdf (accessed on 17 March 2015).
- Rodriguez, M.D.E.; del Puerto, A.M.G.; Montealegre, M.L.M.; Adamsen, A.P.S.; Gullov, P.; Sommer, S.G. Separation of phosphorus from pig slurry using chemical additives. Appl. Eng. Agric. 2005, 21, 739–742. [Google Scholar] [CrossRef]
- Xiu, S.; Zhang, Y.; Shahbazi, A. Swine manure solids separation and thermochemical conversion to heavy oil. BioResources 2009, 4, 458–470. [Google Scholar]
- Hjorth, M.; Christensen, M.L.; Christensen, P.V. Flocculation, coagulation, and coagulation of manure affecting three separation techniques. Bioresour. Technol. 2008, 99, 8598–8604. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Flatow, L.A. Flocculation of swine manure: Influence of flocculant, rate of addition, and diet. Appl. Eng. Agric. 2002, 18, 609–614. [Google Scholar] [CrossRef]
- Christensen, M.L.; Hjorth, M.; Keidling, K. Characterization of pig slurry with reference to flocculation and separation. Water Res. 2009, 43, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Wnetrzak, R.; Kwapinski, W.; Peters, K.; Sommer, S.G.; Jensen, L.S.; Leahy, J.J. The influence of the pig manure separation system on the energy production potentials. Bioresour. Technol. 2013, 136, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Egyir, I.S.; Donkor, A.K.; Amoah, P.; Nyarko, S.; Boateng, K.K.; Ziwu, C. Feasibility study for biogas integration into waste treatment plants in Ghana. Egypt. J. Pet. 2017, 26, 695–703. [Google Scholar] [CrossRef]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Conventional Mesophilic vs. Thermophilic anaerobic digestion: A trade-off between performance and stability? Water Res. 2014, 53, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Sweeten, J.M.; Korenberg, J.; LePori, W.A.; Annamalai, K.; Parnell, C.B. Combustion of cattle feedlot manure for energy production. Energy Agric. 1986, 5, 55–72. [Google Scholar] [CrossRef]
- Bernal, M.P.; Sánchez-Monedero, M.A.; Paredes, C.; Roig, A. Carbon mineralization from organic wastes at different composting stages during their incubation with soil. Agric. Ecosyst. Environ. 1998, 69, 175–189. [Google Scholar] [CrossRef]
- Finestein, M.S.; Miller, F.C.; Strom, P.F. Monitoring and evaluating composting process performance. J. WPCF 1986, 58, 272–278. [Google Scholar]
- Tiquia, S.; Richard, T.; Honeyman, M. Effect of windrow turning and seasonal temperatures on composting of hog manure from hoop structures. Environ. Technol. 2000, 21, 1037–1046. [Google Scholar] [CrossRef]
- Sadaka, S.S.; Richard, T.L.; Loecke, T.D.; Liebman, M. Determination of Compost Respiration Rates Using Pressure Sensors. Compos. Sci. Util. 2006, 14, 124–131. [Google Scholar] [CrossRef]
- Gamroth, M.J. Composting: An Alternative for Livestock Manure Management and Disposal of Dead Animals; The Ohio State University: Columbus, OH, USA, 2012. [Google Scholar]
- Gajalakshmi, S.; Abbasi, S.A. Solid Waste Management by Composting: State of the Art. Crit. Rev. Environ. Sci. Technol. 2008, 38, 311–400. [Google Scholar] [CrossRef]
- Lau, A.K.; Lo, K.V.; Liao, P.H.; Yu, J.C. Aeration experiments for swine waste composting. Bioresour. Technol. 1992, 41, 145–152. [Google Scholar] [CrossRef]
- Huang, G.; Wu, Q.; Wong, J.; Nagar, B. Transformation of organic matter during co-composting of pig manure with sawdust. Bioresour. Technol. 2006, 97, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.; Alburquerque, J.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.K.; Mulbry, W.; White, J.; Kondrad, S. Pile mixing increases greenhouse gas emissions during composting of dairy manure. Bioresour. Technol. 2011, 102, 2904–2909. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Operating Anaerobic Digester Projects December 2014; U.S. EPA: Washington, DC, USA, 2014.
- EPA. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2011; EPA 430-R-13-001; EPA: Washington, DC, USA, 2013.
- Wang, Q.; Kuninobu, M.; Ogawa, H.I.; Kato, Y. Degradation of volatile fatty acids in highly efficient anaerobic digestion. Biomass Bioenergy 1999, 16, 407–416. [Google Scholar] [CrossRef]
- Ahring, B.K.; Sandberg, M.; Angelidaki, I. Volatile fatty acids as indicators of process imbalance in anaerobic digestors. Appl. Microbiol. Biotechnol. 1995, 43, 559–565. [Google Scholar] [CrossRef]
- Chen, H.; Li, B.; Yang, H.; Yang, G.; Zhang, S. Experimental investigation of biomass gasification in a fluidized bed reactor. Energy Fuels 2008, 22, 3493–3498. [Google Scholar]
- Angelidaki, I.; Ahring, B. Thermophilic anaerobic digestion of livestock waste: The effect of ammonia. Appl. Microbiol. Biotechnol. 1993, 38, 560–564. [Google Scholar] [CrossRef]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- Wu, X.; Yao, W.; Zhu, J.; Miller, C. Biogas and CH4 productivity by co-digesting swine manure with three crop residues as an external carbon source. Bioresour. Technol. 2010, 101, 4042–4047. [Google Scholar] [CrossRef] [PubMed]
- Lyberatos, G.; Skiadas, I. Modelling of anaerobic digestion—A review. Glob. Nest Int. J. 1999, 1, 63–76. [Google Scholar]
- Gallert, C.; Winter, J. Mesophilic and thermophilic anaerobic digestion of source-sorted organic wastes: Effect of ammonia on glucose degradation and methane production. Appl. Microbiol. Biotechnol. 1997, 48, 405–410. [Google Scholar] [CrossRef]
- Vindis, P.; Mursec, B.; Janzekovic, M.; Cus, F. The impact of mesophilic and thermophilic anaerobic digestion on biogas production. J. Achiev. Mater. Manuf. Eng. 2009, 36, 192–198. [Google Scholar]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, S.; Goodrich, B. Dairy Biomass as a Renewable Fuel Source; Texas Agri-Life Extension Service Publication No. L-5494; Texas A&M University: College Station, TX, USA, 2008. [Google Scholar]
- Jenkins, B.; Baxter, L.; Miles, T., Jr.; Miles, T. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Energie Centrum Nederland (ECN). Phyllis, the Composition of Biomass and Waste; ECN: Sint Maartensvlotbrug, The Netherlands, 2007; Available online: www.ecn.nl/phyllis2 (accessed on 12 October 2014).
- Adani, F.; Baido, D.; Calcaterra, E.; Genevini, P. The influence of biomass temperature on biostabilization—Biodrying of municipal solid waste. Bioresour. Technol. 2002, 83, 173–179. [Google Scholar] [CrossRef]
- Suler, D.J.; Finstein, M.S. Effect of temperature, aeration, and moisture on CO2 formation in bench-scale, continuously Thermophilic composting of solid waste. Appl. Environ. Microbiol. 1977, 33, 345–350. [Google Scholar] [PubMed]
- Sadaka, S.; Ahn, H. Evaluation of a biodrying process for beef, swine, and poultry manures mixed separately with corn stover. Appl. Eng. Agric. 2012, 28, 457–463. [Google Scholar] [CrossRef]
- Li, X.; Zhang, R.; Pang, Y. Characteristics of dairy manure composting with rice straw. Bioresour. Technol. 2008, 99, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Matsuda, J.; Ikeuchi, Y. High rapid composting of dairy cattle manure with crop and forest residues. Trans. ASAE 1983, 26, 533–541. [Google Scholar] [CrossRef]
- Gao, M.; Li, B.; Yu, A.; Liang, F.; Yang, L.; Sun, Y. The effect of aeration rate on forced-aeration composting of chicken manure and sawdust. Bioresour. Technol. 2010, 101, 1899–1903. [Google Scholar] [CrossRef] [PubMed]
- Sharara, M.A.; Sadaka, S.; Costello, T.A.; VanDevender, K. Influence of aeration rate on the physio-chemical characteristics of biodried dairy manure-wheat straw mixture. Appl. Eng. Agric. 2012, 28, 407–415. [Google Scholar] [CrossRef]
- Mason, D.M.; Gandhi, K.N. Formulas for calculating the calorific value of coal and coal chars: Development, tests, and uses. Fuel Process. Technol. 1983, 7, 11–22. [Google Scholar] [CrossRef]
- Cordero, T.; Marquez, F.; Rodriguez-Mirasol, J.; Rodriguez, J. Predicting heating values of lignocellulosics and carbonaceous materials from proximate analysis. Fuel 2001, 80, 1567–1571. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Li, H.; Liu, H. Thermodynamic evaluation of biomass gasification with air in autothermal gasifiers. Thermochim. Acta 2011, 519, 65–71. [Google Scholar] [CrossRef]
- Thygesen, A.; Wernberg, O.; Skou, E.; Sommer, S.G. Effect of incineration temperature on phosphorus availability in bio-ash from manure. Environ. Technol. 2011, 32, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Kumar, S.; Ra, C. Solid Waste from Swine Wastewater as a Fuel Source for Heat Production. Asian-Aust. J. Anim. Sci. 2012, 25, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.; Sánchez, M.E.; Gómez, X. Co-firing of coal and manure biomass: A TG–MS approach. Bioresour. Technol. 2011, 102, 8304–8309. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, M.; Puig-Gamero, M.; Lopez-Gonzalez, D.; Avalos-Ramirez, A.; Valverde, J.; Sanchez-Silva, L. Life cycle assessment of swine and dairy manure: Pyrolysis and combustion processes. Bioresour. Technol. 2015, 182, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Xiu, S.; Rojanala, H.K.; Shahbazi, A.; Fini, E.H.; Wang, L. Pyrolysis and combustion characteristics of Bio-oil from swine manure. J. Therm. Anal. Calorim. 2011, 107, 823–829. [Google Scholar] [CrossRef]
- Komiyama, T.; Kobayashi, A.; Yahagi, M. The chemical characteristics of ashes from cattle, swine and poultry manure. J. Mater. Cycles Waste Manag. 2013, 15, 106–110. [Google Scholar] [CrossRef]
- Petrus, L.; Noordermeer, M.A. Biomass to biofuels, a chemical perspective. Green Chem. 2006, 8, 861–867. [Google Scholar] [CrossRef]
- Yung, M.M.; Jablonski, W.S.; Magrini-Bair, K.A. Review of catalytic conditioning of biomass-derived syngas. Energy Fuels 2009, 23, 1874–1887. [Google Scholar] [CrossRef]
- Sadaka, S.S.; Ghaly, A.E.; Sabbah, M.A. Two-phase biomass air-steam gasification model for fluidized bed reactors: Part III—Model validation. Biomass Bioenergy 2002, 22, 479–487. [Google Scholar] [CrossRef]
- Lv, P.; Xiong, Z.; Chang, J.; Wu, C.; Chen, Y.; Zhu, J. An experimental study on biomass air–steam gasification in a fluidized bed. Bioresour Technol. 2004, 95, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Wang, L.; Dzenis, Y.A.; Jones, D.D.; Hanna, M.A. Thermogravimetric characterization of corn stover as gasification and pyrolysis feedstock. Biomass Bioenergy 2008, 32, 460–467. [Google Scholar] [CrossRef]
- González, J.; Román, S.; Bragado, D.; Calderón, M. Investigation on the reactions influencing biomass air and air/steam gasification for hydrogen production. Fuel Process. Technol. 2008, 89, 764–772. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Thermodynamics of gas-char reactions: First and second law analysis. Chem. Eng. Sci. 2003, 58, 1003–1011. [Google Scholar] [CrossRef]
- Reed, T.; Reed, T.B.; Das, A.; Das, A. Handbook of Biomass Downdraft Gasifier Engine Systems: Biomass Energy Foundation; National Technical Information Service, U.S. Department of Commerce: Alexandria, VA, USA, 1988.
- Balat, M.; Balat, M.; Kırtay, E.; Balat, H. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 2: Gasification systems. Energy Convers. Manag. 2009, 50, 3158–3168. [Google Scholar] [CrossRef]
- Di Blasi, C.; Signorelli, G.; Portoricco, G. Countercurrent fixed-bed gasification of biomass at laboratory scale. Ind. Eng. Chem. Res. 1999, 38, 2571–2581. [Google Scholar] [CrossRef]
- Van der Walt, I.J.; Nel, J.T.; Glasser, D.; Hildebrandt, D.; Ngubevana, L. An Economic Evaluation for Small Scale Thermal Plasma Waste-to-Energy Systems. 2012, pp. 1–4. Available online: https://www.yumpu.com/en/document/view/24349230/an-economic-evaluation-for-small-scale-plasma-waste-to-energy (accessed on 14 February 2018).
- Rajasekhar, M.; Rao, N.V.; Rao, G.C.; Priyadarshini, G.; Kumar, N.J. Energy generation from municipal solid waste by innoviative technologies—Plasma gasification. Procedia Mater. Sci. 2015, 10, 513–518. [Google Scholar] [CrossRef]
- Bosmans, A.; Wasan, S.; Helsen, L. Waste to clean syngas: Avoiding tar problems. In Proceedings of the 2nd International Academic Symposium on Enhanced Landfill Mining, Houthalen-Helchteren, Belgium, 15–16 October 2013. [Google Scholar]
- Campos, U.; Zamenian, H.; Koo, D.D.; Goodman, D.W. Waste-to-energy technology applications for municipal solid waste (MSW) treatment in the urban environment. Int. J. Emerg. Technol. Adv. Eng. 2015, 5, 504–508. [Google Scholar]
- Gray, L. Plasma gasification as a viable waste-to-energy treatment of municipal solid waste. In MANE 6960-Solid and Hazardous Waste Prevention and Control Engineering; Rensselaer Hartford: Hartford, CT, USA, 2014; pp. 1–15. [Google Scholar]
- Heberlein, J.; Murphy, A.B. Thermal plasma waste treatment. J. Phys. D Appl. Phys. 2008, 41, 1–20. [Google Scholar] [CrossRef]
- Sirillova, I.L. Waste treatment by plasma technology. Transf. Inovacii 2015, 31, 40–42. [Google Scholar]
- Cairns, E.; Tevebaugh, A. CHO Gas Phase Compositions in Equilibrium with Carbon, and Carbon Deposition Boundaries at One Atmosphere. J. Chem. Eng. Data 1964, 9, 453–462. [Google Scholar] [CrossRef]
- Narvaez, I.; Orio, A.; Aznar, M.P.; Corella, J. Biomass gasification with air in an atmospheric bubbling fluidized bed. Effect of six operational variables on the quality of the produced raw gas. Ind. Eng. Chem. Res. 1996, 35, 2110–2120. [Google Scholar]
- Gai, C.; Dong, Y. Experimental study on non-woody biomass gasification in a downdraft gasifier. Int. J. Hydrog. Energy 2012, 37, 4935–4944. [Google Scholar] [CrossRef]
- Baker, E.; Brown, M.; Elliott, D.; Mudge, L. Characterization and treatment of tars and biomass gasifiers. In Proceedings of the American Institute of Chemical Engineers Summer National Meeting, Denver, CO, USA, 21–24 August 1988. [Google Scholar]
- Boerrigter, H.; den Uil, H.; Calis, H. Green diesel from biomass via Fischer-Tropsch synthesis: New insights in gas cleaning and process design. In Proceedings of the Pyrolysis and Gasification of Biomass and Waste Expert Meeting, Strasbourg, France, 30 September–1 October 2002; pp. 371–383. [Google Scholar]
- Tijmensen, M.J.; Faaij, A.P.; Hamelinck, C.N.; van Hardeveld, M.R. Exploration of the possibilities for production of Fischer Tropsch liquids and power via biomass gasification. Biomass Bioenergy 2002, 23, 129–152. [Google Scholar] [CrossRef]
- Higman, C.; Van der Burgt, M. Gasification; Gulf Professional Publishing: Houston, TX, USA, 2003. [Google Scholar]
- Cantrell, K.; Ro, K.; Mahajan, D.; Anjom, M.; Hunt, P.G. Role of thermochemical conversion in livestock waste-to-energy treatments: Obstacles and opportunities. Ind. Eng. Chem. Res. 2007, 46, 8918–8927. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, J.; Takarada, T. Effect of pretreatment with different washing methods on the reactivity of manure char. Bioresour. Technol. 2010, 101, 6130–6135. [Google Scholar] [CrossRef] [PubMed]
- Arvelakis, S.; Gehrmann, H.; Beckmann, M.; Koukios, E.G. Preliminary results on the ash behavior of peach stones during fluidized bed gasification: Evaluation of fractionation and leaching as pre-treatments. Biomass Bioenergy 2005, 28, 331–338. [Google Scholar] [CrossRef]
- Roy, P.C.; Datta, A.; Chakraborty, N. Assessment of cow dung as a supplementary fuel in a downdraft biomass gasifier. Renew. Energy 2010, 35, 379–386. [Google Scholar] [CrossRef]
- Gautam, G.; Adhikari, S.; Brodbeck, C.; Bhavnani, S.; Fasina, O.; Taylor, S. Gasification of wood chips, agricultural residues, and waste in a commercial downdraft gasifier. Trans. ASABE 2011, 54, 1801–1807. [Google Scholar] [CrossRef]
- Morf, P.; Hasler, P.; Nussbaumer, T. Mechanisms and kinetics of homogeneous secondary reactions of tar from continuous pyrolysis of wood chips. Fuel 2002, 81, 843–853. [Google Scholar] [CrossRef]
- Evans, R.J.; Milne, T.A. Molecular characterization of the pyrolysis of biomass. Energy Fuels 1987, 1, 123–137. [Google Scholar] [CrossRef]
- Vreugdenhil, B.; Zwart, R.; Neeft, J.P.A. Tar Formation in Pyrolysis and Gasification; ECN: Sint Maartensvlotbrug, The Netherlands, 2009. [Google Scholar]
- Kuligowski, K.; Poulsen, T.G.; Rubæk, G.H.; Sørensen, P. Plant-availability to barley of phosphorus in ash from thermally treated animal manure in comparison to other manure based materials and commercial fertilizer. Eur. J. Agron. 2010, 33, 293–303. [Google Scholar] [CrossRef]
- Bridgwater, A.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Meier, D.; Faix, O. State of the art of applied fast pyrolysis of lignocellulosic materials—A review. Bioresour. Technol. 1999, 68, 71–77. [Google Scholar] [CrossRef]
- He, B.J.; Zhang, Y.; Yin, Y.; Funk, T.L.; Riskowski, G. Preliminary characterization of raw oil products from the thermochemical conversion of swine manure. Trans. ASAE 2001, 44, 1865–1871. [Google Scholar]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Mullen, C.A.; Boateng, A.A. Chemical Composition of Bio-oils Produced by Fast Pyrolysis of Two Energy Crops. Energy Fuels 2008, 22, 2104–2109. [Google Scholar] [CrossRef]
- Maggi, R.; Delmon, B. Comparison between ‘slow’and ‘flash’ pyrolysis oils from biomass. Fuel 1994, 73, 671–677. [Google Scholar] [CrossRef]
- Demirbaş, A. Mechanisms of liquefaction and pyrolysis reactions of biomass. Energy Convers. Manag. 2000, 41, 633–646. [Google Scholar] [CrossRef]
- Diebold, J.P. A Review of the Chemical and Physical Mechanisms of the Storage Stability of Fast Pyrolysis Bio-Oils; National Renewable Energy Laboratory Golden: Golden, CO, USA, 2000.
- Elliott, D.C. Historical developments in hydroprocessing bio-oils. Energy Fuels 2007, 21, 1792–1815. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Rioche, C.; Kulkarni, S.; Meunier, F.C.; Breen, J.P.; Burch, R. Steam reforming of model compounds and fast pyrolysis bio-oil on supported noble metal catalysts. Appl. Catal. B Environ. 2005, 61, 130–139. [Google Scholar] [CrossRef]
- Vagia, E.C.; Lemonidou, A.A. Thermodynamic analysis of hydrogen production via steam reforming of selected components of aqueous bio-oil fraction. Int. J. Hydrog. Energy 2007, 32, 212–223. [Google Scholar] [CrossRef]
- Takanabe, K.; Aika, K.; Seshan, K.; Lefferts, L. Sustainable hydrogen from bio-oil—Steam reforming of acetic acid as a model oxygenate. J. Catal. 2004, 227, 101–108. [Google Scholar] [CrossRef]
- Hossain, A.K.; Davies, P.A. Pyrolysis liquids and gases as alternative fuels in internal combustion engines–A review. Renew. Sustain. Energy Rev. 2013, 21, 165–189. [Google Scholar] [CrossRef]
- Hossain, A.K.; Serrano, C.; Brammer, J.B.; Omran, A.; Ahmed, F.; Smith, D.I.; Davies, P.A. Combustion of fuel blends containing digestate pyrolysis oil in a multi-cylinder compression ignition engine. Fuel 2016, 171, 18–28. [Google Scholar] [CrossRef]
- Vihar, R.; Seljak, T.; Oprešnik, S.R.; Katrašnik, T. Combustion characteristics of tire pyrolysis oil in turbo charged compression ignition engine. Fuel 2015, 150, 226–235. [Google Scholar] [CrossRef]
- Martin, J.A.; Boateng, A.A. Combustion performance of pyrolysis oil/ethanol blends in a residential-scale oil-fired boiler. Fuel 2014, 133, 34–44. [Google Scholar] [CrossRef]
- Shudo, T.; Nagano, T.; Kobayashi, M. Combustion characteristics of waste-pyrolysis gases in an internal combustion engine. Int. J. Automot. Technol. 2003, 4, 1–8. [Google Scholar]
- Shah, A.; Srinivasan, R.; To, S.D.F.; Columbus, E.P. Performance and emissions of a spark-ignited engine driven generator on biomass based syngas. Bioresour. Technol. 2010, 101, 4656–4661. [Google Scholar] [CrossRef] [PubMed]
- Hagos, F.Y.; Aziz, A.R.A.; Sulaiman, S.A. Trends of syngas as a fuel in internal combustion engines. Adv. Mech. Eng. 2014, 6, 401587. [Google Scholar] [CrossRef]
- Kim, S.; Agblevor, F.A.; Lim, J. Fast pyrolysis of chicken litter and turkey litter in a fluidized bed reactor. J. Ind. Eng. Chem. 2009, 15, 247–252. [Google Scholar] [CrossRef]
- Mante, O.D.; Agblevor, F.A. Parametric study on the pyrolysis of manure and wood shavings. Biomass Bioenergy 2011, 35, 4417–4425. [Google Scholar] [CrossRef]
- Cao, J.; Xiao, X.; Zhang, S.; Zhao, X.; Sato, K.; Ogawa, Y.; Wei, X.Y.; Takarada, T. Preparation and characterization of bio-oils from internally circulating fluidized-bed pyrolyses of municipal, livestock, and wood waste. Bioresour. Technol. 2011, 102, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Dolan, R.; Harris, M.; Tan, Z. Subcritical hydrothermal liquefaction of cattle manure to bio-oil: Effects of conversion parameters on bio-oil yield and characterization of bio-oil. Bioresour. Technol. 2010, 101, 3657–3664. [Google Scholar] [CrossRef] [PubMed]
- Biller, P.; Ross, A.B. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour. Technol. 2011, 102, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of suband supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Duan, P.; Savage, P.E. Upgrading of crude algal bio-oil in supercritical water. Bioresour. Technol. 2011, 102, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhang, L.; Zhang, S.; Fu, H.; Chen, J. Hydrothermal liquefaction of macroalgae Enteromorpha prolifera to bio-oil. Energy Fuels 2010, 24, 4054–4061. [Google Scholar] [CrossRef]
- Demirbas, F. Biorefineries for biofuel upgrading: A critical review. Appl. Energy 2009, 86, S151–S161. [Google Scholar] [CrossRef]
- He, B.J.; Zhang, Y.; Funk, T.L.; Riskowski, G.L.; Yin, Y. Thermochemical conversion of swine manure: An alternative process for waste treatment and renewable energy production. Trans. ASAE 2000, 43, 1827–1833. [Google Scholar]
- Xiu, S.; Shahbazi, A.; Wang, L.; Wallace, C.W. Supercritical ethanol liquefaction of swine manure for bio-oils production. Am. J. Eng. Appl. Sci. 2010, 3, 494–500. [Google Scholar] [CrossRef]
- Bergman, P.C.; Kiel, J.H. Torrefaction for biomass upgrading. In Proceedings of the 14th European Biomass Conference & Exhibition, Paris, France, 17–21 October 2005. [Google Scholar]
- Qiu, Y.; Zheng, Z.; Zhou, Z.; Sheng, G.D. Effectiveness and mechanisms of dye adsorption on a straw-based biochar. Bioresour. Technol. 2009, 100, 5348–5351. [Google Scholar] [CrossRef] [PubMed]
- Sohi, S.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Ro, K.S.; Cantrell, K.B.; Hunt, P.G.; Ducey, T.F.; Vanotti, M.B.; Szogi, A.A. Thermochemical conversion of livestock wastes: Carbonization of swine solids. Bioresour. Technol. 2009, 100, 5466–5471. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Soil Res. 2008, 45, 629–634. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Bruun, E.W.; Hauggaard-Nielsen, H.; Ibrahim, N.; Egsgaard, H.; Ambus, P.; Jensen, P.A.; Dam-Johansen, K. Influence of fast pyrolysis temperature on biochar labile fraction and short-term carbon loss in a loamy soil. Biomass Bioenergy 2011, 35, 1182–1189. [Google Scholar] [CrossRef]
- Unger, R.; Killorn, R. Effect of Three Different Qualities of Biochar on Selected Soil Properties. Commun. Soil Sci. Plant Anal. 2011, 42, 2274–2283. [Google Scholar] [CrossRef]
- Shinogi, Y.; Yoshida, H.; Koizumi, T.; Yamaoka, M.; Saito, T. Basic characteristics of low-temperature carbon products from waste sludge. Adv. Environ. Res. 2003, 7, 661–665. [Google Scholar] [CrossRef]
- Marchetti, R.; Castelli, F.; Orsi, A.; Sghedoni, L.; Bochicchio, D. Biochar from swine manure solids: Influence on carbon sequestration and Olsen phosphorus and mineral nitrogen dynamics in soil with and without digestate incorporation. Ital. J. Agron. 2012, 7, e26. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Martin, J.H. Stochastic state-space temperature regulation of biochar production. Part II: Application to manure processing via pyrolysis. J. Sci. Food Agric. 2012, 92, 490–495. [Google Scholar] [PubMed]
- Tsai, W.T.; Liu, S.C.; Chen, H.R.; Chang, Y.M.; Tsai, Y.L. Textural and chemical properties of swine-manure-derived biochar pertinent to its potential use as a soil amendment. Chemosphere 2012, 89, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Glaser, B.; Quicker, P. Technical, economical, and climate-related aspects of biochar production technologies: A literature review. Environ. Sci. Technol. 2011, 45, 9473–9483. [Google Scholar] [CrossRef] [PubMed]

| Component | Units | Gestating Sow | Lactating Sow | Boar |
|---|---|---|---|---|
| Animal weight | kg | 200 | 192 | 200 |
| Total Manure | kg·day−1animal−1 | 5.00 | 12.00 | 3.80 |
| Moisture Cont. | %, w.b. | 90.00 | 90.00 | 90.00 |
| TS | kg·day−1animal−1 | 0.50 | 1.20 | 0.38 |
| VS | kg·day−1animal−1 | 0.45 | 1.00 | 0.34 |
| BOD | kg·day−1animal−1 | 0.17 | 0.38 | 0.13 |
| N | kg·day−1animal−1 | 0.03 | 0.09 | 0.03 |
| P | kg·day−1animal−1 | 0.01 | 0.03 | 0.01 |
| K | kg·day−1animal−1 | 0.02 | 0.05 | 0.02 |
| Manure | Dry Matter (g·kg−1) | Organic C (g·kg−1) | Total N (g·kg−1) | NH4-N (g·kg−1) | pH - |
|---|---|---|---|---|---|
| Liquid | 4.9–152 | 1.0–65 | 0.6–7.8 | 0.3–6.6 | 6.7–8.9 |
| Solid | 150–330 | 42–132 | 3.5–11 | 0.5–6.0 | 8.1 |
| Feedstock | HHV (MJ·kg−1)-Dry Basis |
|---|---|
| Bituminous coal | 31.60 |
| Peat | 21.22 |
| Cellulose | 17.30 |
| Lignin | 26.70 |
| Poplar wood chips | 20.75 |
| Oil shale | 12.44 |
| Wheat straw | 17.55 |
| Corn stover | 18.10 |
| Rice straw | 15.95 |
| Poultry litter | 17.14 |
| Cattle manure | 17.36 |
| Swine manure | 19.70 |
| Class | Tar Class | |||
|---|---|---|---|---|
| Primary | Secondary | Tertiary (Alkyl) | Tertiary (Condensed) | |
| Compounds | Levoglucosan, hydroxyacetaldehyde, furfurals, and methoxyphenols | Phenolics, moreover, olefins | Methyl acenaphthylene, methylnaphthalene, toluene and indene | Benzene, naphthalene, acenaphthylene, pyrene |
| Temperature range | 500–800 °C | 500–1000 °C | 700–1000 °C | 700 °C ≥ 1000 °C |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharara, M.A.; Sadaka, S.S. Opportunities and Barriers to Bioenergy Conversion Techniques and Their Potential Implementation on Swine Manure. Energies 2018, 11, 957. https://doi.org/10.3390/en11040957
Sharara MA, Sadaka SS. Opportunities and Barriers to Bioenergy Conversion Techniques and Their Potential Implementation on Swine Manure. Energies. 2018; 11(4):957. https://doi.org/10.3390/en11040957
Chicago/Turabian StyleSharara, Mahmoud A., and Sammy S. Sadaka. 2018. "Opportunities and Barriers to Bioenergy Conversion Techniques and Their Potential Implementation on Swine Manure" Energies 11, no. 4: 957. https://doi.org/10.3390/en11040957




