Italian Experience on Electrical Storage Ageing for Primary Frequency Regulation
Abstract
:1. Introduction
2. Brief Description of the Storage Labs in Sardinia and Sicily
3. Ageing Tests
- a standard cycle (these tests are performed by the manufacturers themselves in their laboratories under the surveillance of Terna personnel and independent certification bodies);
- afrequency regulation cycle (performed upon independent, ISO 17025 certified, Italian laboratories); and
- a current step cycle (performed upon independent, ISO 17025 certified Italian laboratories).
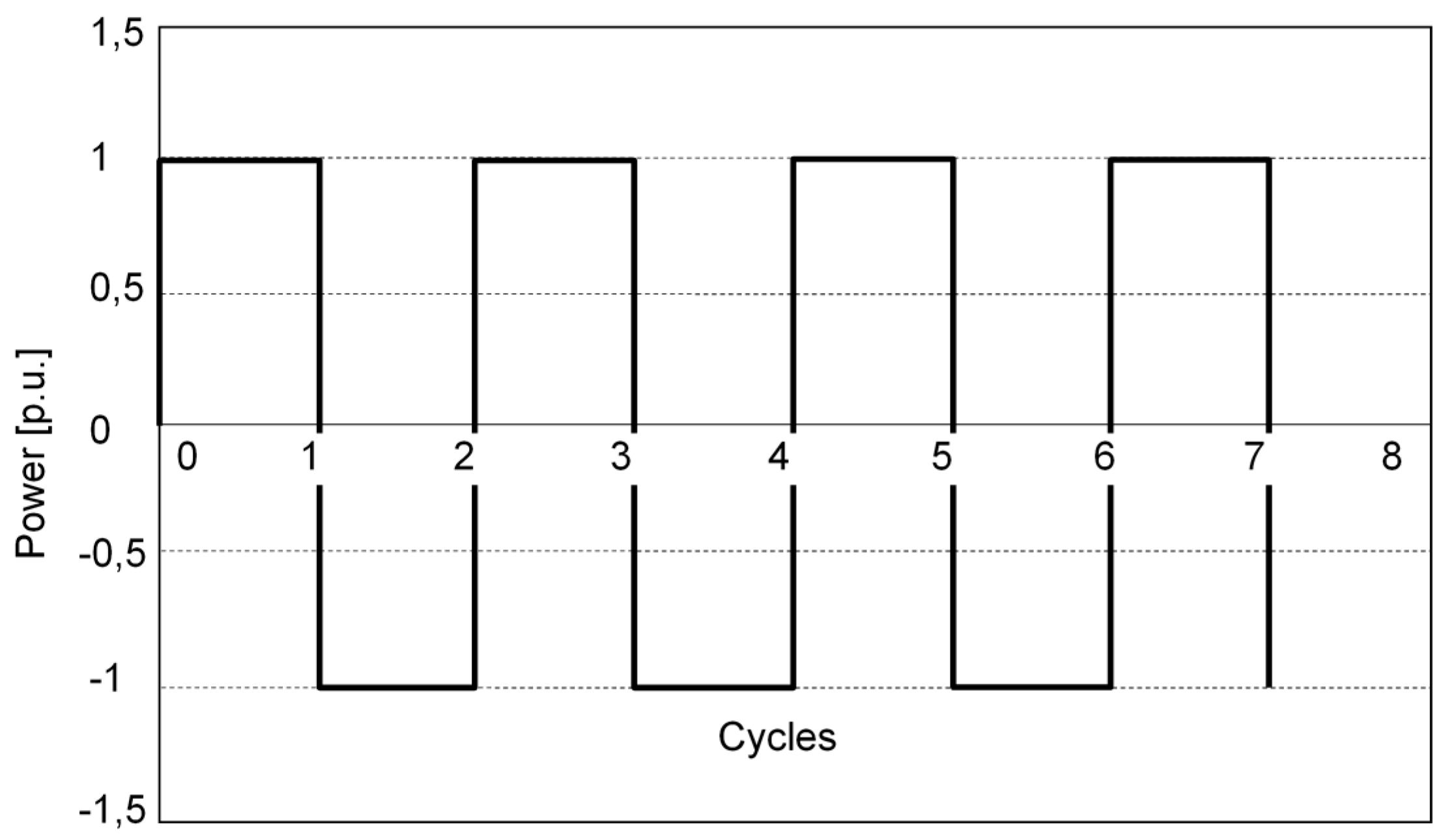
3.1. The Standard Cycle
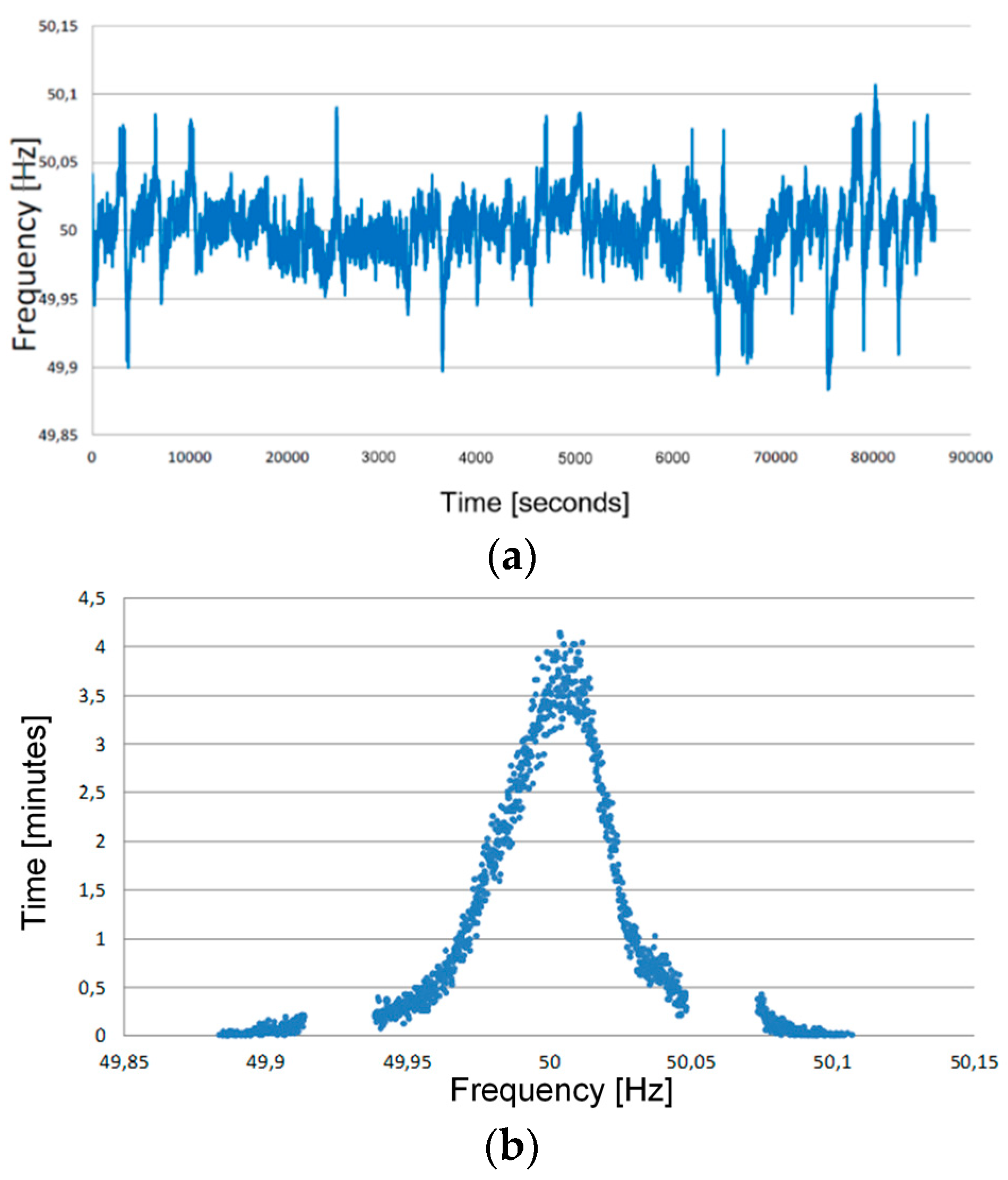
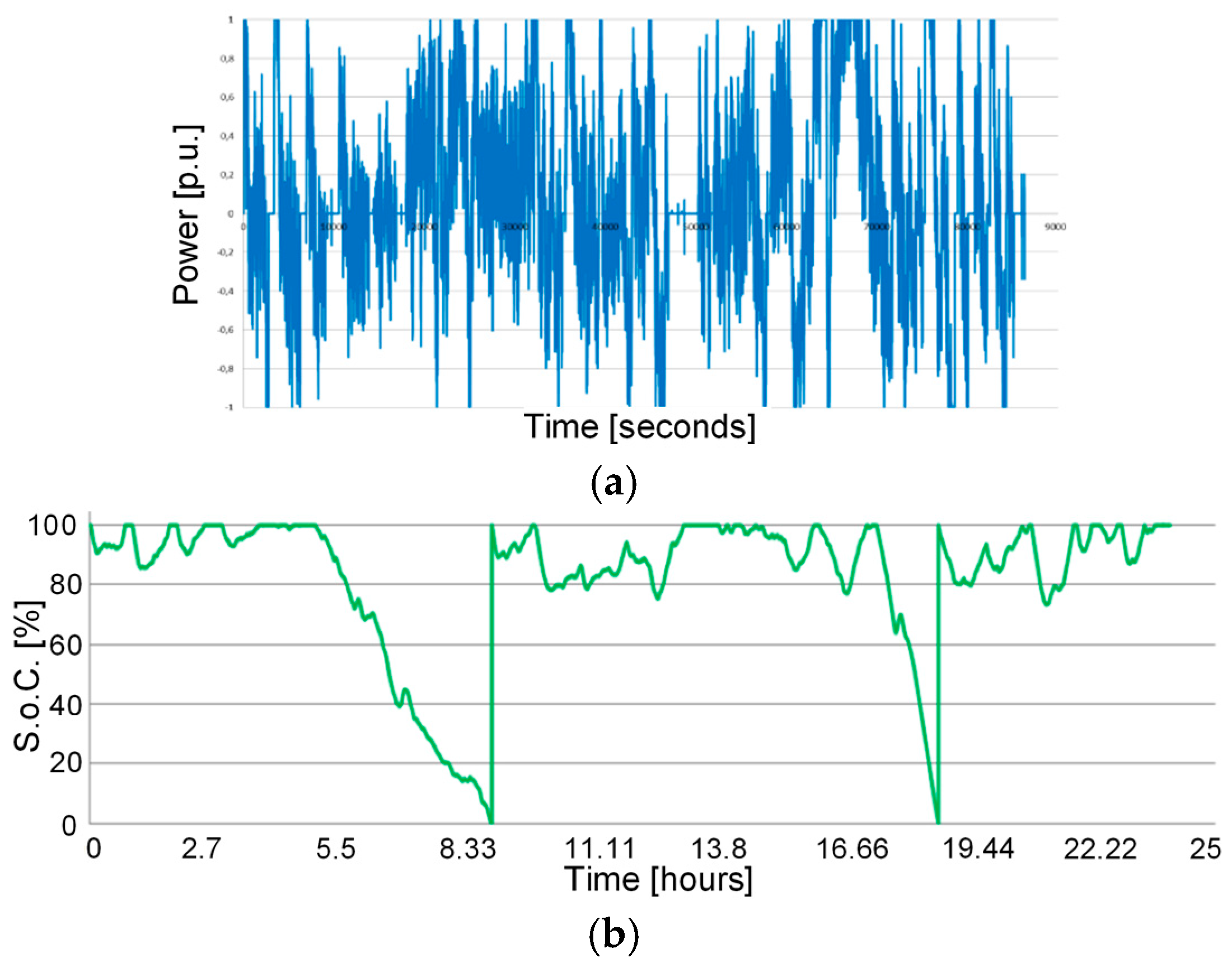
3.2. The Frequency Regulation Cycle
- A frequency droop of 0.075%;
- Deadband equal to 0 Hz;
- Initial S.o.C. equal to 100%;
- Maximum S.o.C. equal to 100% (no over-charge phases are foreseen);
- Minimum S.o.C. equal to 0% (no over-discharge phases are foreseen);
- Once the minimum S.o.C. is reached, the charge phase starts up to S.o.C. = 100% (with a charge current of 1 C for lithium-based technologies and of C/5 and C/4 for M1 and M2 sodium-nickel chloride technologies, respectively) and regulation cycle re-starts from the point where it was interrupted;
- Every 10 days for lithium-based technologies (28 days for sodium-nickel chloride ones), a full charge–discharge cycle is executed in order to determine the electrochemical parameters of the batteries, that is:
- -
- the total discharged capacity [Ah];
- -
- the total discharged energy [kWh];
- -
- the total charged capacity [Ah];
- -
- the total charged energy [kWh];
- -
- the temperature inside the modules [°C];
- -
- the ambient temperature [°C].
3.3. New Cycle with Current Steps
- 30 s of discharge at 1 C;
- 30 s of charge at 1 C;
- 30 s of discharge at 0.2 C;
- 30 s of charge at 0.2 C;
- 30 s of discharge at 0.2 C; and
- 30 s of charge at 0.2 C.
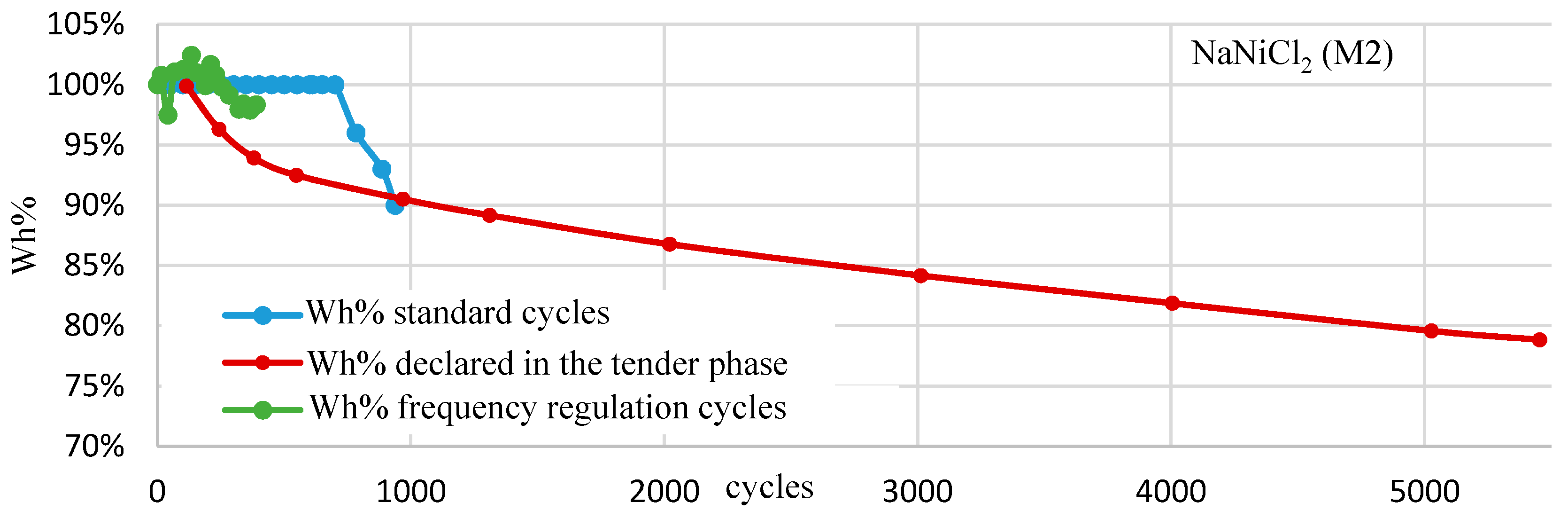
4. Ageing Test Results
5. Conclusions
- Some lithium-based technologies were not influenced by the cycle type and by its features in terms of both energy and power. In particular, the LTO and LMO technologies showed better performances among the tested lithium-based technologies.
- The capacity of the lithium nickel cobalt aluminium technology decreased by 20% at the end of the described test. It is the storage technology with the worst performance in terms of ageing, because of the standard cycle.
- The sodium-nickel chloride technologies did not show any ageing in terms of the dischargeable capacity reduction, with respect to the nominal one, when they are subjected to the frequency regulation cycle.
- With the same considered cycles (700), the LFP and LCNM batteries suffered more markedly in the continuous execution of the frequency regulation cycle than the standard one.
Author Contributions
Conflicts of Interest
References
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of current development in electrical energy storage technologies and the application potential in power system operation. Appl. Energy 2015, 137, 511–536. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, Q.; Hu, S.; Xu, H.; Rasmussen, C.N. Review of energy storage system for wind power integration support. Appl. Energy 2015, 137, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Benato, R.; Bruno, G.; Palone, F.; Polito, R.M.; Rebolini, M. Large-scale electrochemical energy storage in high voltage grids: Overview of the Italian experience. Energies 2017, 10, 108. [Google Scholar] [CrossRef]
- Andriollo, M.; Benato, R.; Dambone Sessa, S.; Di Pietro, N.; Hirai, N.; Nakanishi, Y.; Senatore, E. Energy intensive electrochemical storage in Italy: 34.8MW sodium–sulphur secondary cells. J. Energy Storage 2016, 5, 146–155. [Google Scholar] [CrossRef]
- Andriollo, M.; Benato, R.; Dambone Sessa, S. 34.8 MW di accumulo elettrochimico di tipo Energy Intensive mediante celle secondarie sodio-zolfo (Na-S). L’Energia Elettr. 2014, 5, 23–35. [Google Scholar]
- Sudworth, J.; Tilley, R. The Sodium/Sulfur Battery; Chapman and Hall: London, UK, 1985. [Google Scholar]
- Linden, D.; Reddy, T.B. Handbook of Batteries, 3rd ed.; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Dustmann, C.-H.; Bito, A. Safety. In Encyclopedia of Electrochemical Power Sources; Garche, J., Dyer, C., Moseley, P., Ogumi, Z., Rand, D., Scrosati, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; Volume 4, pp. 324–333. [Google Scholar]
- Ohima, T.; Kajita, M.; Okuno, A. Development of sodium-sulfur Batteries. Int. J. Appl. Ceram. Technol. 2004, 1, 269–276. [Google Scholar] [CrossRef]
- Benato, R.; Dambone Sessa, S.; Bevilacqua, F.; Palone, F. Measurement-based lithium-manganese oxide battery model. In Proceedings of the 2017 AEIT International Annual Conference, Cagliari, Italy, 20–22 September 2017. [Google Scholar]
- Mehr, T.H.; Masoum, M.A.S.; Jabalameli, N. Grid-connected lithium-ion battery energy storage system for load leveling and peak shaving. In Proceedings of the 2013 Australasian Universities Power Engineering Conference (AUPEC), Hobart, TAS, Australia, 29 September–3 October 2013. [Google Scholar]
- Winter, M.; Passerini, S. Lithium ion batteries as key component for energy storage in automotive and stationary applications. In Proceedings of the 2011 IEEE 33rd International Telecommunications Energy Conference (INTELEC), Amsterdam, The Netherlands, 9–13 October 2011. [Google Scholar]
- Enache, S.D.; Mircea, I.; Mircea, P.M. Study of the influence of lithium batteries on the power network. In Proceedings of the International Conference of Applied and Theoretical Electricity (ICATE), Craiova, Romania, 25–27 October 2012. [Google Scholar]
- Stroe, D.-I.; Knap, V.; Swierczynski, M.; Stroe, A.-I.; Teodorescu, R. Operation of a grid-connected lithium-ion battery energy storage system for primary frequency regulation: A battery lifetime perspective. IEEE Trans. Ind. Appl. 2016, 53, 430–438. [Google Scholar] [CrossRef]
- Yao, L.W.; Aziz, J.A.; Kong, P.Y.; Idris, N.R.N. Modeling of lithium-ion battery using MATLAB/Simulink. In Proceedings of the IECON 2013-39th Annual Conference of the IEEE Industrial Electronics Society, Vienna, Austria, 10–13 November 2013. [Google Scholar]
- Skoog, S. Electro-thermal modeling of high-performance lithium-ion energy storage systems including reversible entropy heat. In Proceedings of the 2017 IEEE Applied Power Electronics Conference and Exposition (APEC), Tampa, FL, USA, 26–30 March 2017. [Google Scholar]
- Dambone Sessa, S.; Palone, F.; Necci, A.; Benato, R. Sodium-nickel chloride battery experimental transient modelling for energy stationary storage. J. Energy Storage 2017, 9, 40–46. [Google Scholar] [CrossRef]
- Benato, R.; Cosciani, N.; Crugnola, G.; Dambone Sessa, S.; Lodi, G.; Parmeggiani, C.; Todeschini, M. Sodium nickel chloride battery technology for large-scale stationary storage in the high voltage network. J. Power Sources 2015, 293, 127–136. [Google Scholar] [CrossRef]
- Dambone Sessa, S.; Crugnola, G.; Todeschini, M.; Zin, S.; Benato, R. Sodium nickel chloride battery steady-state regime model for stationary electrical energy storage. J. Energy Storage 2016, 6, 105–115. [Google Scholar] [CrossRef]
- Dustmann, C.-H. Advances in ZEBRA batteries. J. Power Sources 2004, 127, 85–92. [Google Scholar] [CrossRef]
- Sudworth, J.L. The sodium/nickel chloride (ZEBRA) battery. J. Power Sources 2001, 100, 149–163. [Google Scholar] [CrossRef]
- Van Zyl, A. Review of the zebra battery system development. Solid State Ion. 1996, 86–88, 883–889. [Google Scholar] [CrossRef]
- Alotto, P.; Guarnieri, M.; Moro, F.; Stella, A. Redox flow batteries for large scale energy storage. In Proceedings of the 2012 IEEE International Energy Conference and Exhibition (ENERGYCON), Florence, Italy, 9–12 September 2012; pp. 293–298. [Google Scholar]
- Alotto, P.; Guarnieri, M.; Moro, F. Redox flow batteries for the storage of renewable energy: A review. Renew. Sustain. Energy Rev. 2014, 29, 325–335. [Google Scholar] [CrossRef]
- Alotto, P.; Guarnieri, M.; Moro, F.; Stella, A. Large scale energy storage with redox flow batteries. COMPEL Int. J. Comput. Math. Electr. Electr. Eng. 2013, 32, 1459–1470. [Google Scholar] [CrossRef]
- Andriollo, M.; Benato, R.; Bressan, M.; Dambone Sessa, S.; Palone, F.; Polito, R.M. Review of power conversion and conditioning systems for stationary electrochemical storage. Energies 2015, 8, 960–975. [Google Scholar] [CrossRef]
- Andriollo, M.; Benato, R.; Dambone Sessa, S.; Nucci, F.; Palone, F.; Rebolini, M.; Siviero, F. Power conversion system model and control for energy stationary storage. In Proceedings of the 2017 AEIT International Annual Conference, Cagliari, Italy, 20–22 September 2017. [Google Scholar]
- Mulder, G.; Omar, N.; Pauwels, S.; Leemans, F.; Verbrugge, B.; De Nijs, W.; Van den Bossche, P.; Six, D.; Van Mierlo, J. Enhanced test methods to characterise automotive battery cells. J. Power Sources 2011, 196, 10079–10087. [Google Scholar] [CrossRef]
- Standard IEC/EN 62660-1. Secondary Batteries for the Propulsion of Electric Road Vehicles—Part 1: Performance Testing For Lithium-Ion Cells; IEC Central Office: Geneva, Switzerland, 2010. [Google Scholar]
- Gyenes, B.; Stevens, D.A.; Chevrier, V.L.; Dahn, J.R. Understanding anomalous behavior in coulombic efficiency measurements on li-ion batteries. J. Electr. Soc. 2015, 162, 278–283. [Google Scholar] [CrossRef]
- Lewerenz, M.; Münnix, J.; Schmalstieg, J.; Käbitz, S.; Knips, M.; Sauer, D.U. Systematic aging of commercial LiFePO4jGraphite cylindrical cells including a theory explaining rise of capacity during aging. J. Power Sources 2017, 345, 254–263. [Google Scholar] [CrossRef]











| ACRONYM | Electrochemistry | Power (MW) | Energy (MWh) |
|---|---|---|---|
| LFP | lithium iron phosphate | 1 | 1.23 |
| NaNiCl2 | sodium nickel chloride | 1.2 | 4.15 |
| NaNiCl2 | sodium nickel chloride | 1 | 2.00 |
| NCA | lithium nickel cobalt aluminium | 1.2 | 0.97 |
| LMO | lithium manganese oxide | 1 | 0.92 |
| LNCM | lithium nickel cobalt manganese | 1 | 0.54 |
| LTO | lithium titanate | 1 | 1.02 |
| VRB | vanadium redox flow | 0.4 | 1.10 |
| ACRONYM | Electrochemistry | Power (MW) | Energy (MWh) |
|---|---|---|---|
| LFP | lithium iron phosphate | 1 | 1.23 |
| NaNiCl2 | sodium nickel chloride | 1.2 | 4.15 |
| NaNiCl2 | lithium nickel cobalt aluminium | 0.9 | 0.54 |
| LMO | lithium manganese oxide | 1 | 0.92 |
| LTO | lithium titanate | 1 | 1.02 |
| VRB | vanadium redox flow | 0.45 | 1.44 |
| Storage Technology | Nominal Voltage | Nominal Capacity | Nominal Energy | Module structure |
|---|---|---|---|---|
| LFP | 12.8 V | 185 Ah | 2.48 kWh | four battery elementary cells in series |
| NCA | 50 V | 80 Ah | 3.77 kWh | two packs composed of 14 cells connected in 2 parallel strings of 7 serial cells |
| NaNiCl2 (M1) | 620 V | 38 Ah | 23.5 kWh | 240 elementary cells in series |
| NaNiCl2 (M2) | 570 V | 35 Ah | 20 kWh | 240 elementary cells in series |
| LMNC | 51.8 V | 62 Ah | 3.2 kWh | 2 parallel strings of 14 elementary cells in series |
| LMO | 59.2 V | 60 Ah | 3.2 kWh | 16 elementary cells in series |
| LTO | 27.6 V | 40 Ah | 1.1 kwh | 2 parallel strings of 12 elementary cells in series |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benato, R.; Dambone Sessa, S.; Musio, M.; Palone, F.; Polito, R.M. Italian Experience on Electrical Storage Ageing for Primary Frequency Regulation. Energies 2018, 11, 2087. https://doi.org/10.3390/en11082087
Benato R, Dambone Sessa S, Musio M, Palone F, Polito RM. Italian Experience on Electrical Storage Ageing for Primary Frequency Regulation. Energies. 2018; 11(8):2087. https://doi.org/10.3390/en11082087
Chicago/Turabian StyleBenato, Roberto, Sebastian Dambone Sessa, Maura Musio, Francesco Palone, and Rosario Maria Polito. 2018. "Italian Experience on Electrical Storage Ageing for Primary Frequency Regulation" Energies 11, no. 8: 2087. https://doi.org/10.3390/en11082087






