Thermochemical Biomass Gasification: A Review of the Current Status of the Technology
Abstract
:1. Bioenergy and the Role of Biomass Gasification
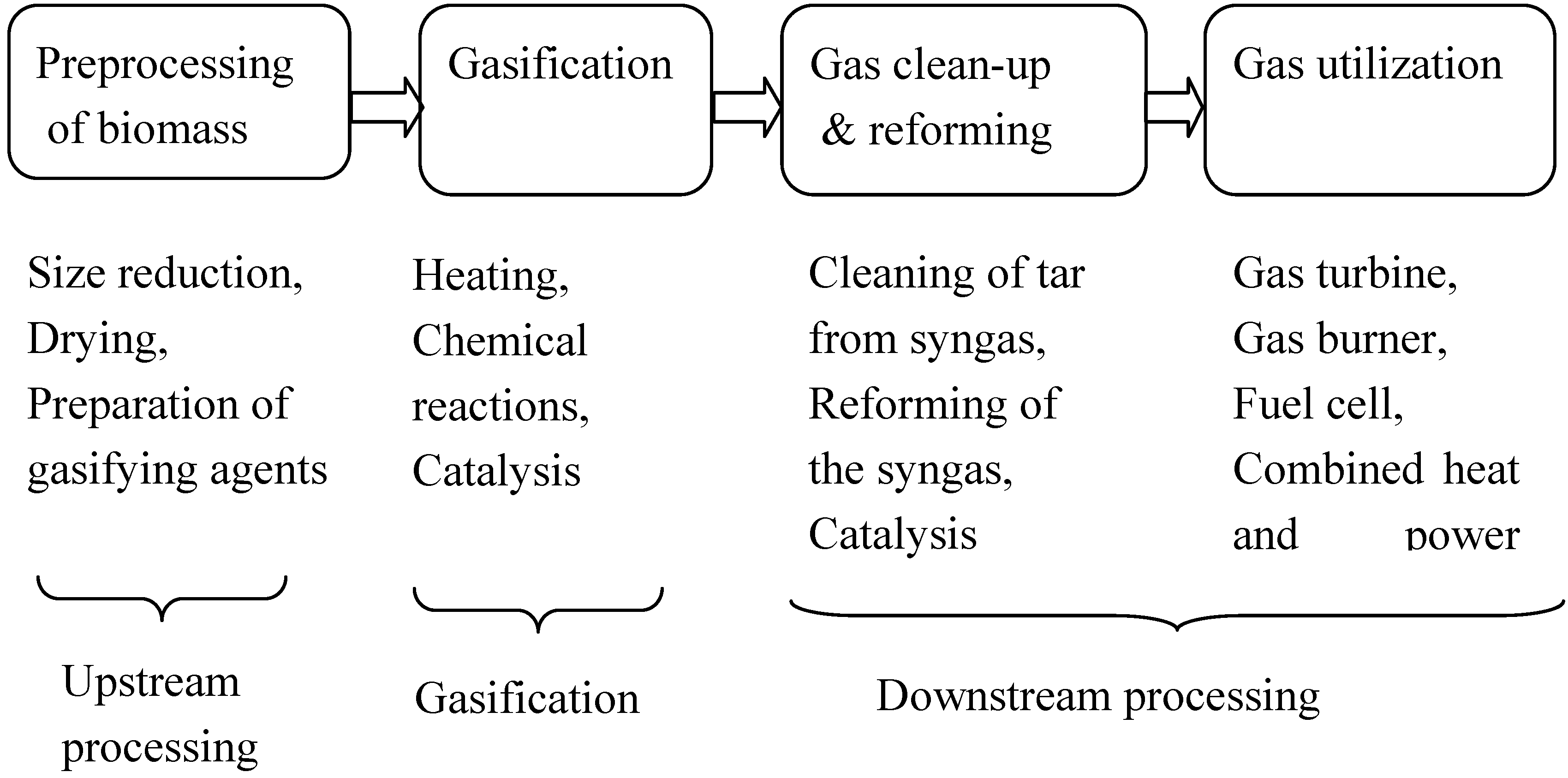
2. Upstream Processing
2.1. Size reduction
2.2. Drying
3. Gasification
3.1. Types of gasifier
3.2. Gasification process
3.3. Effects of gasification operating conditions on the product properties
3.3.1. Biomass flow rate, type and properties
3.3.2. Air flow rate (equivalence ratio, ER or superficial velocity, SV)
3.3.3. Steam flow rate (steam to biomass ratio, S/B)
3.3.4. Gasification temperature profile
4. Downstream Processing
4.1. Syngas cleaning
4.1.1. Particulate removal
4.1.2. Alkali removal
4.1.3. Nitrogen compounds
4.1.4. Sulfur compounds
4.1.5. Tar removal
Effects of operating conditions (A primary removal technique)
In-bed catalyst (A primary removal technique)
Wet cleaning (A secondary removal technique)
Reforming for secondary tar removal and increased H2 production
4.2. Conversion of syngas to biofuels, bioproducts and biopower
4.2.1. Biopower
4.2.2. Hydrogen
4.2.3. Catalytic synthesis of methanol and higher alcohols
4.2.4. Fischer-Tropsch Synthesis (FTS) diesel and gasoline
4.2.5. Fermentation of syngas to ethanol
5. Conclusions
Acknowledgements
References and Notes
- Fischer, G.; Schrattenholzer, L. Global bioenergy potentials through 2050. Biomass Bioenergy 2001, 20, 151–159. [Google Scholar] [CrossRef]
- Hoogwijk, M.; Faaij, A.; van den Broek, R.; Berndes, G.; Gielen, D.; Turkenburg, W. Exploration of the ranges of the global potential of biomass for energy. Biomass Bioenergy 2003, 25, 119–133. [Google Scholar] [CrossRef]
- Parikka, M. Global biomass fuel resources. Biomass Bioenergy 2004, 27, 613–620. [Google Scholar] [CrossRef]
- Perlack, R.D.; Wright, L.L.; Turhollow, A.F.; Graham, R.L.; Stokes, B.J.; Erbach, D.C. Biomass as feedstock for a bioenergy and bio-products industry: The technical feasibility of billion-ton annual supply; DOE/G0-102005-2135; ORNL/TM-2005/66; Oak Ridge National Laboratory: Oak Ridge, TN, USA, April 2005.
- Gates, B.C.; Huber, G.W.; Marshall, C.L.; Ross, P.N.; Siirola, J.; Wang, Y. Catalysts for emerging energy applications. MRS Bull. 2008, 33, 429–435. [Google Scholar] [CrossRef]
- Thorsell, S.; Epplin, F.M.; Huhnke, R.L.; Taliaferro, C.M. Economics of a coordinated biorefinery feedstock harvest system: Lignocellulosic biomass harvest cost. Biomass Bioenergy 2004, 27, 327–337. [Google Scholar] [CrossRef]
- Stiegel, G.J.; Maxwell, R.C. Gasification technologies: The path to clean, affordable energy in the 21st century. Fuel Process. Technol. 2001, 71, 79–97. [Google Scholar] [CrossRef]
- Sipilä, K. New power production technologies: various options for biomass and cogeneration. Bioresour. Technol. 1993, 46, 5–12. [Google Scholar] [CrossRef]
- Whitty, K.J.; Zhang, H.R.; Eddings, E.G. Emission from syngas combustion. Combust. Sci. Technol. 2008, 180, 1117–1136. [Google Scholar] [CrossRef]
- Petrus, L.; Noordermeer, M.A. Biomass to biofuels, a chemical perspective. Green Chem. 2006, 8, 861–867. [Google Scholar] [CrossRef]
- Wu, M.; Wu, Y.; Wang, M. Energy and emission benefits of alternative transportation liquid fuels derived from switchgrass: A fuel life cycle assessment. Biotechnol. Progr. 2006, 22, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Kirubakaran, V.; Sivaramakrishnan, V.; Nalini, R.; Sekar, T.; Premalatha, M.; Subramanian, P. A review on gasification of biomass. Renewable Sustainable Energy Rev. 2009, 13, 179–186. [Google Scholar] [CrossRef]
- Lv, P.; Chang, J.; Wang, T.; Fu, Y.; Chen, Y. Hydrogen-rich gas production from biomass catalytic gasification. Energy Fuels 2004, 18, 228–233. [Google Scholar] [CrossRef]
- Rapagna, S.; Latif, A. Steam gasification of almond shells in a fluidised bed reactor: The influence of temperature and particle size on product yield and distribution. Biomass Bioenergy 1997, 12, 281–288. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Guo, X.; Hu, Z.; Liu, S.; He, M. Hydrogen-rich gas from catalytic steam gasification of biomass in a fixed bed reactor: Influence of particle size on gasification performance. Int. J. Hydrogen Energy 2009, 34, 1260–1264. [Google Scholar] [CrossRef]
- Cummer, K.R.; Brown, R.C. Ancillary equipment for biomass gasification. Biomass Bioenergy 2002, 23, 113–128. [Google Scholar] [CrossRef]
- Mani, S.; Tabil, L.G.; Sokhansanj, S. Grinding performance and physical properties of wheat and barley straws, corn stover and switchgrass. Biomass Bioenergy 2004, 27, 339–352. [Google Scholar] [CrossRef]
- Brammer, J.G.; Bridgwater, A.V. The influence of feedstock drying on the performance and economics of a biomass gasifier-engine CHP system. Biomass Bioenergy 2002, 22, 271–281. [Google Scholar] [CrossRef]
- Ciferno, J.P.; Marano, J.J. Benchmarking biomass gasification technologies for fuels, chemicals and hydrogen production; US DOE National Energy Technology Laboratory: Pittsburgh, PA, USA, June 2002.
- Warnecke, R. Gasification of biomass: comparison of fixed bed and fluidized bed gasifier. Biomass Bioenergy 2000, 18, 489–497. [Google Scholar] [CrossRef]
- Mansaray, K.G.; Ghaly, A.E. Physical and thermochemical properties of rice husk. Energy Sources Part A 1997, 19, 989–1004. [Google Scholar] [CrossRef]
- Kumar, A.; Wang, L.; Dzenis, Y.A.; Jones, D.D.; Hanna, M.A. Thermogravimetric characterization of corn stover as gasification and pyrolysis feedstock. Biomass Bioenergy. 2008, 32, 460–467. [Google Scholar]
- Varhegyi, G.; Antal, M.J.; Jakab, E.; Szabó, P. Kinetic modeling of biomass pyrolysis. J. Anal. Appl. Pyrolysis 1997, 42, 73–87. [Google Scholar] [CrossRef] [Green Version]
- Biagini, E.; Barontini, F.; Tognotti, L. Devolatilization of biomass fuels and biomass components studied by TG/FTIR technique. Ind. Eng. Chem. Res. 2006, 45, 4486–4493. [Google Scholar] [CrossRef]
- Hanaoka, T.; Inoue, S.; Uno, S.; Ogi, T.; Minowa, T. Effect of woody biomass components on air-steam gasification. Biomass Bioenergy 2005, 28, 69–76. [Google Scholar] [CrossRef]
- Barneto, A.G.; Carmona, J.A.; Gálvez, A.; Conesa, J. Effects of the compositing and the heating rate on biomass gasification. Energy Fuels 2009, 23, 951–957. [Google Scholar] [CrossRef]
- Kumabe, K.; Hanaoka, T.; Fujimoto, S.; Minowa, T.; Sakanishi, K. Co-gasification of woody biomass and coal with air and steam. Fuel 2007, 86, 684–689. [Google Scholar] [CrossRef]
- Lapuerta, M.; Hernández, J.J.; Pazo, A.; López, J. Gasification and co-gasification of biomass wastes: Effect of the biomass origin and the gasifier operating conditions. Fuel Process. Technol. 2008, 89, 828–837. [Google Scholar] [CrossRef]
- Yamazaki, T.; Kozu, H.; Yamagata, S.; Murao, N.; Ohta, S.; Shiya, S.; Ohba, T. Effect of superficial velocity on tar from downdraft gasification of biomass. Energy Fuels 2005, 19, 1186–1191. [Google Scholar] [CrossRef]
- Narváez, I.; Orío, A.; Aznar, M.P.; Corella, J. Biomass gasification with air in an atmospheric bubbling fluidized bed. Effects of six operational variables on the quality of the produced raw gas. Ind. Eng. Chem. Res. 1996, 35, 2110–2120. [Google Scholar] [CrossRef]
- Lv, P.M.; Xiong, Z.H.; Chang, J.; Wu, C.Z.; Chen, Y.; Zhu, J.X. An experimental study on biomass air- steam gasification in a fluidized bed. Bioresour. Technol. 2004, 95, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yoshikawa, K.; Namioka, T.; Hashimoto, Y. Performance optimization of two-staged gasification system for woody biomass. Fuel Process. Technol. 2007, 88, 243–250. [Google Scholar] [CrossRef]
- Kumar, A.; Eskridge, K.; Jones, D.D.; Hanna, M.A. Steam-air fluidized bed gasification of distillers grains: effects of steam to biomass ratio, equivalence ratio and gasification temperature. Bioresour. Technol. 2009, 100, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Turn, S.; Kinoshita, C.; Zhang, Z.; Ishimura, D.; Zhou, J. An experimental investigation of hydrogen production from biomass gasification. Int. J. Hydrogen Energy 1998, 23, 641–648. [Google Scholar] [CrossRef]
- Lucas, C.; Szewczyk, D.; Blasiak, W.; Mochida, S. High-temperature air and steam gasification of densified biofuels. Biomass Bioenergy 2004, 27, 563–575. [Google Scholar] [CrossRef]
- Gupta, A.K.; Cichonski, W. Ultrahigh temperature steam gasification of biomass and solid wastes. Environ. Eng. Sci. 2007, 24, 1179–1189. [Google Scholar]
- Maschio, G.; Lucchesi, A.; Stoppato, G. Production of syngas from biomass. Bioresour. Technol. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Lv, P.M.; Yuan, Z.H.; Wu, C.Z.; Ma, L.L.; Chen, Y.; Tsubaki, N. Bio-syngas production from biomass catalytic gasification. Energy Convers. Manage. 2007, 48, 1132–1139. [Google Scholar] [CrossRef]
- Gonzalez, J.F.; Roman, S.; Bragado, D.; Calderon, M. Investigation on the reactions influencing biomass air and air/steam gasification for hydrogen production. Fuel Process. Technol. 2008, 89, 764–772. [Google Scholar] [CrossRef]
- Hanping, C.; Bin, L.; Haiping, Y.; Guolai, Y.; Shihong, Z. Experimental investigation of biomass gasification in a fluidized bed reactor. Energy Fuels. 2008, 22, 3493–3498. [Google Scholar] [CrossRef]
- Boateng, A.A.; Walawender, W.P.; Fan, L.T.; Chee, C.S. Fluidized-bed steam gasification of rice hull. Bioresour. Technol. 1992, 40, 235–239. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D.W.; Maloney, J.O. Perry’s chemical engineers’ handbook, 7th ed.; McGraw-Hill Professional: New York, NY, USA, 1997. [Google Scholar]
- Parnell, C.B.; Guzman, F. Cyclone design methodology for agricultural processing. In Winter Meeting of American Society of Agricultural Engineering, Chicago, IL, USA, 1982. Paper No. 82-3582.
- Parnell, C.B. Cyclone design for cotton gins. In International winter meeting of American Society of Agricultural Engineering, Chicago, IL, USA, 1990. Paper No. 90-5102.
- Baker, E.G.; Brown, M.D.; Moore, R.H.; Mudge, L.K.; Elliot, D.C. Engineering analysis of biomass gasifier product gas cleaning technology; PNL-5534; Pacific Northwest Laboratory: Richland, WA, USA, 1986.
- Stevens, D.J. Hot gas conditioning: recent progress with larger-scale biomass gasification systems; NREL/SR-510-29952; National Renewable Energy Laboratory: Golden, CO, USA, September 2001.
- Turn, S.Q.; Kinoshita, C.M.; Ishimura, D.M.; Hiraki, T.T.; Zhou, J.; Masutani, S.M. An experimental investigation of alkali removal from biomass producer gas using a fixed bed of solid sorbent. Ind. Eng. Chem. Res. 2001, 40, 1960–1967. [Google Scholar] [CrossRef]
- Goldschmidt, B.; Padban, N.; Cannon, M.; Kelsall, G.; Neergaard, M.; Ståhl, K.; Odenbrand, I. Ammonia formation and NOx emissions with various biomass and waste fuels at Värnamo 18 MWth IGCC plant. In Progress in thermochemical biomass conversion; Bridgewater, A.V., Ed.; Blackwell Science Ltd.: Malden, MA, USA, 2001; Vol. 1, pp. 524–535. [Google Scholar]
- Leppälahti, J.; Koljonen, T. Nitrogen evolution from coal, peat and wood during gasification: Literature review. Fuel Process. Technol. 1995, 43, 1–45. [Google Scholar] [CrossRef]
- Vamvuka, D.; Arvanitidis, C.; Zachariadis, D. Flue gas resulfurization at high temperature: a review. Environ. Eng. Sci. 2004, 21, 525–546. [Google Scholar] [CrossRef]
- Bergman, P.C.A.; van Passen, S.V.B. The novel “OLGA” technology for complete tar removal from biomass producer gas. In Pyrolysis and gasification of biomass and waste. Expert meeting, Strasbourg, France, September–October, 2002.
- Han, J.; Kim, H. The reduction and control technology of tar during biomass gasification/pyrolysis: an overview. Renewable Sustainable Energy Rev. 2008, 12, 397–416. [Google Scholar] [CrossRef]
- Neeft, J.P.A.; Knoef, H.A.M.; Buffinga, G.J.; Zielke, U. Guidelines for sampling and analysis of tars and particles in biomass producer gases. In Progress in thermochemical biomass conversion; Bridgewater, A.V., Ed.; Blackwell Science Ltd.: Malden, MA, USA, 2001; Vol. 1, pp. 162–175. [Google Scholar]
- Sanchez, J.M.; Ruiz, E.; Cillero, E.; Otero, J.; Cabanillas, A. Design of a biomass gasification gas sampling system. In Progress in thermochemical biomass conversion; Bridgwater, A.V., Ed.; Blackwell Science Ltd.: Malden, MA, USA, 2001; Vol. 1, pp. 137–149. [Google Scholar]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Rapagna, S.; Jand, N.; Kiennemann, A.; Foscolo, P.U. Steam-gasification of biomass in a fluidised-bed of olivine particles. Biomass Bioenergy 2000, 19, 187–197. [Google Scholar] [CrossRef]
- Asadullah, M.; Ito, S.; Kunimori, K.; Tomishige, K. Role of catalyst and its fluidization in the catalytic gasification of biomass to syngas at low temperature. Ind. Eng. Chem. Res. 2002, 41, 4567–4575. [Google Scholar] [CrossRef]
- Asadullah, M.; Miyazawa, T.; Ito, S.; Kunimori, K.; Koyama, S.; Tomishige, K. A comparison of Rh/CeO2/SiO2 catalysts with steam reforming catalysts, dolomite and inert materials as bed materials in low throughput fluidized bed gasification systems. Biomass Bioenergy 2004, 26, 269–279. [Google Scholar] [CrossRef]
- El-Rub, Z.A.; Bramer, E.A.; Brem, G. Review of catalysts for tar elimination in biomass gasification processes. Ind. Eng. Chem. Res. 2004, 43, 6911–6919. [Google Scholar] [CrossRef]
- Wang, T.; Chang, J.; Lv, P. Novel catalyst for cracking of biomass tar. Energy Fuels 2005, 19, 22–27. [Google Scholar] [CrossRef]
- Aznar, M.P.; Caballero, M.A.; Gil, J.; Martin, J.A.; Corella, J. Commercial steam reforming catalysts to improve biomass gasification with steam–oxygen mixtures. 2. catalytic tar removal. Ind. Eng. Chem. Res. 1998, 37, 2668–2680. [Google Scholar] [CrossRef]
- Corella, J.; Orio, A.; Toledo, J.M. Biomass gasification with air in a fluidized bed: exhaustive tar elimination with commercial steam reforming catalysts. Energy Fuels 1999, 13, 702–709. [Google Scholar] [CrossRef]
- Inaba, M.; Murata, K.; Saito, M.; Takahara, I. Hydrogen production by gasification of cellulose over Ni catalysts supported on zeolites. Energy Fuels 2006, 20, 432–438. [Google Scholar] [CrossRef]
- Nordgreen, T.; Liliedahl, T.; Sjöström, K. Elemental iron as a tar breakdown catalyst in conjunction with atmospheric fluidized bed gasification of biomass: a thermodynamic study. Energy Fuels 2006, 20, 890–895. [Google Scholar] [CrossRef]
- Corella, J.; Toledo, J.M.; Padilla, R. Catalytic hot gas cleaning with monoliths in biomass gasification in fluidized beds. 1. their effectiveness for tar elimination. Ind. Eng. Chem. Res. 2004, 43, 2433–2445. [Google Scholar] [CrossRef]
- Sato, K.; Fujimoto, K. Development of new nickel based catalyst for tar reforming with superior resistance to sulfur poisoning and coking in biomass gasification. Catal. Commun. 2007, 8, 697–1701. [Google Scholar] [CrossRef]
- Kimura, T.; Miyazawa, T.; Nishikawa, J.; Kado, S.; Okumura, K.; Miyao, T.; Naito, S.; Kunimori, K.; Tomishige, K. Development of Ni catalysts for tar removal by steam gasification of biomass. Appl. Catal. B 2006, 68, 160–170. [Google Scholar] [CrossRef]
- Fernando, S.; Adhikari, S.; Chaudrapal, C.; Murali, N. Biorefineries: current status, challenges, and future direction. Energy Fuels 2006, 20, 1727–1737. [Google Scholar] [CrossRef]
- Bain, R.L. Electricity from biomass in the United States: status and future direction. Bioresour. Technol. 1993, 46, 86–93. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 2): conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Kinoshita, C.M.; Turn, S.Q.; Overend, R.P.; Bain, R.L. Power generation potential of biomass gasification systems. J. Energy Eng. 1997, 88, 88–99. [Google Scholar] [CrossRef]
- Ståhl, K.; Waldheim, L.; Morris, M.; Johnsson, U.; Gårdmark, L. Biomass IGCC at Värnamo, Sweden – past and future. In GCEP Energy Workshop; Stanford University: Palo Alto, CA, USA, 27 April 2004. [Google Scholar]
- Yin, X.L.; Wu, C.Z.; Zheng, S.P.; Chen, Y. Design and operation of a CFB gasification and power generation system for rice husk. Biomass Bioenergy 2002, 23, 181–187. [Google Scholar] [CrossRef]
- Wu, C.; Yin, X.; Ma, L.; Zhou, Z.; Chen, H. Design and operation of a 5.5 MWe biomass integrated gasification combined cycle demonstration plant. Energy Fuels 2008, 22, 4259–4264. [Google Scholar] [CrossRef]
- Craig, K.R.; Mann, M.K. Cost and performance analysis of biomass-based integrated gasification combined-cycle (BIGCC) power systems; NREL/TP-430–21657; National Renewable Energy Laboratory: Golden, CO, USA, 1996.
- Uddin, S.K.; Barreto, L. Biomass-fired cogeneration systems with CO2 capture and storage. Renewable Energy 2007, 32, 1006–1019. [Google Scholar] [CrossRef]
- Rhodes, J.S.; Keith, D.W. Engineering economic analysis of biomass IGCC with carbon capture and storage. Biomass Bioenergy 2005, 29, 440–450. [Google Scholar] [CrossRef]
- Tiffany, D.G.; Morey, R.V.; Kam, M.J.D. Economic of biomass gasification/combustion at fuel ethanol plants. In 2007 ASABE Annual International Meeting, Minneapolis, MN, USA, June 2007. paper No. 076233.
- Farrell, A.E.; Gopal, A.R. Bioenergy research needs for heat, electricity, and liquid fuels. MRS Bull. 2008, 33, 373–380. [Google Scholar] [CrossRef]
- Abe, H.; Katayama, A.; Sah, B.P.; Toriu, T.; Samy, S.; Pheach, P.; Adams, M.A.; Grierson, P.F. Potential for rural electrification based on biomass gasification in Cambodia. Biomass Bioenergy 2007, 31, 656–664. [Google Scholar] [CrossRef]
- Siemons, R.V. Identifying a role for biomass gasification in rural electrification in developing countries: the economic perspective. Biomass Bioenergy 2001, 20, 271–285. [Google Scholar] [CrossRef]
- Faaji, A.P.C. Bio-energy in Europe: changing technology choices. Energy Policy 2006, 34, 322–342. [Google Scholar] [CrossRef]
- Marbán, G.; Valdés-Solís, T. Towards the hydrogen economy? Int. J. Hydrogen Energy 2007, 32, 1625–1637. [Google Scholar] [CrossRef]
- Spath, P.; Aden, A.; Eggeman, T.; Ringer, M.; Wallace, B.; Jechura, J. Biomass to hydrogen production detailed design and economics utilizing the Battelle Columbus Laboratory indirectly-heated gasifier; NREL/TP-510-37408; National Renewable Energy Lab: Golden, CO, USA, 2005.
- Milne, T.M.; Elam, C.C.; Evans, R.J. Hydrogen from biomass: State of the art and research challenges; IEA/H2/TR-02/001; National Renewable Energy Laboratory: Golden, CO, USA, 2001.
- Demibras, A. Hydrogen production from biomass by the gasification processes. Energy Sources 2002, 24, 59–68. [Google Scholar]
- Gerber, M.A.; White, J.F.; Stevens, D.J. Mixed alcohol synthesis catalyst screening; PNNL-16763; Pacific Northwest National Laboratory: Richland, WA, USA, 2007.
- Gerber, M.A.; Gray, M.; White, J.F.; Stevens, D.J. Evaluation of promoters for rhodium-based catalysts for mixed alcohol synthesis; PNNL-17857; Pacific Northwest National Laboratory: Richland, WA, USA, 2007.
- Dry, M.E. High quality diesel via the Fischer-Tropsch process - a review. J. Chem. Technol. Biotechnol. 2001, 77, 43–50. [Google Scholar] [CrossRef]
- Tijmensen, M.J.A.; Faaij, A.P.C.; Hamelinck, C.N.; van hardeveld, M.R.M. Exploration of the possibilities for production of fischer tropsch liquids and power via biomass gasification. Biomass Bioenergy 2002, 23, 129–152. [Google Scholar] [CrossRef]
- Ereña, J.; Arandes, J.M.; Bilbao, J.; Olazar, M.; de Lasa, H.I. Effect of the operating conditions on the conversion of syngas into liquid hydrocarbons over a Cr2O3-ZnO/ZSM5 bifunctional catalysts. J. Chem. Technol. Biotechnol. 1998, 72, 190–196. [Google Scholar] [CrossRef]
- Ereña, J.; Arandes, J.M.; Bilbao, J.; Aguayo, A.; de Lasa, H.I. Study of physical mixtures of Cr2O3-ZnO and ZSM-5 catalysts for the transformation of syngas into liquid hydrocarbons. Ind. Eng. Chem. Res. 1998, 37, 1211–1219. [Google Scholar] [CrossRef]
- Klasson, K.T.; Ackerson, M.; Clausan, E.C.; Gaddy, J.L. Bioconversion of synthesis gas into liquid or gaseous fuels. Enzyme Microb. Technol. 1992, 14, 602–608. [Google Scholar] [CrossRef]
- Henstra, A.M.; Sipma, J.; Rinzema, A.; Stams, A. Microbiology of synthesis gas fermentation for biofuel production. Curr. Opin. Biotechnol. 2007, 18, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Datar, R.P.; Shenkman, R.M.; Cateni, B.G.; Huhnke, R.L.; Lewis, R.S. Fermentation of biomass-generated producer gas to ethanol. Biotechnol. Bioeng. 2004, 86, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Bredwell, M.D.; Srivastava, P.; Worden, R.M. Reactor design issue for synthesis-gas fermentations. Biotechnol. Progr. 1999, 15, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Do, Y.S.; Smeenk, J.; Broer, K.M.; Kisting, C.J.; Brown, R.; Heindel, T.J.; Bobik, T.A.; DiSpirito, A.A. Growth of Rhodospirillum rubrum on synthesis gas: conversion of CO to H2 and Poly-B-hydroxyalkanoate. Biotechnol. Bioeng. 2007, 97, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.R.; Marcuschamer, D.K.; Stephanopoulos, G. Selection and optimization of microbial hosts for biofuels production. Metab. Eng. 2008, 10, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Lewis, R.S. Fermentation of biomass-generated synthesis gas: effects of nitric oxide. Biotechnol. Bioeng. 2007, 97, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556-581. https://doi.org/10.3390/en20300556
Kumar A, Jones DD, Hanna MA. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies. 2009; 2(3):556-581. https://doi.org/10.3390/en20300556
Chicago/Turabian StyleKumar, Ajay, David D. Jones, and Milford A. Hanna. 2009. "Thermochemical Biomass Gasification: A Review of the Current Status of the Technology" Energies 2, no. 3: 556-581. https://doi.org/10.3390/en20300556



