Strategies for Lowering Solid Oxide Fuel Cells Operating Temperature
Abstract
:1. Introduction
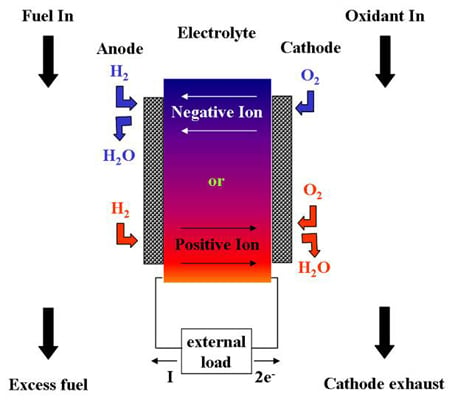
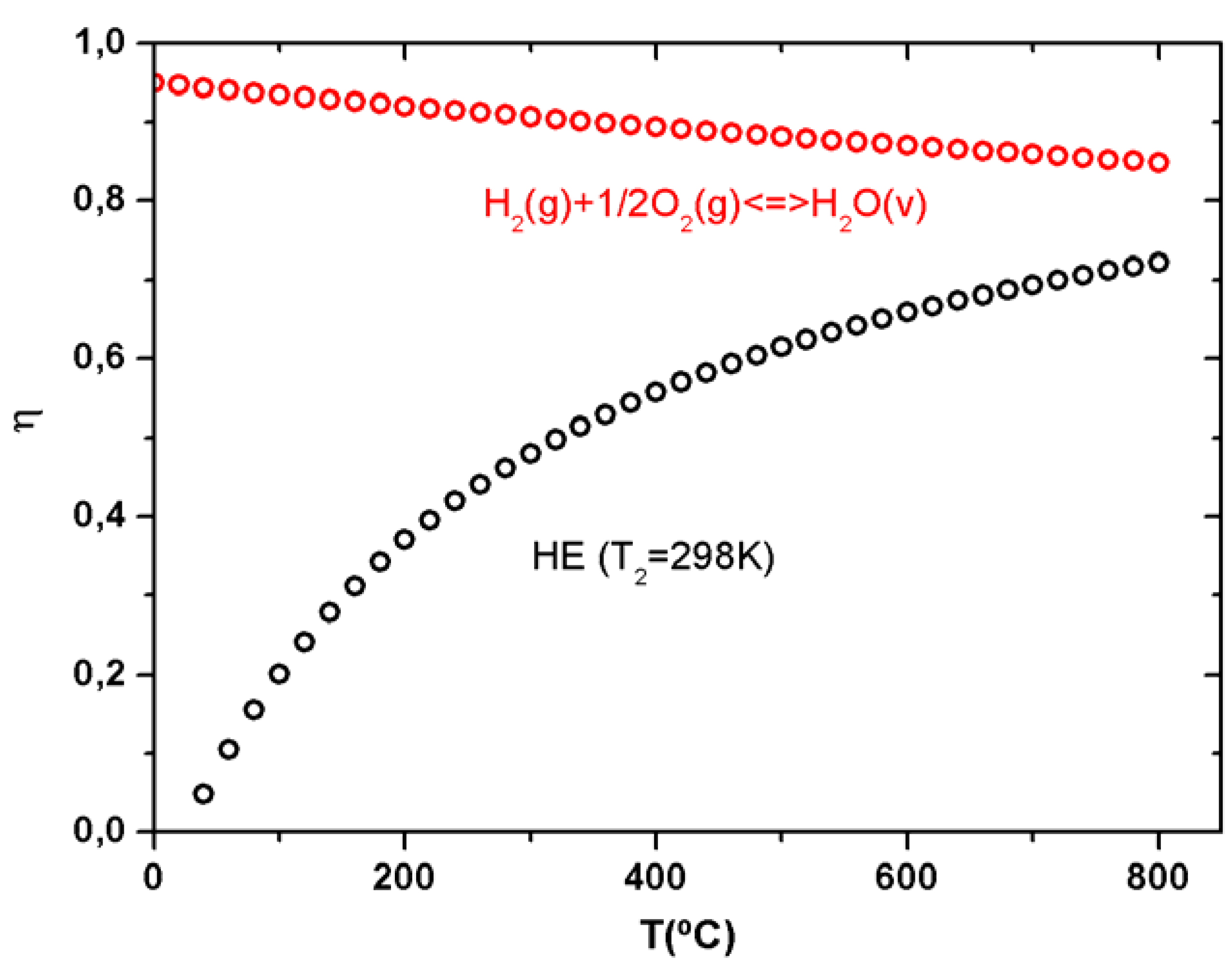
1.1. Effect of Low Operating Temperatures in Fuel Cell Efficiency
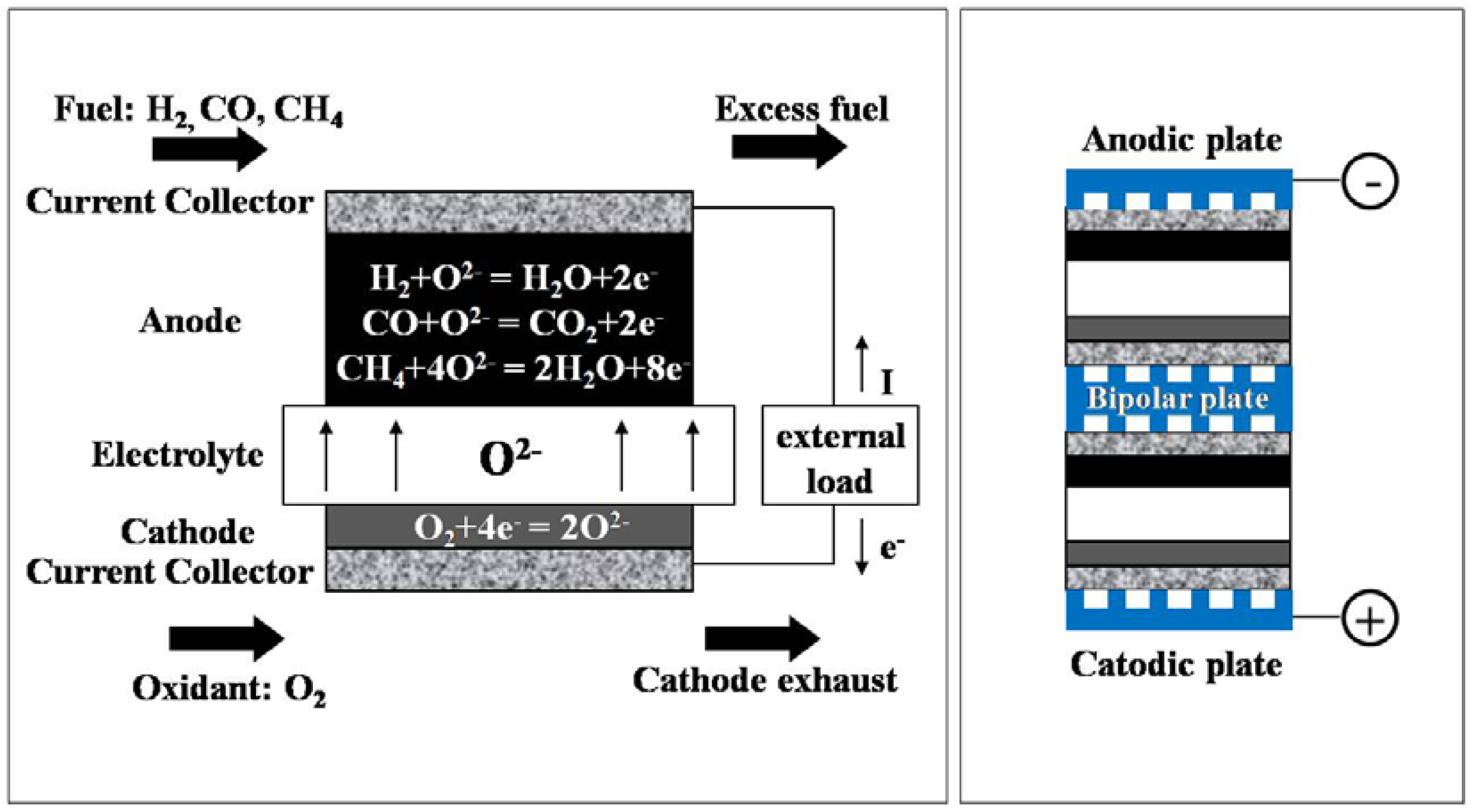
1.2. Solid Oxide Fuel Cells
2. The State-of-the-Art Materials for Solid Oxide Fuel Cells
| Component | ca. 1965 | ca. 1975 | At Present |
|---|---|---|---|
| Anode | Porous Pt | Ni/ZrO2 cermet | Ni/ZrO2 cermet (~150 μm thickness) 20%–40% porosity TEC~ 12.5 × 10−6 cm/cm K−1 |
| Cathode | Porous Pt | Stabilized ZrO2 impregnated with Pr2O3 and covered with SnO doped In2O3 | Doped LaMnO3 (~2 mm thickness) 30%–40% porosity TEC~ 11 × 10−6 cm/cm K−1 |
| Electrolyte | YSZ (~0.5-mm thickness) | YSZ | YSZ (~30–40 μm thickness) TEC~ 10.5 × 10−6 cm/cm K−1 |
| Interconnector | Pt | Mn doped cobalt chromite | Doped LaCrO3 (~100 μm thickness) TEC~ 10 × 10−6 cm/cm K−1 |
3. The Relevance of the Temperature for Solid Oxide Fuel Cells
3.1. Thermo-Mechanical Mismatch
3.2. Materials Stability
3.3. Thermal Management and Sulphur Tolerance
3.4. Interconnect Materials (and Bipolar Plates)
4. Reduction of the Operation Temperature: Materials for Intermediate Temperature Solid Oxide Fuel Cells
4.1. Electrolyte Thickness Effect
4.2. Oxide Ion Conductors Limitations

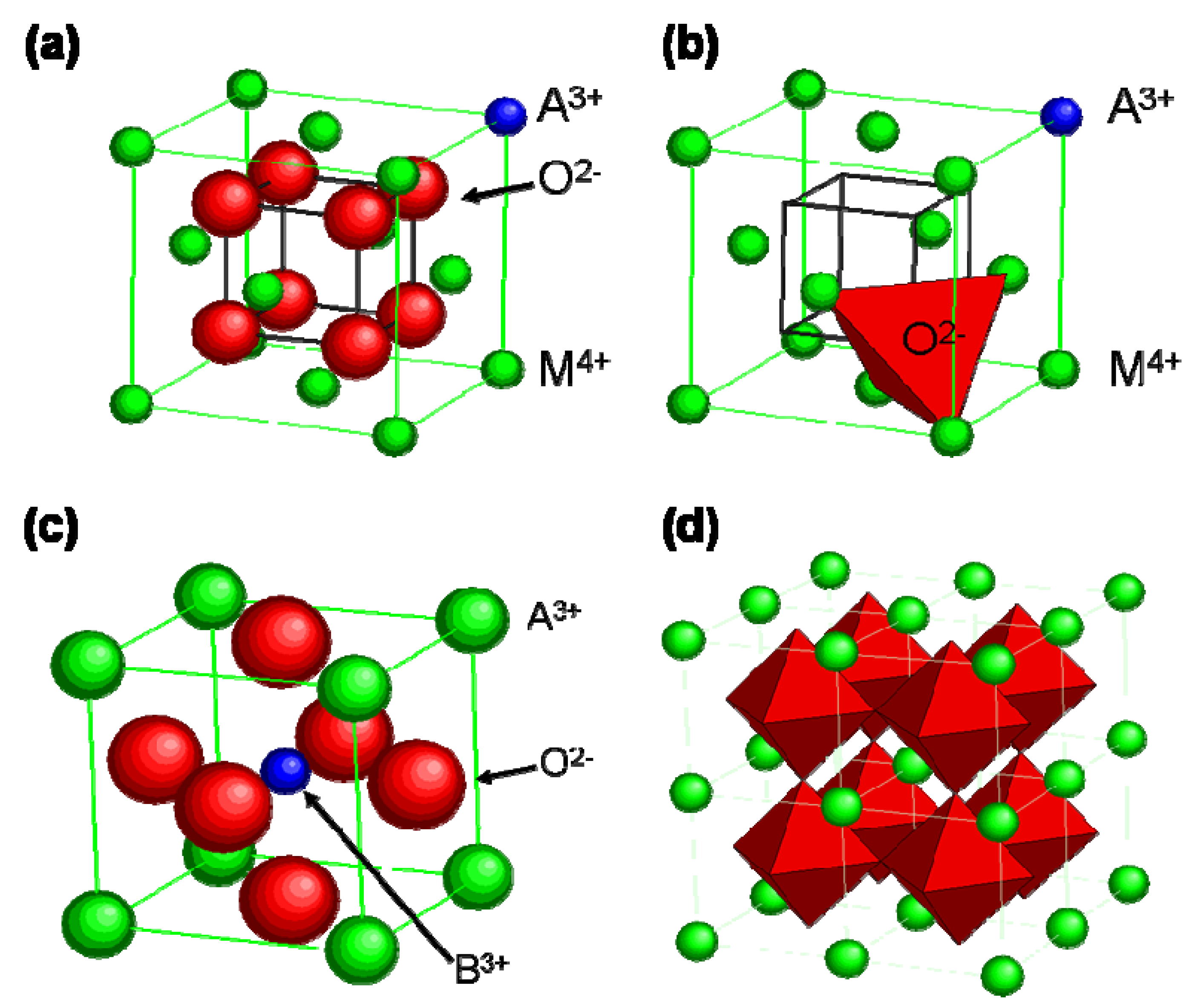
4.3. The Role of the Electrodes
| D*/cm2s–1 T = 500 °C | k*/cm·s–1 T = 500 °C | s/Scm–1 T = 500–750 °C | Ref. | |
|---|---|---|---|---|
| La0.8Sr0.2MnO3–δ | 4.5 × 10–20 | 3.1 × 10–11 | 120–130 | [ 78–80] |
| La0.8Sr0.2CoO3–δ | 9.0 × 10–14 | 2.8 × 10–9 | 1,500–1,600 | [ 78,79,81] |
| La0.5Sr0.5CoO3–δ | 1.5 × 10–10 | 3.9 × 10–7 | 1,300–1,800 | [ 78,79,81] |
| Ba0.5Sr0.5Co0.8Fe0.2O3–δ | 1.2 × 10–7 | 1.1 × 10–6 | 10–55 | [ 82,83] |
| La2NiO4+δ* | 3.3 × 10–9 | 7.0 × 10–9 | 55–65 | [ 67,84] |
| La2CoO4+δ* | 2.5 × 10–8 | 3.2 × 10–6 | 1–5 | [ 66,85] |
| GdBaCo2O5+δ* | 2.8 × 10–10 | 7.5 × 10–8 | 550–925 | [ 74,86] |
| PrBaCo2O5+δ* | 3.6 × 10–7 | 6.9 × 10–5 | 400–700 | [ 87] |
| *Layered o × ide structures | ||||
5. New Strategies for Operation Temperature Reduction Based on Micro and Nanotechnologies
6. Conclusions
- -
- Reduction of the electrolyte thickness by using new techniques like dense screen printing.
- -
- Development of new electrolyte materials, e.g., LAMOX, BIMEVOX or apatite families, and understanding of mass transport properties, where simulation work could play a main role.
- -
- Development of new anodes based on oxides to substitute classical cermets, e.g., La-SrTiO3 based materials.
- -
- Development of new cathodes presenting layered structures, e.g. La2NiO4+d and GdBaCo2O5+d.
- -
- Adaptation of the fabrication processes for using interconnections based on metals (metal supported SOFC).
- -
- New strategies based on micro- and nano-technolgies (micro-SOFC and nanoionics concepts).
Acknowledgements
References and Notes
- Fuel Cell Handbook, 7th ed.; E.G.&G. Services for the U.S.; Department of Energy, National Technical Information Service: Springfield, VA, USA, 2004.
- Appleby, A.J.; Foulkes, F.R. Fuel Cell Handbook; Van Nostrand Reinhold: New York, NY, USA, 1989. [Google Scholar]
- Yamamoto, O. Solid oxide fuel cells: Fundamental aspects and prospects. Electrochim. Acta 2000, 45, 2423–2435. [Google Scholar] [CrossRef]
- Haber, F.; Moser, A. Das Generatorgas- und das Kohlenelement. Z. Elektrochem. 1905, 11, 593–609. [Google Scholar] [CrossRef]
- Nernst, W. Über die elektrolytische Leitung fester Körper bei sehr hohen Temperaturen. Z. Elektrochem. 1899, 6, 41–43. [Google Scholar]
- Wagner, C. Über den Mechanismus der elektrischen Stromleitung im Nernststift. Naturwissenschaften 1943, 31, 265–268. [Google Scholar] [CrossRef]
- Baur, E.; Preis, H. Über Brennsto-Ketten mit Festleitern. Z. Elktrochem 1937, 43, 727–732. [Google Scholar]
- Möbius, H.H. On the history of solid electrolyte fuel cells. J. Solid State Electrochem. 1997, 1, 2–16. [Google Scholar]
- Final Report, Project Fuel Cell, Rep. No. 57; Westinghouse Electric Corp: Monroeville, PA, USA, 1970.
- Etsell, T.H.; Flengas, S.N. The electrical properties of solid oxide electrolytes. Chem. Rev. 1970, 70, 339–376. [Google Scholar] [CrossRef]
- Terauchi, S.; Takizawa, H.; Endo, T.; Uchida, S.; Terui, T.; Shimada, M. High ionic conductivity and high fracture strength of cubic zirconia, (Y0.16- xScx)Zr0.84O1.92/alumina composites. Mat. Letts. 1995, 23, 273–275. [Google Scholar] [CrossRef]
- Li, Z.; Behruzi, M.; Fuerst, L.; Stöver, D. Crystalline structure and electrical conductivity of bulk-sintered and plasma-sprayed La1-xSrxMnO3-8 with 0 < x < 0.9. In Proceedings of the 3rd International Symposium of Solid Oxide Fuel Cells, Honolulu, HI, USA, May 1993; Singhal, S.C., Ed.; The Electrochemical Society: Pennington, NJ, USA, 1995; p. 171. [Google Scholar]
- Mitterdorfer, A.; Gauckler, L.J. La2Zr2O7 formation and oxygen reduction kinetics of the La0.85Sr0.15MnyO3, O2 (g)|YSZ system. Solid State Ionics 1998, 111, 185–218. [Google Scholar] [CrossRef]
- Fleig, J. Solid oxide fuel cell cathodes: Polarization mechanisms and modeling of the electrochemical performance. Ann. Rev. Mat. Res. 2003, 33, 361–382. [Google Scholar] [CrossRef]
- Fleig, J.; Maier, J. The polarization of mixed conducting SOFC cathodes: Effects of surface reaction coefficient, ionic conductivity and geometry. J. Eur. Ceram. Soc. 2004, 24, 1343–1347. [Google Scholar] [CrossRef]
- Dees, D.W.; Claar, T.D.; Easler, T.E.; Fee, D.C.; Mrazek, F.C. Conductivity of porous Ni/ZrO//2-Y//2O//3 cermets. J. Electrochem. Soc. 1987, 134, 2141–2146. [Google Scholar] [CrossRef]
- Majumdar, S.; Claar, T.; Flandermeyer, B. Stress and fracture behavior of monolithic fuel cell tapes. J. Am. Ceram. Soc. 1986, 69, 628–633. [Google Scholar] [CrossRef]
- Hsiao, Y.C.; Selman, J.R. The degradation of SOFC electrodes. Solid State Ionics 1997, 98, 33–38. [Google Scholar] [CrossRef]
- Ralph, J.M.; Schoeler, A.C.; Krumpelt, M. Materials for lower temperature solid oxide fuel cells. J. Mater. Sci. 2001, 36, 1161–1172. [Google Scholar] [CrossRef]
- Kuscer, D.; Holc, J.; Hrovat, M.; Bernik, S.; Samardzija, Z.; Kolar, D. Interactions between a thick film LaMnO3 cathode and YSZ SOFC electrolyte during high temperature ageing. Solid State Ionics 1995, 78, 79–85. [Google Scholar] [CrossRef]
- Elangovan, S.; Hartvigsen, J.; Khandkar, A.; Privette, R.M.; Kneidel, K.E.; Perna, M.A.; Rowley, D.R. Planar solid oxide fuel cell integrated system technology development. J. Power Sources 1998, 71, 354–360. [Google Scholar] [CrossRef]
- Nakamura, T.; Petzow, G.; Gauckler, L.J. Stability of the perovskite phase LaBO3 (B = V, Cr, Mn, Fe, Co, Ni) in reducing atmosphere I. Experimental results. Mater.Res. Bull. 1979, 14, 649–659. [Google Scholar]
- Zhu, W.Z.; Deevi, S.C. A review on the status of anode materials for solid oxide fuel cells. Mater. Sci. Eng. 2003, 227, 227–239. [Google Scholar] [CrossRef]
- Badwal, P.S.; Deller, R.; Foger, K.; Ramprakash, Y.; Zhang, J.P. Interaction between chromia forming alloy interconnects and air electrode of solid oxide fuel cells. Solid State Ionics 1997, 99, 297–310. [Google Scholar] [CrossRef]
- Brandon, N.P.; Skinner, S.J.; Steele, B.C.H. Recent advances in materials for fuel cells. Ann. Rev. Mat. Res. 2003, 33, 183–213. [Google Scholar] [CrossRef]
- Blum, L.; Meulenberg, W.A.; Nabielek, H.; Steinberger-Wilckens, R. Worldwide SOFC technology overview and benchmark. Int. J. Appl. Ceram. Technol. 2005, 2, 482–492. [Google Scholar] [CrossRef]
- Steele, B.C.H. Ceramic ion conducting membranes. Curr. Opin. Solid State Mater. Sci. 1996, 1, 684–691. [Google Scholar] [CrossRef]
- Steele, B.C.H. Interfacial reactions associated with ceramic ion transport membranes. Solid State Ionics 1995, 75, 157–165. [Google Scholar] [CrossRef]
- Kilner, J.A. Isotopic exchange in mixed and ionically conducting oxides. In Proceedings of the 2nd International Symposium. on Ionic and Mixed Conducting Ceramics, San Francisco, CA, USA, May 1994; Ramanarayanan, T.A., Worrell, W.L., Tuller, H.L., Eds.; Electrochemical Society: Pennington, NJ, USA, 1994; Vol. 94–12, p. 174. [Google Scholar]
- Minh, N.Q. Ceramic fuel cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Wang, L.S.; Barnett, S.A. Lowering the air-electrode interfacial resistance in medium-temperature solid oxide fuel cells. J. Electrochem. Soc. 1992, 139, L89–L91. [Google Scholar] [CrossRef]
- Kueper, T.W.; Visco, S.J.; de Jonghe, L.C. Thin-film ceramic electrolytes deposited on porous and non-porous substrates by sol-gel techniques. Solid State Ionics 1992, 52, 251–259. [Google Scholar] [CrossRef]
- Negishi, A.; Nozaki, K.; Ozawa, T. Thin-film technology for solid electrolyte fuel cells by the RF sputtering technique. Solid State Ionics 1981, 3, 443–446. [Google Scholar] [CrossRef]
- de Souza, S.; Visco, S.J.; De Jonghe, L.C. Thin-film solid oxide fuel cell with high performance at low-temperature. Solid State Ionics 1997, 98, 57–61. [Google Scholar] [CrossRef]
- Xia, C.; Chen, F.; Liu, M. Reduced-temperature solid oxide fuel cells fabricated by screen printing. Electr. Solid State Lett. 2001, 4, A52–A54. [Google Scholar] [CrossRef]
- Tarancón, A.; Dezanneau, G.; Arbiol, J.; Peiró, F.; Morante, J.R. Synthesis of nanocrystalline materials for SOFC applications by acrylamide polymerisation. J. Power Sources 2003, 118, 256–264. [Google Scholar] [CrossRef]
- Morata, A.; Tarancón, A.; Dezanneau, G.; Peiró, F.; Morante, J.R. Optimized screen-printing and SEM-FIB characterization of YSZ thin films for solid oxide fuel cells and gas sensors devices. Mater. Res. Soc. Symp. Proc. 2004, 822, 109–114. [Google Scholar] [CrossRef]
- Mistler, R.E.; Twiname, E.R. Tape Casting: Theory and Practice; The American Ceramic Society: Westerville, OH, USA, 2000. [Google Scholar]
- Boivin, J.C.; Mairesse, G. Recent material developments in fast oxide ion conductors. Chem. Mater. 1998, 10, 2870–2888. [Google Scholar] [CrossRef]
- Goodenough, J.B. Oxide-ion electrolytes. Annu. Rev. Mater. Res. 2003, 33, 91–128. [Google Scholar] [CrossRef]
- Zacate, M.; Minervini, L.; Bradfield, D.J.; Grimes, R.W.; Sickafus, K.E. Defect cluster formation in M2O3-doped cubic ZrO2. Solid State Ionics 2000, 128, 243–254. [Google Scholar] [CrossRef]
- Lu, T.; Steele, B.C.H. Electrical conductivity of polycrystalline BiVO4 samples having the scheelite structure. Solid State Ionics 1986, 21, 339–342. [Google Scholar] [CrossRef]
- Lacorre, P.; Goutenoire, F.; Bohnke, O.; Retoux, R.; Lagilant, Y. Designing fast oxide-ion conductors based on La2Mo2O9. Nature 2000, 404, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Tarancón, A.; Norby, T.; Dezanneau, G.; Morata, A.; Peiró, F.; Morante, J.R. Conductivity dependence on oxygen partial pressure and oxide-ion transport numbers determination for La2Mo2O9. Electrochem. Solid-State Lett. 2004, 7, A373–A375. [Google Scholar] [CrossRef]
- Marrero-López, D.; Canales-Vázquez, J.; Ruiz-Morales, J.C.; Irvine, J.T.S.; Núñez, P. Electrical conductivity and redox stability of La2Mo2-xWxO9 materials. Electrochim. Acta 2005, 50, 4385–4395. [Google Scholar] [CrossRef]
- Marrero-López, D.; Peña-Martínez, J.; Ruiz-Morales, J.C.; Pérez-Coll, D.; Martín-Sedeño, M.C.; Núñez, P. Applicability of La2Mo2-xWxO9 materials as solid electrolyte for SOFCs. Solid State Ionics 2007, 178, 1366–1378. [Google Scholar] [CrossRef]
- Norby, T. The promise of protonics. Nature 2001, 410, 877–878. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre-Joud, F.; Gauthier, G.; Mougin, J. Current status of proton-conducting solid oxide fuel cells development. J. Appl. Electrochem. 2009, 39, 535–543. [Google Scholar] [CrossRef]
- Boivin, J.C. Structural and electrochemical features of fast oxide ion conductors. Int. J. Inorg. Mater. 2001, 3, 1261–1266. [Google Scholar] [CrossRef]
- Keen, D.A. Disordering phenomena in superionic conductors. J. Phys. Condens. Matter. 2002, 14, R819–R857. [Google Scholar] [CrossRef]
- Minervini, L.; Zacate, M.; Grimes, R.W. Defect cluster formation in M2O3-doped CeO2. Solid State Ionics 1999, 116, 339–349. [Google Scholar] [CrossRef]
- Zacate, M.; Minervini, L.; Bradfield, D.J.; Grimes, R.W.; Sickafus, K.E. Defect cluster formation in M2O3-doped cubic ZrO2. Solid State Ionics 2000, 128, 243–254. [Google Scholar] [CrossRef]
- Kilo, M.; Argirusis, C.; Borchardta, G.; Jackson, R.A. Oxygen diffusion in yttria stabilised zirconia–Experimental results and molecular dynamics calculations. Phys. Chem. Chem. Phys. 2003, 5, 2219–2224. [Google Scholar] [CrossRef]
- Lashtabeg, A.; Skinner, S.J. Solid oxide fuel cells–A challenge for materials chemists? J. Mater. Chem. 2006, 16, 3161–3170. [Google Scholar] [CrossRef]
- Costa-Nunes, O.; Gorte, R.J.; Vohs, J.M. Comparison of the performance of Cu-CeO2-YSZ and Ni-YSZ composite SOFC anodes with H2, CO, and syngas. J. Power Sources 2005, 141, 241–249. [Google Scholar] [CrossRef]
- He, H.P.; Gorte, R.J.; Vohs, J.M. Highly sulfur tolerant Cu-ceria anodes for SOFCs. Electrochem. Solid-State Lett. 2005, 8, A279–A280. [Google Scholar] [CrossRef]
- Ruiz-Morales, J.C.; Canales-Vázquez, J.; Marrero-López, D.; Peña-Martínez, J.; Tarancón, A.; Irvine, J.T.S.; Núñez, P. Is YSZ stable in the presence of Cu? J. Mater. Chem. 2008, 18, 5072–5077. [Google Scholar] [CrossRef]
- Naito, H.; Arashi, H. Electrical properties of ZrO2-TiO2-Y2O3 system. Solid State Ionics 1992, 53, 436–441. [Google Scholar] [CrossRef]
- Sfeir, J. LaCrO3-based anodes: Stability considerations. J. Power Sources 2003, 118, 276–285. [Google Scholar] [CrossRef]
- Hui, S.; Petric, A. Evaluation of yttrium-doped SrTiO3 as an anode for solid oxide fuel cells. J. Eur. Ceram. Soc. 2002, 22, 1673–1681. [Google Scholar] [CrossRef]
- Ruiz-Morales, J.C.; Canales-Vázquez, J.; Savaniu, C.; Marrero-López, D.; Zhou, W.; Irvine, J.T.S. Disruption of extended defects in solid oxide fuel cell anodes for methane oxidatio. Nature 2006, 439, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Skinner, S.J. Recent advances in perovskite-type materials for solid oxide fuel cell cathodes. Int. J. Inorg. Mater. 2001, 3, 113–121. [Google Scholar] [CrossRef]
- Shao, Z.; Haile, S.M.; Ahn, J.; Ronney, P.D.; Zhan, Z.; Barnett, S.A. A thermally self-sustained micro solid-oxide fuel-cell stack with high power density. Nature 2005, 435, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.B. Mechanism and kinetics of oxygen reduction on porous La1-xSrxCoO3-δ electrodes. Solid State Ionics 1998, 111, 125–134. [Google Scholar] [CrossRef]
- Adler, S.B.; Lane, J.A.; Steele, B.C.H. Electrode kinetics of porous mixed-conducting oxygen electrodes. J. Electrochem. Soc. 1996, 143, 3554–3564. [Google Scholar] [CrossRef]
- Munnings, C.N.; Skinner, S.J.; Amow, G.; Whitfield, P.S.; Davidson, I.J. Oxygen transport in the La2Ni1-xCoxO4+δ system. Solid State Ionics 2005, 176, 1895–1901. [Google Scholar] [CrossRef]
- Boehm, E.; Bassat, J.M.; Dordor, P.; Mauvy, F.; Grenier, J.C.; Stevens, P. Oxygen diffusion and transport properties in non-stoichiometric Ln2-xNiO4+δ oxides. Solid State Ionics 2005, 176, 2717–2725. [Google Scholar] [CrossRef]
- Boehm, E.; Bassat, J.M.; Dordor, P.; Mauvy, F.; Grenier, J.C. Oxygen transport properties of La2Ni1-xCuxO4+δ mixed conducting oxides. Solid State Sci. 2003, 5, 973–981. [Google Scholar] [CrossRef]
- Skinner, S.J.; Kilner, J.A. Oxygen diffusion and surface exchange in La2-xSrxNiO4+δ. Solid State Ionics 2000, 135, 709–712. [Google Scholar] [CrossRef]
- Burriel, M.; Garcia, G.; Santiso, J.; Kilner, J.A.; Chater, R.J.; Skinner, S.J. Anisotropic oxygen diffusion properties in epitaxial thin films of La2NiO4+δ. J. Mater. Chem. 2008, 18, 416–422. [Google Scholar] [CrossRef]
- Taskin, A.A.; Lavrov, A.N.; Ando, Y. Achieving fast oxygen diffusion in perovskites by cation ordering. Appl. Phys. Lett. 2005, 86, 1–3. [Google Scholar] [CrossRef]
- Taskin, A.A.; Lavrov, A.N.; Ando, Y. Transport and magnetic properties of GdBaCo2O5+x single crystals: A cobalt oxide with square-lattice Co O2 planes over a wide range of electron and hole doping. Phys. Rev. B 2005, 71, 1–28. [Google Scholar] [CrossRef]
- Chang, A.; Skinner, S.J.; Kilner, J.A. Electrical properties of GdBaCo2O5+x for ITSOFC applications. Solid State Ionics 2006, 177, 2009–2011. [Google Scholar] [CrossRef]
- Tarancón, A.; Skinner, S.J.; Kilner, J.A. yered perovskites as promising cathodes for Intermediate Temperature SOFCs. In Proceedings of the 7th European SOFC Forum, Lucerne, Switzerland, July 2006; Bossel, U., Ed.; The European Solid Oxide Fuel Cell Forum: Oberrohrdorf, Switzerland, 2006; pp. 78–88. [Google Scholar]
- Tarancón, A.; Skinner, S.J.; Chater, R.J.; Hernández-Ramírez, F.; Kilner, J.A. Layered perovskites as promising cathodes for intermediate temperature solid oxide fuel cells. J. Mater. Chem. 2007, 17, 3175–3181. [Google Scholar] [CrossRef]
- Kim, G.; Wang, S.; Jacobson, A.J.; Yuan, Z.; Donner, W.; Chen, C.L.; Reimus, L.; Brodersen, P.; Mims, C.A. Oxygen exchange kinetics of epitaxial PrBaCo2O5+x thin films. Appl. Phys. Lett. 2006, 88, 1–3. [Google Scholar]
- Kim, G.; Wang, S.; Jacobson, A.J.; Reimus, L.; Brodersen, P.; Mims, C.A. Rapid oxygen ion diffusion and surface exchange kinetics in PrBaCo2O5+x with a perovskite related structure and ordered a cations. J. Mater. Chem. 2007, 17, 2500–2505. [Google Scholar] [CrossRef]
- Bowmeester, H.J.M.; Burggraaf, A.J. Fundamentals of Inorganic Membrane Science and Technology; Burggraaf, A.J., Cot, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 1, Chapter 10. [Google Scholar]
- Mauvy, F.; Bassat, J.M.; Boehm, E.; Dordor, P.; Grenier, J.C.; Loup, J.P. Chemical oxygen diffusion coefficient measurement by conductivity relaxation-correlation between tracer diffusion coefficient and chemical diffusion coefficient. J. Eur. Ceram. Soc. 2004, 24, 1265–1269. [Google Scholar] [CrossRef]
- Ahlgren, E.O.; Poulsen, F.W. Thermoelectric power and electrical conductivity of strontium-doped lanthanum manganite. Solid State Ionics 1996, 86, 1173–1178. [Google Scholar] [CrossRef]
- Petric, A.; Huang, P.; Tietz, F. Evaluation of La-Sr-Co-Fe-O perovskites for solid oxide fuel cells and gas separation membranes. Solid State Ionics 2000, 135, 719–725. [Google Scholar] [CrossRef]
- Wang, L.; Merkle, R.; Maier, J.; Acartürk, T.; Starke, U. Oxygen tracer diffusion in dense Ba0.5Sr0.5Co0.8Fe0.2O3-δ films. Appl. Phys. Lett. 2009, 94, 1–3. [Google Scholar]
- Peña-Martínez, J.; Tarancón, A.; Marrero-López, D.; Ruiz-Morales, J.C.; Núñez, P. Evaluation of GdBaCo2O5+x as cathode material for doped lanthanum gallate electrolyte IT-SOFCs. Fuel Cells 2008, 5, 351–359. [Google Scholar]
- Sayers, R.; De Souza, R.A.; Kilner, J.A.; Skinner, S.J. Low temperature diffusion and oxygen stoichimoetry in lanthanum nickelate. Solid State Ionics 2009, in press. [Google Scholar]
- Matsuura, T.; Tabuchi, J.; Mizusaki, J.; Yamamuchi, S.; Fueki, K. Electrical properties of La2-xSrxCoO4-δ: Structure, electrical conductivity, and Seebeck coefficient of single crystals x = 0.0, 0.5, 1.0 and 1.5. J. Phys. Chem. Solids 1988, 49, 1403–1408. [Google Scholar] [CrossRef]
- Tarancón, A.; Marrero-López, D.; Peña-Martínez, J.; Ruiz-Morales, J.C.; Núñez, P. Effect of phase transition on high-temperature electrical properties of GdBaCo2O5+x layered perovskite. Solid State Ionics 2008, 179, 611–618. [Google Scholar] [CrossRef]
- Evans, A.; Bieberle-Hütter, A.; Rupp, J.L.M.; Gauckler, L.J. J. Power Sources 2009, 194, 119. [CrossRef]
- Bieberle-Hutter, A.; Beckel, D.; Infortuna, A.; Muecke, U.P.; Rupp, J.L.M.; Gauckler, L.J.; Rey-Mermet, S.; Muralt, P.; Bieri, M.R.; Hotz, N.; Stutz, M.J.; Poulikakos, D.; Heeb, P.; Müller, P.; Bernard, A.; Gmür, R.; Hocker, T. Review on microfabricated micro-solid oxide fuel cell membranes. J. Power Sources 2008, 177, 123–129. [Google Scholar] [CrossRef]
- Huang, H.; Nakamura, M.; Su, P.; Fasching, R.; Saito, Y.; Prinz, F.B. High-performance ultrathin solid oxide fuel cells for low-temperature operation. J. Electrochem. Soc. 2007, 154, 20–24. [Google Scholar] [CrossRef]
- Tarancón, A.; Sabaté, N.; Cavallaro, A.; Gràcia, I.; Roqueta, J.; Garbayo, I.; Esquivel, J.P.; Garcia, G.; Cané, C.; Santiso, J. Residual stress of free-standing membranes of yttria-stabilized zirconia for micro solid oxide fuel cell applications. J. Nanosci. Nanotechnol. 2010, 10, 1. [Google Scholar] [CrossRef]
- Garbayo, I.; Tarancón, A.; Santiso, J.; Peiró, F.; Alarcón-LLadó, E.; Cavallaro, A.; Gràcia, I.; Cané, C.; Sabaté, N. Electrical characterization of thermomechanically stable YSZ membranes for micro Solid Oxide Fuel Cells applications. Solid State Ionics 2009. submitted. [Google Scholar] [CrossRef]
- Maier, J. Nanoionics: Ion transport and electrochemical storage in confined systems. Nat. Mater. 2005, 4, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Maier, J. Thermodynamic aspects and morphology of nano-structured ion conductors: Aspects of nano-ionics Part I. Solid State Ionics 2002, 154, 291–301. [Google Scholar] [CrossRef]
- Maier, J. Defect chemistry and ion transport in nanostructured materials: Part II. Aspects of nanoionics. Solid State Ionics 2003, 157, 327–334. [Google Scholar] [CrossRef]
- Maier, J. Nano-sized mixed conductors (Aspects of nano-ionics. Part III). Solid State Ionics 2002, 148, 367–374. [Google Scholar] [CrossRef]
- Maier, J. Ionic transport in nano-sized systems. Solid State Ionics 2004, 175, 7–12. [Google Scholar] [CrossRef]
- Tuller, H.L. Ionic conduction in nanocrystalline materials. Solid State Ionics 2000, 131, 143–157. [Google Scholar] [CrossRef]
- Van de Krol, R.; Tuller, H.L. Electroceramics-The role of interfaces. Solid State Ionics 2002, 150, 167–179. [Google Scholar] [CrossRef]
- Kosacki, I.; Rouleau, C.M.; Becher, P.F.; Bentley, J.; Lowndes, D.H. Surface/interface-related conductivity in nanometer thick YSZ films. Electrochem. Solid-State Lett. 2004, 7, A459–A461. [Google Scholar] [CrossRef]
- Kosacki, I.; Rouleau, C.M.; Becher, P.F.; Bentley, J.; Lowndes, D.H. Nanoscale effects on the ionic conductivity in highly textured YSZ thin films. Solid State Ionics 2005, 176, 1319–1326. [Google Scholar] [CrossRef]
- Garcia-Barriocanal, J.; Rivera-Calzada, A.; Varela, M.; Sefrioui, Z.; Iborra, E.; Leon, C.; Pennycook, S.J.; Santamaría, J. Colossal ionic conductivity at interfaces of epitaxial ZrO2:Y2O3/SrTiO3 heterostructures. Science 2008, 321, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.M.; Lavik, E.B.; Kosacki, I.; Tuller, H.L.; Ying, J.Y. Nonstoichiometry and electrical conductivity of nanocrystalline CeO2-x. J. Electroceram. 1997, 1, 7–14. [Google Scholar] [CrossRef]
- Tschope, A.; Birringer, R. Grain size dependence of electrical conductivity in polycrystalline cerium oxide. J. Electroceram. 2001, 7, 169–177. [Google Scholar] [CrossRef]
- Kilner, J.A. Ionic conductors: Feel the strain. Nat. Mater. 2008, 7, 838–839. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Korte, C.; Hesse, D.; Zakharov, N.; Janek, J. Ionic conductivity and activation energy for oxygen ion transport in superlattices-The multilayer system CSZ (ZrO2 + CaO) / Al2O3. Solid State Ionics 2007, 178, 67–76. [Google Scholar] [CrossRef]
- Korte, C.; Peters, A.; Janek, J.; Hesse, D.; Zakharov, N. Ionic conductivity and activation energy for oxygen ion transport in superlattices-The semicoherent multilayer system YSZ (ZrO2 + 9.5 mol% Y2O3)/Y2O3. Phys. Chem. Chem. Phys. 2008, 10, 4623–4635. [Google Scholar] [CrossRef] [PubMed]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tarancón, A. Strategies for Lowering Solid Oxide Fuel Cells Operating Temperature. Energies 2009, 2, 1130-1150. https://doi.org/10.3390/en20401130
Tarancón A. Strategies for Lowering Solid Oxide Fuel Cells Operating Temperature. Energies. 2009; 2(4):1130-1150. https://doi.org/10.3390/en20401130
Chicago/Turabian StyleTarancón, Albert. 2009. "Strategies for Lowering Solid Oxide Fuel Cells Operating Temperature" Energies 2, no. 4: 1130-1150. https://doi.org/10.3390/en20401130