1. Introduction
An investigation of the performance of a combustor for possible use in micro- and ultra-micro gas turbines for both stationary and propulsion applications is presented in this paper. The field of micro-meso thermo-electric/electronic devices is rapidly developing under the pressure of evermore stringent requirements posed by the increasing need for portable power generation. There are three main areas of interest for micro-meso-combustion: terrestrial and marine propulsion, UAV (“Unmanned Aerial Vehicles”) for tactical and meteorological recognissance, and portable electrical power generation.
In the current literature [
1,
2] a mesoscale combustion regime is defined as one in which the flame scale is of the same order of magnitude as the thickness of the flame preheating zone. This implies that one of the characteristic dimensions of the combustor is of the order of the quenching diameter (millimeters to centimeters): According to this definition, the device studied here belongs to the class of
meso-combustion chambers. Meso-scale systems behave differently than macro-scale ones, since in the former both the Reynolds and Peclet numbers are low, and consequently viscous and diffusive effects become more important.
Low Reynolds implies low turbulent intensities or even laminar flow fields, so that the turbulent contribution to mixing processes is scarce or totally absent. At the same time, purely diffusive processes are too slow to be effective.
Therefore, in scaled-down conventional combustors the conversion of chemical- into thermal energy can be severely limited by the decreased residence time, and in the worst case it can be totally prevented. The size reduction implies also larger heat losses that bring about a decline in the overall combustion efficiency and a reduction of the reaction temperatures, which in turn narrows the stability limits of the combustor by increasing the kinetic reaction times. For the above reasons, a proper management and understanding of the thermo chemical issues connected to size reduction are mandatory in order to build operationally efficient meso-combustors.
Previous work in micro/meso-propulsion related to scaling laws issues [
3,
4,
5,
6] and focuses predominantly on fluid dynamics and combustion [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18]. Three dimensional numerical simulations of methane-air combustion in swirling micro/meso-combustors are not common: the majority of the references available in the literature investigate the combustion process of
propane in micro/meso sized combustor by assuming 2-D axi-symmetrical geometry and reduced kinetic mechanism [
7,
8,
9,
10,
11,
12,
13,
16,
17,
18].
Six of the above references deal with methane fuel, and while five of them [
10,
11,
14,
15,
16,
17,
18] consider 2-D combustion chambers, only one of them analyzes 3-D swirling combustion chambers as in the present work; in [
19] flame stabilization and flow evolution are analyzed mostly by means of experiments on swirling combustion chambers fueled by hydrogen, methane and propane. Microchambers are much smaller than the one presented here. Combustion efficiencies in excess of 90%, in particular with hydrogen, were reported; in combustion chambers smaller than 124 mm
3, stable methane combustion could be achieved only by enriching air with oxygen. Results were compared with CFD simulations employing the global 1-step Westbrook and Dryer kinetic scheme [
20]. Our work differs from that of [
19] in two aspects: the chamber dimension and the accuracy of the chemical model.
In the following, two simulation campaigns on the same combustion chamber are reported. In the “Flamelet simulation”, combustion is modeled by means of 67 “flamelets” pre-calculated and stored in look-up tables [
21,
22,
23,
24,
25,
26,
27,
28] using detailed chemistry [
29] (GRIMech3.0, 53 species and 325 reactions, using Arrhenius relations).
The “EDC simulation” employs the Eddy Dissipation Concept [
30,
31], and models chemistry with a detailed mechanism (GRIMech1.2) defined by 35 species and 177 reactions, using Arrhenius relations [
32].
The EDC solves the species- and NS equations simultaneously. The GRIMech1.2 mechanism has been chosen to reproduce the chemistry as accurately as possible while abiding by the constraints posed by the CFD software (FLUENT6.3) [
33] capabilities that limit the species equations to a maximum of 50.
Both simulations are performed by means of a 3D unsteady LES method with the “WALE” (Wall Adapting Local Eddy Viscosity) subgrid scale model [
34], following the results provided in [
35].
Temperature, species maps and combustion efficiency values are the most important results presented and discussed. The “Flamelet simulation” results are provided after 0.03 s of physical time while those of the “EDC simulation” are provided at two different times, namely after 0.03 s (3 residence times) and 0.05 s (five residence times).
The “EDC simulation” provides more realistic results than the first one, and it will be argued in the following that the differences result from the different assumptions posited by the two combustion models.
This paper is structured as follows:
Section 2 describes the domain geometry and the specified operating conditions;
Section 3 contains a brief discussion of the turbulence models;
Section 4 discusses the combustion and chemistry models;
Section 5 reports the numerical models while
Section 6 presents and discusses the results.
This work is part of a broader work that includes an experimental analysis carried out at the Sapienza University of Roma, which will be reported and compared in a follow-up paper for a deeper understanding of combustion phenomena in microcombustion chambers.
2. Combustion Chamber and Operating Conditions
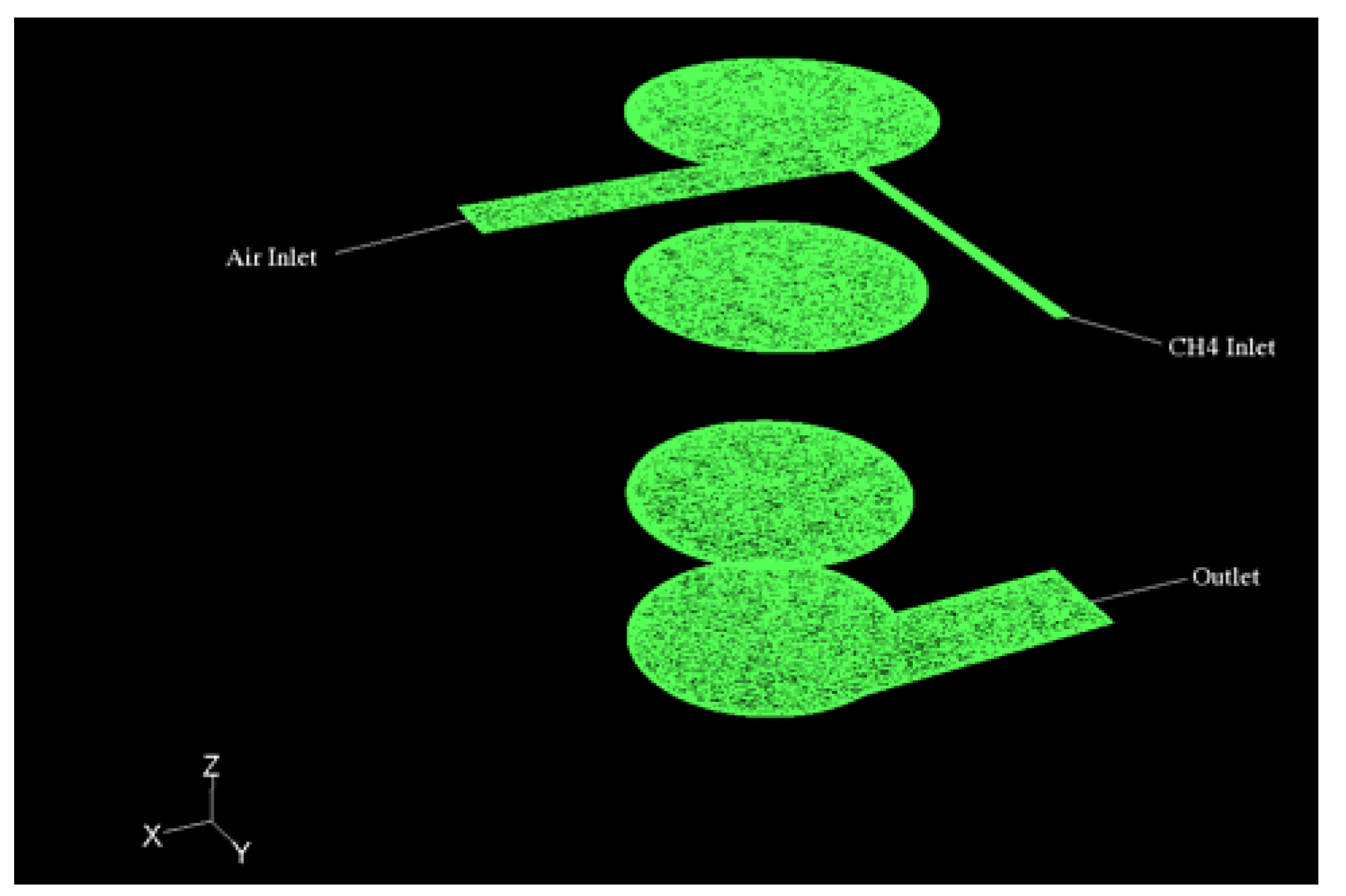
The combustion chamber geometry is reported in
Figure 1 (divided into slices for ease of visualization): The domain is discretized by 870,000 unstructured cells, so that
y+ ≈ 5 and Δ
y+ ≈ 1; that is, the first point away from the wall is inside the viscous sublayer, where the velocity profiles, in spite of the high stretching imposed by the elongated geometry, are still parabolic.
The diameter of the chamber is 0.025 m, its height 0.06 m (aspect ratio = 0.416), for a total volume of 29 cm3. The methane feeding duct has a diameter of 0.0015 m, the air duct 0.005 m. The exhaust duct on the lower side of the chamber has a diameter of 0.01 m.
To improve mixing the gaseous methane is injected in the radial direction at 90° with respect to the air flow which is tangential to the chamber. The dimensions of the chamber have a strong influence on the mixing dynamics, and consequently on the combustion efficiency, due to the strong effects of the aspect ratio (S/V) on the mean streamline curvature and on the swirling motions.
Operating and boundary conditions are reported in
Table 1; mass flows at inlets are imposed, together with the pressure of 2 atm at the outlet section, while the walls are at constant temperature: Two different values were specified depending on the device zones: the chamber and air duct walls are at 700 K while the methane and exhaust ducts are at 300 K.
The fuel inlet Reynolds number is in the transitional regime (ReCH4 = 1,500), while the Reair is fully turbulent (ReAIR = 14,700); this feature is characteristic (and peculiar) of microcombustors; it is well known that the possibility of relaminarization is enhanced by combustion that causes an increase of the effective viscosity of the burning mixture; on the other hand, the slight expansion inside of the chamber introduces a contrasting effect, so that the final regime is determined by the dynamic competition between these two effects.
Figure 1.
Representative geometry of the micro-combustion chamber.
Figure 1.
Representative geometry of the micro-combustion chamber.
Table 1.
Operating Conditions.
Table 1.
Operating Conditions.
| Parameter | Methane | Air |
|---|
| Mass Flow Rate (kg/s) | 3.93 × 10−5 | 1.9 × 10−3 |
| Inlet Temperature (K) | 300 | 700 |
| Outlet Pressure (atm) | 2 | 2 |
| Inlet Velocity (m/s) | 18 | 100 |
| Inlet Viscosity (kg/m-s) | 2 × 10−5 | 3.29 × 10−5 |
| Re | 1,500 | 14,700 |
| Chamber Wall Temperature (K) | 700 |
| Methane Duct Wall Temperature (K) | 300 |
| Air Duct Wall Temperature (K) | 700 |
| Exhaust Duct Wall Temperature (K) | 300 |
| Kinetic Energy Ratio, M | 0.038 |
| Global equivalence ratio | 0.355 |
The species isobaric specific heats are described by polynomials in T as per the GRIMech Thermo Data file [
36] that has been included in the CFD code, while kinetic theory [
37] is assumed to predict transport properties.
5. Results and Discussion
In the following a comparison between the two simulations is provided. For the sake of clarity all figures show the flowfields on slices located at 0.01 m, 0.03 m and 0.055 m, (the first and last being the inlet- and outlet plane respectively) and they are provided, wherever possible, using the same scale.
The results shown here correspond to two different physical times, namely 0.03 s and 0.05 s (three and five residence times, respectively).
The mean residence time can be defined as the time a parcel of gas spends inside the combustor, its value estimated as the ratio between the mass of gas in the chamber volume,
, and the mass flow rate,
:
The total mass flow rate was computed from the data in
Table 1, while the averaged gas density,
, is assumed equal to that of air at 1,100 K and 2 bar (0.62 kg/m
3). Assuming this residence time as the characteristic combustion chamber fluid dynamics time, and comparing it with data in
Table 3 and
Table 4, it is possible to deduce a “
worst case Damkoehler number”.
The Damkoehler number, Da, defines the fluid-dynamics/chemical times ratio and indicates whether the reactions are completed within a given time (Da >> 1), or are frozen (Da << 1).
Table 3 and
Table 4 report the ignition delay times,
tid, predicted at the combustion chamber pressure by the detailed GRIMech 3.0 mechanism [
32] run for different values of the equivalence ratio Φ and for a temperature range from 1,000 K to 2,300 K (1,000 K being just above the auto ignition temperature and 2,300 K is the maximum temperature inside the chamber, for both simulations, at steady state).
In the worst case, to obtain Da >> 1 (complete reactions), combustion temperatures must be ≥1,300 K with Φ ~ 0.9. Figure 6 shows that wide zones of the chamber are well above 2,000 K.
The iterative solution was assumed converged when the difference between inlet and outlet mass flowrate, ∆
m/∆
t, was <1 × 10
−7 kg/s (two and three orders of magnitude smaller than the methane and air mass flow rates, respectively). The integration time step was 1 × 10
−7s, that is, 2 orders of magnitude smaller than the shortest ignition time (1 × 10
−5 s) occurring for T = 2300 K and Φ ~ 1, see
Table 3 and
Table 4.
Table 3.
Ignition Delay, s, 0.3 ≤ Φ ≤ 1.0.
Table 3.
Ignition Delay, s, 0.3 ≤ Φ ≤ 1.0.
| Ignition Temperature (K) | Φ = 0.3 | Φ = 0.5 | Φ = 0.7 | Φ = 0.9 | Φ = 1 |
|---|
| 1000 | 0.608 | 0.772 | 0.982 | 1.03 | 1.03 |
| 1100 | 0.111 | 0.143 | 0.131 | 0.192 | 0.202 |
| 1200 | 0.0244 | 0.0314 | 0.0331 | 0.0424 | 0.044 |
| 1300 | 0.00649 | 0.00815 | 0.000967 | 0.011 | 0.0114 |
| 1400 | 0.00211 | 0.00247 | 0.00289 | 0.00323 | 0.00336 |
| 1500 | 0.000881 | 0.000915 | 0.00103 | 0.00112 | 0.00114 |
| 1600 | 0.000422 | 0.000398 | 0.000423 | 0.000439 | 0.000456 |
| 1700 | 0.000246 | 0.000197 | 0.000203 | 0.000203 | 0.000211 |
| 1800 | 0.000174 | 0.000108 | 0.000106 | 0.000107 | 0.000109 |
| 1900 | 0.000148 | 0.000067 | 0.0000638 | 0.0000627 | 0.0000628 |
| 2000 | 0.000139 | 0.0000398 | 0.0000381 | 0.0000375 | 0.0000377 |
| 2100 | 0.00017 | 0.0000263 | 0.0000247 | 0.0000241 | 0.000024 |
| 2200 | 0.000233 | 0.0000183 | 0.0000167 | 0.0000162 | 0.0000161 |
| 2300 | - | 0.0000156 | 0.0000118 | 0.0000114 | 0.0000113 |
Table 4.
Ignition Delay, s, 1.1 ≤ Φ ≤ 1.9.
Table 4.
Ignition Delay, s, 1.1 ≤ Φ ≤ 1.9.
| Ignition Temperature (K) | Φ = 1.1 | Φ = 1.3 | Φ = 1.5 | Φ = 1.7 | Φ = 1.9 |
|---|
| 1000 | 1.15 | 1.26 | 1.22 | 1.43 | 1.53 |
| 1100 | 0.216 | 0.237 | 0.232 | 0.234 | 0.292 |
| 1200 | 0.0473 | 0.0522 | 0.0524 | 0.0323 | 0.0643 |
| 1300 | 0.0122 | 0.0134 | 0.0142 | 0.0155 | 0.0163 |
| 1400 | 0.00355 | 0.0039 | 0.00425 | 0.00445 | 0.00433 |
| 1500 | 0.00121 | 0.00127 | 0.00133 | 0.00143 | 0.00152 |
| 1600 | 0.00044 | 0.000492 | 0.000525 | 0.000529 | 0.000533 |
| 1700 | 0.000213 | 0.000221 | 0.00023 | 0.000236 | 0.000244 |
| 1800 | 0.000111 | 0.000112 | 0.000112 | 0.000116 | 0.00012 |
| 1900 | 0.0000623 | 0.0000623 | 0.0000623 | 0.0000627 | 0.0000638 |
| 2000 | 0.0000374 | 0.0000376 | 0.0000384 | 0.0000385 | 0.0000392 |
| 2100 | 0.0000239 | 0.000024 | 0.0000241 | 0.0000245 | 0.000025 |
| 2200 | 0.0000161 | 0.0000161 | 0.0000162 | 0.0000164 | 0.0000168 |
| 2300 | 0.0000112 | 0.0000112 | 0.0000113 | 0.0000115 | 0.0000118 |
Flamelet results are provided at 0.03 s while EDC’s results at both 0.03 s and 0.05 s: This has been done to highlight the main characteristics of the two models.
The EDC model reproduces “in-evolution chemistry phenomena” while the flamelet model reaches a steady configuration much faster. This is due to the two different assumptions by the combustion models.
The flamelet model does not need to simulate the spark (the first ignition): flamelet calculations are function only of mixture ratio and scalar dissipation and then the initial solution is the equilibrium condition in every cell.
The EDC, on the contrary, needs to simulate the spark because it assumes an ignition temperature equal to the local cell temperature, so respecting reaction times as defined by the Arrhenius law. The “numerical spark” consisted of few cells close to the impingement region at temperature of 2,000 K.
Moreover, the EDC model explicitly accounts for species transport properties while the Flamelet assumes an overall transport property, in this case equal to that of the nitrogen that is the species with the highest mass fraction.
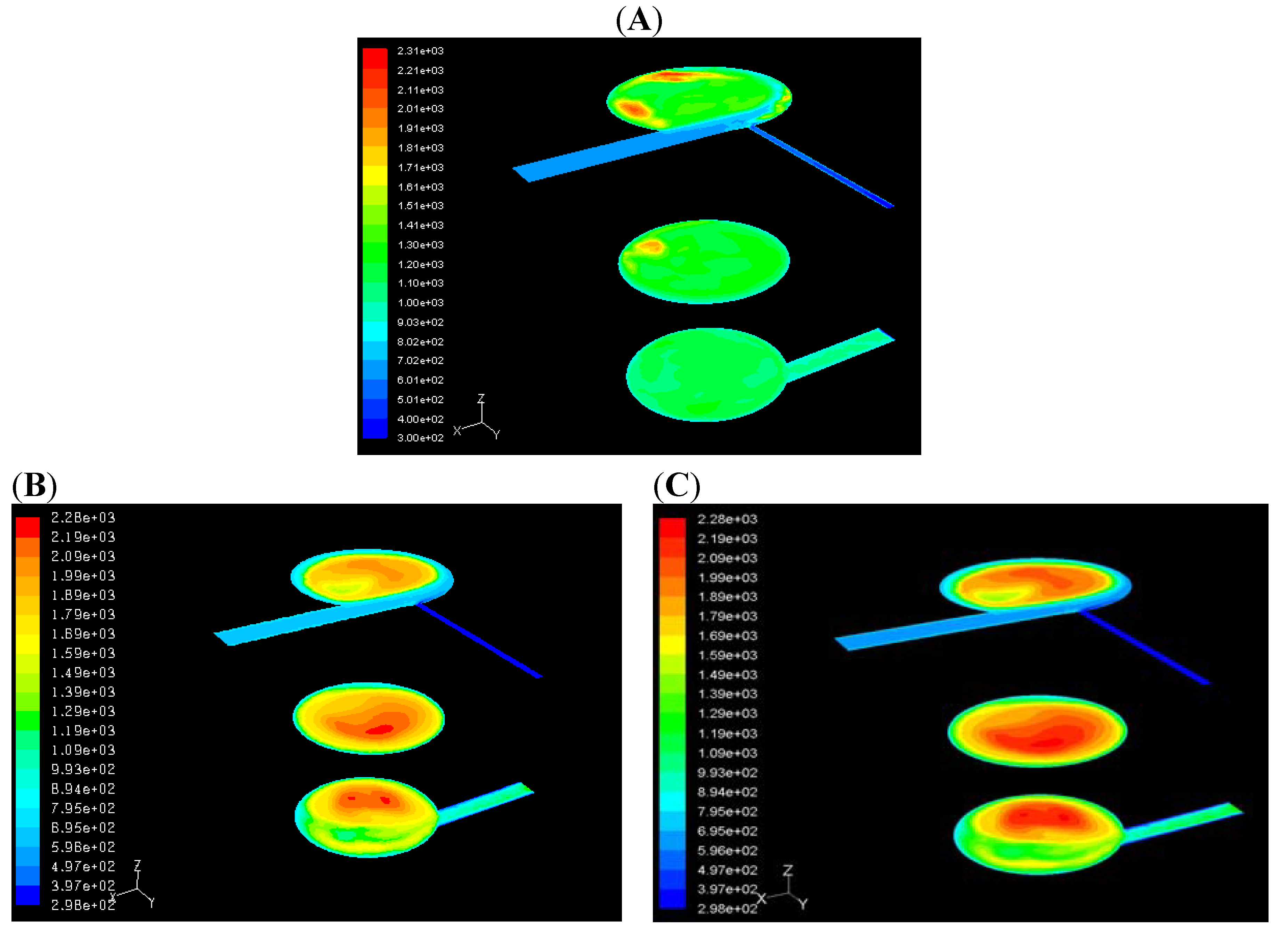
The consequences of such an assumption are clearly exposed by observing the species maps inside the chamber (
Figures 2), which are completely different: with Flamelet, the CO and CO
2 production is essentially concentrated at the inlet section and close to the wall, while the “EDC simulation” predicts, at both times, that the CO-CO
2 conversion takes place well downstream of the inlet section.
This means that
the Flamelet model predicts complete combustion earlier than the EDC model; in fact, this is confirmed by the combustion efficiency, which is defined by the exhaust anhydrous molar fractions ratio,
:
Table 5 reports the combustion efficiency values for the three cases.
Figure 2.
(A) Flamelet: CO map after 0.03 s; (B) EDC: CO map after 0.03 s; (C) EDC: CO map after 0.05 s; (D) Flamelet: CO2 map after 0.03 s; (E) EDC: CO2 map after 0.03 s; (F) EDC: CO2 map after 0.05 s.
Figure 2.
(A) Flamelet: CO map after 0.03 s; (B) EDC: CO map after 0.03 s; (C) EDC: CO map after 0.05 s; (D) Flamelet: CO2 map after 0.03 s; (E) EDC: CO2 map after 0.03 s; (F) EDC: CO2 map after 0.05 s.
Table 5.
Combustion Efficiency.
Table 5.
Combustion Efficiency.
| Flamelet at 0.03 s | EDC at 0.03 s | EDC at 0.05 s |
|---|
| 0.999 | 0.27 | 0.986 |
The “EDC simulation” needs five residence times to reach a combustion efficiency higher than 0.98, while the “flamelet simulation” needs just three residence times.
Even if the calculated combustion efficiencies are known to be higher than real ones, these orders of magnitude are confirmed by the experiments published by Wu
et al. [
19], even though those experiments were on much smaller chambers and with oxygen enriched air.
A similar investigation carried out on a smaller chamber (0.25 cm
3) [
41,
42] provided a lower predicted combustion efficiency,
η, of 0.8, showing the influence of the higher S/V ratio on
η and confirmed the different behavior of the flamelet and EDC models. The fact that even the EDC models predicts an unusually high combustion efficiency is due to the combustion chamber dimensions that are large enough to attain complete reactions.
Different species maps, and therefore different combustion efficiencies, lead to different temperature maps, as shown in
Figure 3. The “evolution feature” is highlighted in
Figures 3 (B and C).
Figure 3.
(A) Flamelet: Temperature, t = 0.03s; (B) EDC: Temperature, t = 0.03 s; (C) EDC: Temperature, t = 0.05 s.
Figure 3.
(A) Flamelet: Temperature, t = 0.03s; (B) EDC: Temperature, t = 0.03 s; (C) EDC: Temperature, t = 0.05 s.
The “EDC simulation” predicts a hot core and a colder outer zone close the wall, due to the heat losses introduced by the low constant wall temperature of 700 K.
Figure 3B clearly displays a noticeable swirling motion as well.
On the contrary, the “flamelet simulation” predicts a more homogeneous temperature field over the entire section, as if the boundary condition at walls were “
quasi adiabatic”.
Figure 3C shows a weaker swirl too.
The two simulations predict similar maximum temperatures inside the chamber: 2,280 K versus 2,310 K. Temperatures at the outlet section are 1,100 K and 1,270 K.
Experimental support for our conclusions about the inherent physical differences between the two combustion models comes from the comparison between the temperature maps shown in
Figure 3 and the time-averaged of CH* chemiluminescence images obtained by [
43] (
Figure 4). Their results pertain to a combustion chamber smaller than the one studied here (exactly 6 mm diameter and 9 mm length), but, even if only in a
qualitative sense, they confirm the general behaviour calculated in the present study.
CH
4 (methane) appears in rich regions of the flame, because it is produced later in methane pyrolysis (after partial CH
2 oxidation has taken place [
44] and thus is associated to heat release and can be related with the flame front. This correlation is only approximate though, because it should be remembered that line-of-sight measurements must be interpreted with caution [
43]. The time averaged CH* image shows the maximum intensity occurring opposite to the air/fuel injection zone. The central hot bubble is characterized by weaker CH* emission; the zone close to the reactant inlets shows the lowest intensity. In both regions CH* is low because either completely oxidized to CO already, or because methane pyrolysis has not yet begun. Similarly, due to wall flame quenching, no CH* emission was observed in the “dead” layer about 0.5 mm thick close to the wall. Thus, even qualitative, in fair agreement with the mean CH* image, the “EDC mean temperature maps” show that the region of highest gas temperature is located away from the wall and away from the air/fuel injection point, see
Figure 3 (B and C).
Figure 4.
EDC: Temperature, t = 0.05 s. The chamber wall is the black line.
Figure 4.
EDC: Temperature, t = 0.05 s. The chamber wall is the black line.
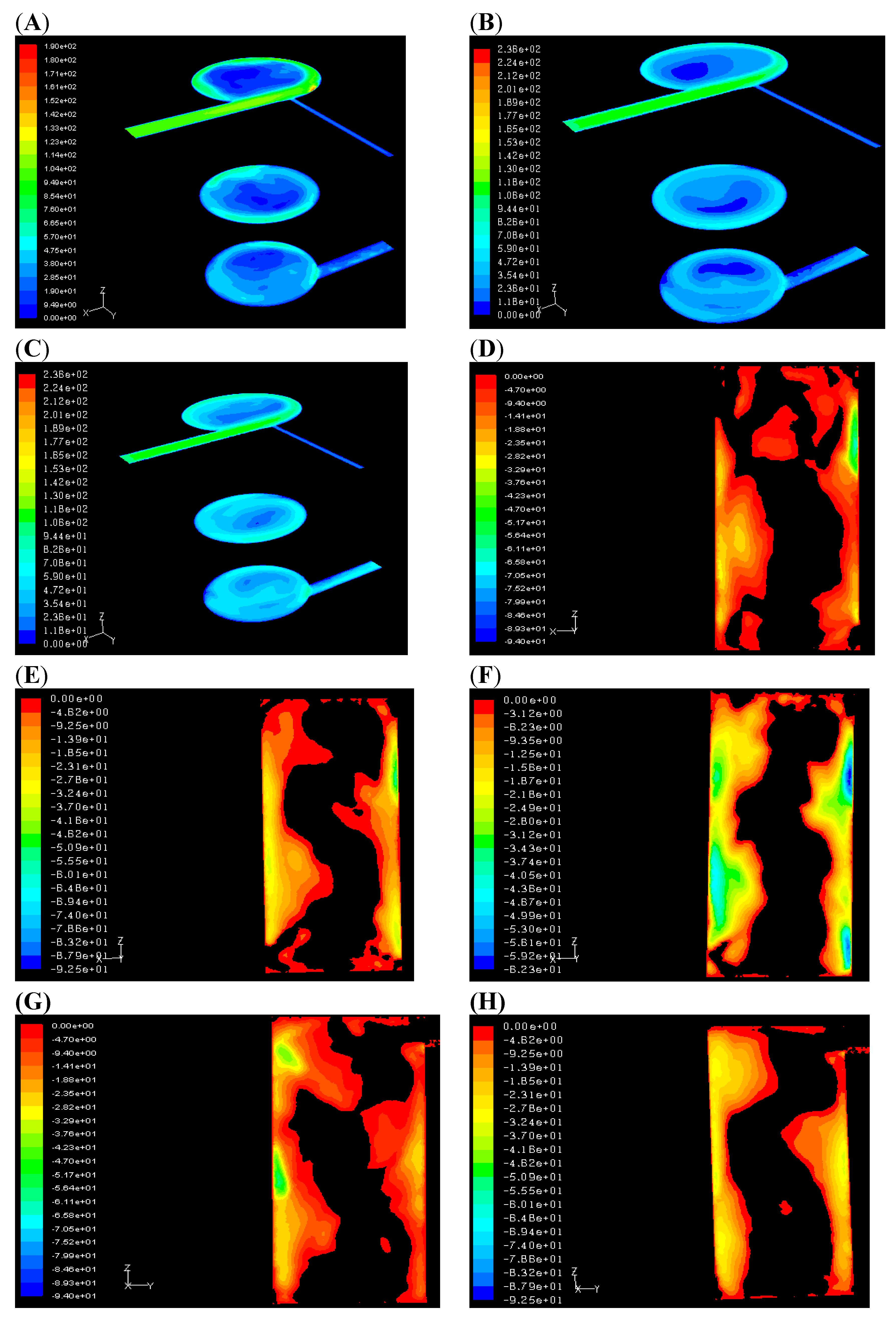
These discrepancies in the prediction of the thermodynamic properties and in the species profiles obviously affect the densities and therefore both the velocity magnitude maps (
Figure 5) and the topology of the recirculation zones (
Figure 5C–
Figure 5I). The zones with the lowest velocity values (
Figure 5A–
Figure 5C) provide a hint to the complex largest vortex structures inside the chamber.
These are more clearly apparent in
Figure 5D–
Figure 5I that show the mean
Z velocity on the “
XZ” and “
YZ” Planes. These figures indicate only negative
Z-velocities, consistent with the “in-out direction” trough the chamber. The black zones (positive
Z-velocities) identify the recirculation bubbles in the chamber that promote the mixing process: These regions are quite wide in both simulations but have a more regular shape in the second one (with the EDC model). This flow reversal is a result of the particular shape of the chamber, and in particular is promoted by the outlet section being perpendicular to the cylinder axis and tangent to its external surface (see
Figure 1).
Figure 5.
(A) Flamelet: Velocity, t = 0.03 s; (B) EDC: Velocity, t = 0.03 s; (C) EDC: Velocity, t = 0.05 s; (D) Flamelet: Z-Velocity, XZ plane, t = 0.03 s; (E) EDC: Z-Velocity, XZ plane, t = 0.03s; (F) EDC: Z-Velocity, XZ plane, t = 0.05 s; (G) Flamelet: Z-Velocity, YZ plane, t = 0.03 s; (H) EDC: Z-Velocity, YZ plane, t = 0.03 s; (I) EDC: Z-Velocity, YZ plane after 0.05 s.
Figure 5.
(A) Flamelet: Velocity, t = 0.03 s; (B) EDC: Velocity, t = 0.03 s; (C) EDC: Velocity, t = 0.05 s; (D) Flamelet: Z-Velocity, XZ plane, t = 0.03 s; (E) EDC: Z-Velocity, XZ plane, t = 0.03s; (F) EDC: Z-Velocity, XZ plane, t = 0.05 s; (G) Flamelet: Z-Velocity, YZ plane, t = 0.03 s; (H) EDC: Z-Velocity, YZ plane, t = 0.03 s; (I) EDC: Z-Velocity, YZ plane after 0.05 s.
These differences are confirmed by the dimensionless swirl number,
S (defined as the axial flux of swirling momentum divided by the product of the axial flux of axial momentum times the equivalent nozzle radius):
Table 7 provides the swirl numbers at the inlet plane (
Z = 0.055 m) and further confirms the above explanation.
Table 7.
Swirl Number.
| Flamelet at 0.03 s | EDC at 0.03 s | EDC at 0.05 s |
|---|
| 0.47 | 1.62 | 0.57 |
The “EDC simulation” displays different flowfields before reaching steadiness: at 0.03 s it predicts an axial flux of the axial momentum smaller than the swirling momentum, while at 0.05 s it is the opposite. The latter result is obtained by the “flamelet simulation”, two residence times earlier.
6. Conclusions
Scope of this work was the simulation of an air/methane ultramicro-combustion chamber with a nominal thermal power of 2 kW, designed and built at the Sapienza University of Roma. The ultimate goal was that of validating suitable computational tools to design miniature electrical power generators.
Two simulations are reported, both use the LES approach with the WALE SGS model and detailed chemistry, but differ for the combustion-chemistry models: the first uses 67 “flamelets”, while the second uses the EDC model.
Results are reported at two different times: 0.03 s and 0.05 s, that is, almost three and five residence times, respectively. Instantaneous maps of different quantities and a combustion efficiency analysis are provided. The work is focused on the analysis of the different assumptions posited by the combustion models.
Flamelets are calculated by an initial ignition temperature equal to the equilibrium condition and they account for overall transport properties, equal in this case to that of nitrogen which is the species with the highest mass fraction. The EDC model solves the species equations with the local cell properties and explicitly accounts for every transport property.
The analysis starts from the species maps, obtaining the relative combustion efficiency values, and then focuses on the temperature maps to deduce their effect on the velocity fields and then on the swirl numbers. The results presented here lead to the conclusion that while an overall analysis on variables like combustion efficiency and maximum temperature may be carried out by a flamelet model with a substantially lower CPU time than with an EDC model, fundamental physical quantities like heat fluxes, local species concentrations and temperature distribution, and in particular their evolution, are completely mispredicted by Flamelets. This is confirmed by values reported in Table 6 and
Table 7, which provide combustion efficiency and swirl number at different times: the ones obtained with the flamelets at 0.03 are in very good agreement with those calculated by EDC at 0.05 s, but the EDC results at 0.03 s are quite different from those at 0.05 s (they show a dynamic evolution absent in the flamelet simulations). A combustion efficiency higher than 0.9 is predicted by both models, but while in the first case this result is caused by the flamelet computation (function only of the fluid dynamics data and not of thermodynamics values), in the EDC model this is due to the fact that the dimensions of the combustion chamber are large enough to allow for the reactions to proceed to completion.
In fact, the “flamelet simulation” predicts complete reactions near the injection section and close to the wall while the second shows more realistic delay times.
Moreover, the difference in the calculated temperature maps is remarkable: the “flamelet simulation” predicts a quite homogenous temperature distribution with the highest value close to the wall, as if a “quasi-adiabatic boundary condition” were imposed on the wall, while the “EDC simulation” predicts -quite on the contrary- a hot core and a colder zone near the wall, respecting the wall heat fluxes due to the constant low wall temperature imposed. We conclude that the EDC approach is more accurate than the flamelets model and it is more suitable to study the evolution phenomena inside combustion chambers of this scale.
A future study will focus on the comparison of the simulation results with suitable experimental data in order to validate the computational model and to obtain useful design information.