Photocatalytic Desulfurization of Waste Tire Pyrolysis Oil
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Proximate and Ultimate Analysis of the Waste Tire Powder Composition
2.3. Pyrolysis of the Waste Tire Powder and Yields of the Resultant Components
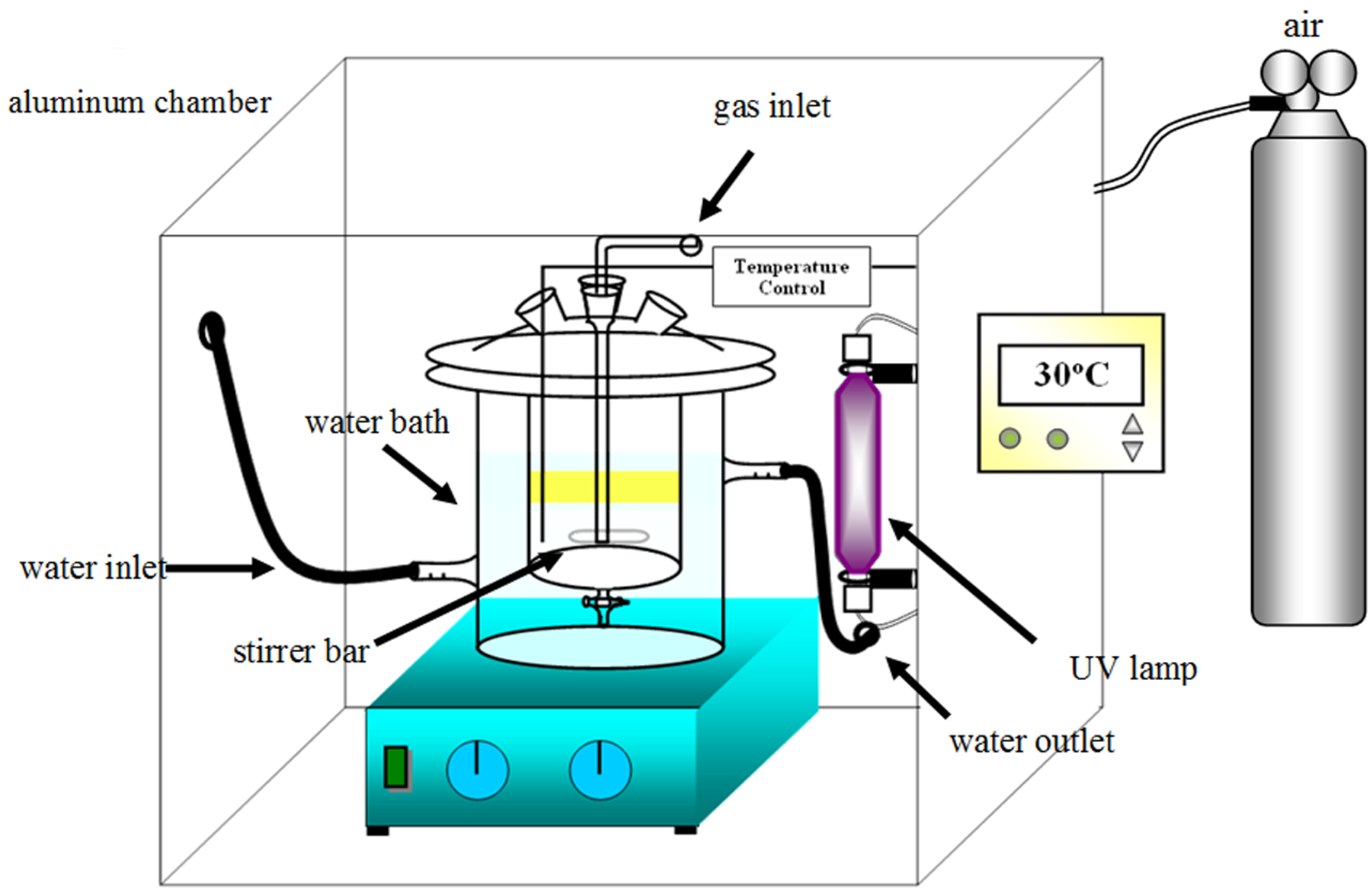
2.4. Photocatalytic Desulfurization of the Waste Tire Pyrolysis Oil
2.5. Determination of Sulfurous Compounds in the Waste Tire Pyrolysis Oil
2.6. Properties of Waste Tire Pyrolysis Oil
3. Results and Discussion
3.1. Composition of the Waste Tire Powder and Its Pyrolysis Products
| Analytical | % (w/w) |
|---|---|
| Proximate Analysis | |
| Volatiles | 62.4 |
| Fixed carbon | 27.6 |
| Ash | 8.73 |
| Moisture | 1.22 |
| Ultimate Analysis | |
| Carbon | 80.1 |
| Hydrogen | 7.48 |
| Nitrogen | 0.42 |
| Sulfur | 1.54 |
| Oxygen | 10.5 |
| Calorific heating value (MJ/kg) | 35.7 |
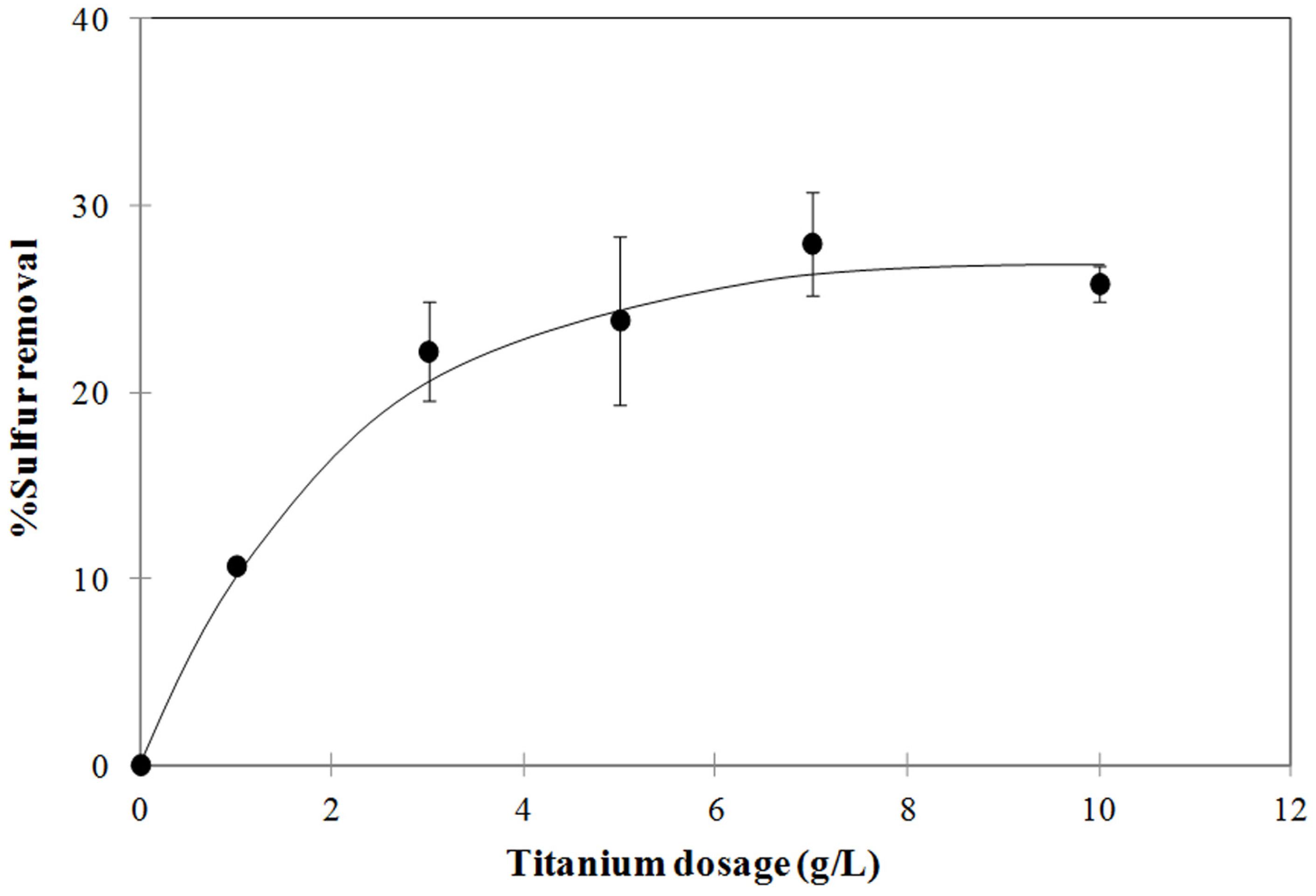
3.2. Influence of the TiO2 Dosage on the Degree of Sulfur Removal


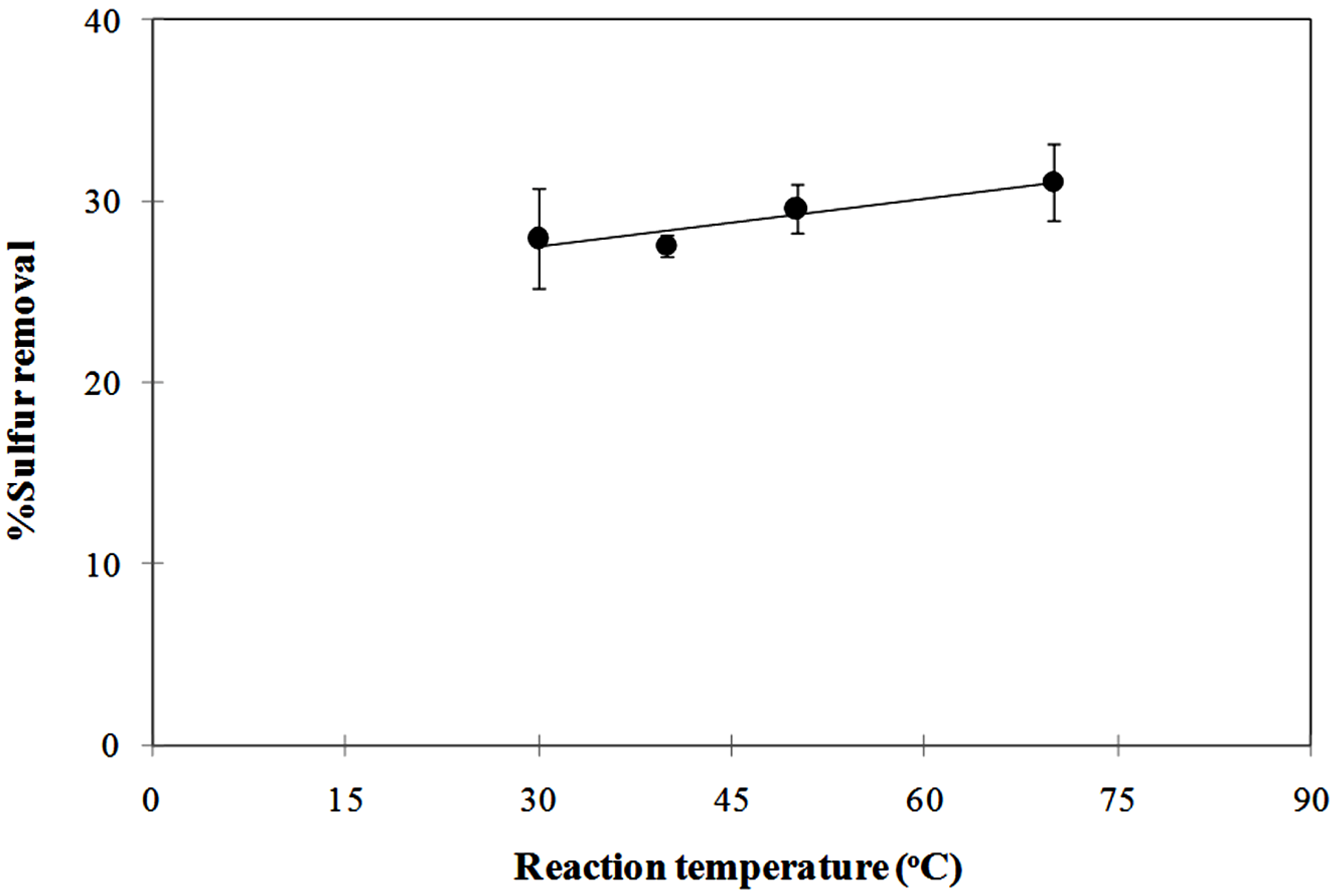
3.3. Influence of the Photoreaction Temperature on the Degree of Sulfur Removal
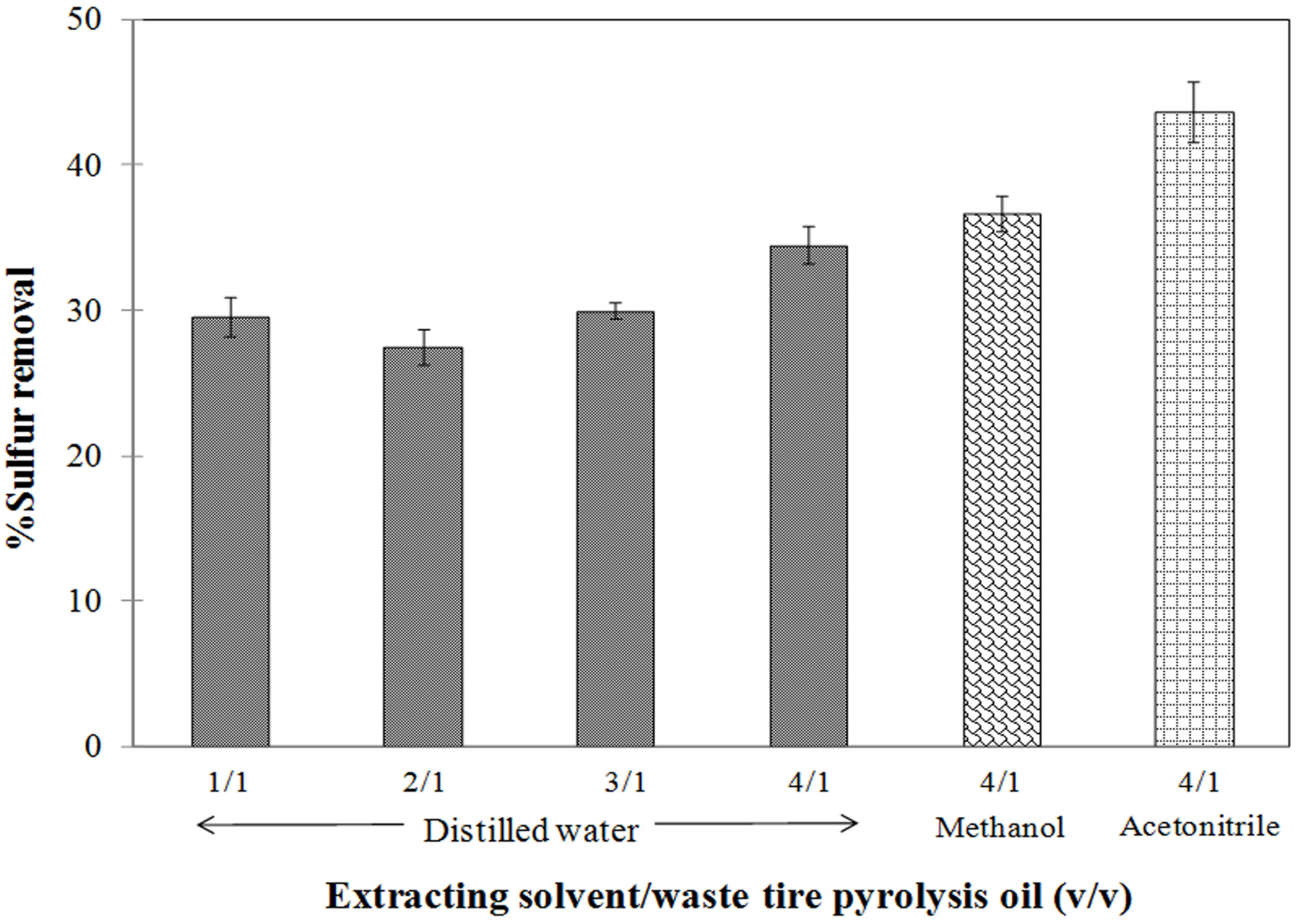
3.4. Influence of the Extracting Solvents on the Degree of Sulfur Removal
3.5. Influence of Oxidizing Agents on the Degree of Sulfur Removal
| Oxidizing Agent | Quantity | % Sulfur Removal |
|---|---|---|
| No addition | 3.22 (2.79) * | |
| Air | 50 mL/min | 22.3 (0.17) |
| 100 mL/min | 32.5 (0.76) | |
| 150 mL/min | 34.5 (1.26) | |
| H2O2 | 3% (w/w) | 11.9 (2.10) |
| 10% (w/w) | 17.7 (0.90) | |
| 30% (w/w) | 32.7 (1.20) |
3.6. Characterization of Sulfurous Compounds Containing in Waste Tire Pyrolysis Oil
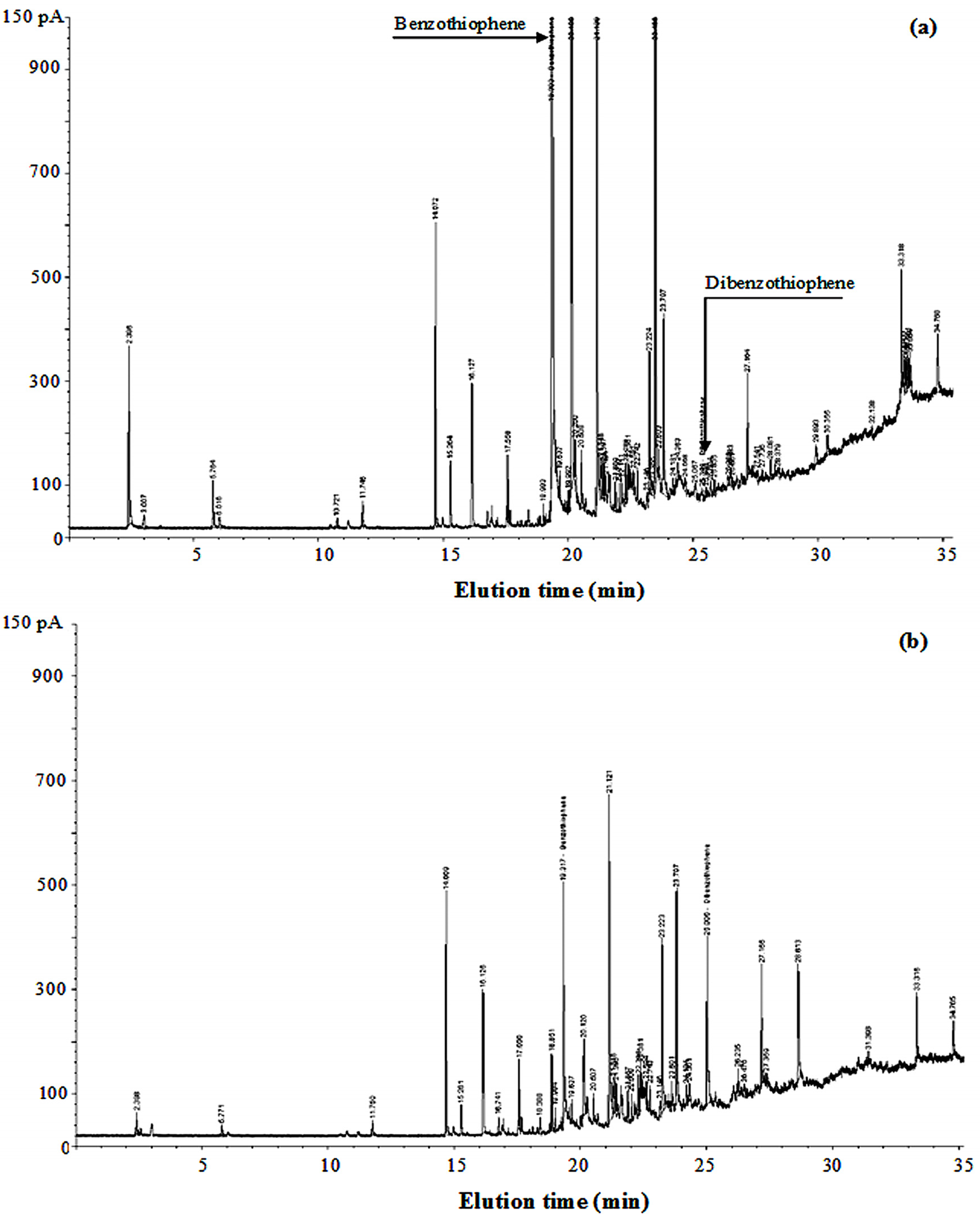
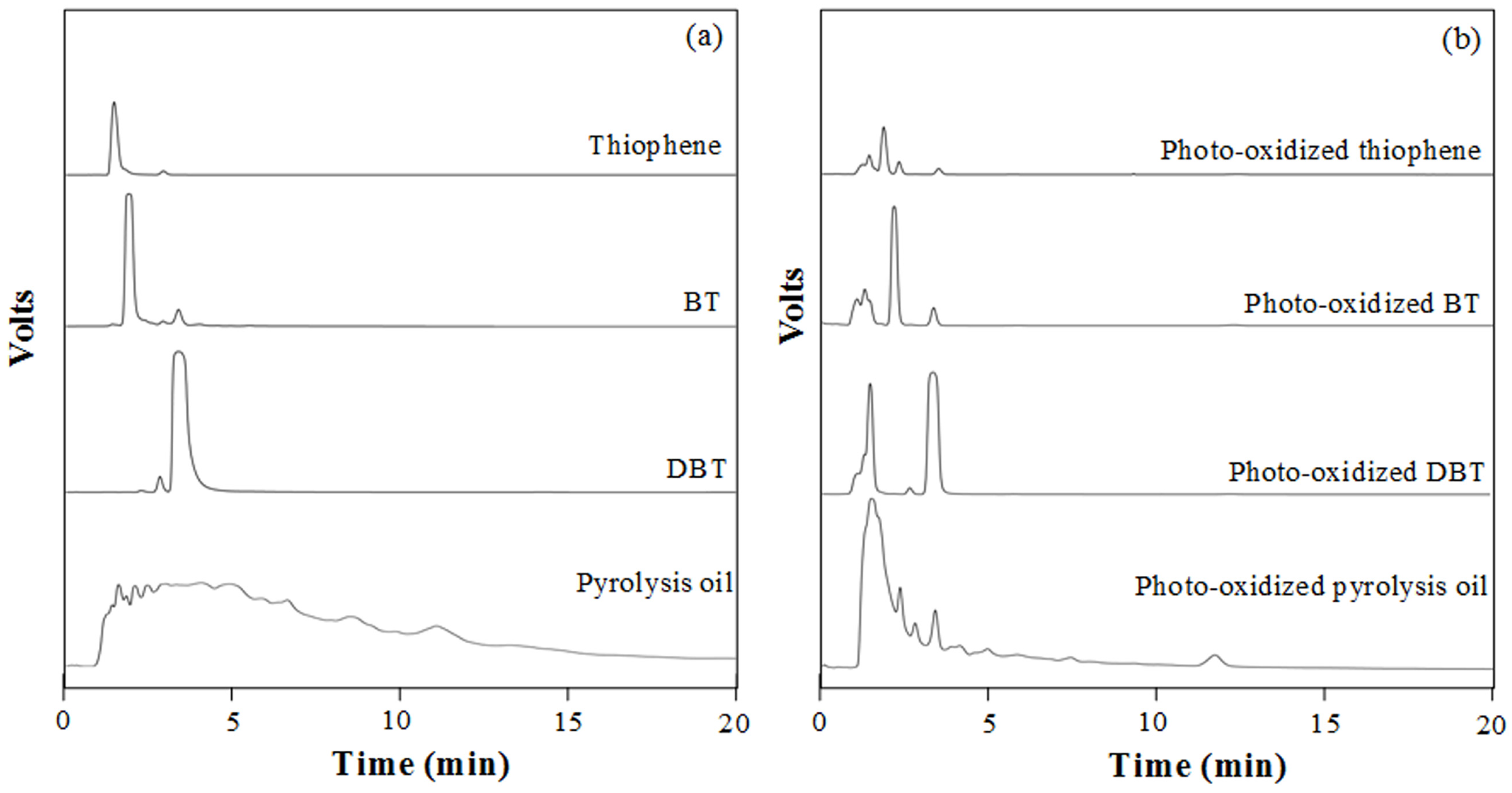
3.7. Properties Consideration of Waste Tire Pyrolysis Oil
| Products | Viscosity @ 100 °F (cSt) | Gross Calorific Heating Value (MJ/kg) | Sulfur Content (% w/w) |
|---|---|---|---|
| Murban crude oil | 2.94 ([44]) | 45.5 ([21]) * | 0.82 ([44]) |
| Waste tire pyrolysis oil | 2.66 | 43 | 0.84 |
| Photo-oxidized waste tire pyrolysis oil ** | 2.85 | 43 | 0.37 |
4. Conclusions
Acknowledgments
References
- United States Environmental Protection Agency. Final Rulemaking: Identification of Nonhazardous Secondary Materials that are Solid Waste Scrap Tires. 2011. Available online: http://www.epa.gov/osw//nonhaz/define/pdfs/tires-final.pdf (accessed on 5 May 2011). [Google Scholar]
- Chang, Y.M. On pyrolysis of waste tire: Degradation rate and product yields. Resour. Conserv. Recycl. 1996, 17, 125–139. [Google Scholar] [CrossRef]
- North Carolina Division of Pollution Prevention and Environmental Assistance. Environmental Problem Associated with Waste Tire. 2011. Available online: http://www.p2pays.org/ref/11/10504/html/intro/ploblems.htm (accessed on 5 May 2011).
- Virginia Department of Environmental Quality. Waste Tire Management. 2011. Available online: http://www.deq.state.va.us/wastetires/ (accessed on 5 May 2011).
- Cao, Q.; Jin, L.; Bao, W.; Lu, Y. Investigations into the characteristics of oils produced from co-pyrolysis of biomass and tire. Fuel Process. Technol. 2009, 90, 337–342. [Google Scholar] [CrossRef]
- Vijaya, S.; Chow, M.C.; Ma, A.N. Energy database of the oil palm, MPOB Palm Oil. Eng. Bull. 2004, 70, 15–22. [Google Scholar]
- Natarajan, E.; Nordin, A.; Rao, A.N. Overview of combustion and gasification of rice husk in fluidized bed reactors. Biomass Bioenergy 1998, 14, 533–546. [Google Scholar] [CrossRef]
- Juma, M.; Koreňová, Z.; Markoš, J.; Annus, J.; Jelemenský, Ĺ. Pyrolysis and combustion of scrap tire. Pet. Coal 2006, 48, 15–26. [Google Scholar]
- Laresgoiti, M.F.; Caballero, B.M.; Marco, I.; Torres, A.; Cabrero, M.A.; Chomón, M.J. Characterization of the liquid products obtained in tyre pyrolysis. J. Anal. Appl. Pyrolysis 2004, 71, 917–934. [Google Scholar] [CrossRef]
- Tao, H.; Nakazato, T.; Sato, S. Energy-efficient ultra-deep desulfurization of kerosene based on selective photooxidation and adsorption. Fuel 2009, 88, 1961–1969. [Google Scholar] [CrossRef]
- Te, M.; Fairbridge, C.; Ring, Z. Oxidation reactivities of dibenzothiophenes in polyoxometalate/H2O2 and formic acid/H2O2 systems. Appl. Catal. A Gen. 2001, 219, 267–280. [Google Scholar] [CrossRef]
- Bunthid, D.; Prasassarakich, P.; Hinchiranan, N. Oxidative desulfurization of tire pyrolysis naphtha in formic acid/H2O2/pyrolysis char system. Fuel 2010, 89, 2617–2622. [Google Scholar] [CrossRef]
- Herrmann, J.M. Heterogeneous photocatalysis: fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Su, Y.; Cheng, B. Template-free fabrication and enhanced photocatalytic activity of hierarchical macro-/mesoporous titania. Adv. Func. Mater. 2007, 17, 1984–1990. [Google Scholar] [CrossRef]
- Zhao, D.; Li, F.; Han, J.; Li, H. Photochemical oxidation of thiophene by O2 in an organic two-phase liquid-liquid extraction system. Petrol. Chem. 2007, 47, 448–451. [Google Scholar] [CrossRef]
- Hirai, T.; Ogawa, K.; Komasawa, I. Desulfurization process for dibenzothiophenes from light oil by photochemical reaction and liquid-liquid extraction. Ind. Eng. Chem. Res. 1996, 35, 586–589. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Hirai, T.; Komasawa, I. Identification of desulfurization products in the photochemical desulfurization process for benzothiophenes and dibenzothiophenes from light oil using an organic two-phase extraction system. Ind. Eng. Chem. Res. 1999, 38, 3300–3309. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, J.; Wang, J.; Liang, W.; Li, H. Photocatalytic oxidation desulfurization of diesel oil using Ti-containing zeolite. Pet. Sci. Technol. 2009, 27, 1–11. [Google Scholar] [CrossRef]
- SRI Instruments. Flame Photometric Detector—FPD. 2011. Available online: http://www.srigc.com/FPDman.pdf (accessed on 16 October 2011).
- Essom Company Limited. Heating Values of Hydrogen and Fuels. 2011. Available online: http://www.essom.com/backend/data-file/engineer/engin21_1.pdf (accessed on 7 May 2011).
- Habibi, M.H.; Vosooghian, H. Photocatalytic degradation of some organic sulfides as environmental pollutants using titanium dioxide suspension. J. Photochem. Photobiol. A Chem. 2005, 174, 45–52. [Google Scholar] [CrossRef]
- Williams, P.T.; Bottrill, R.P. Sulfur-polycyclic aromatic hydrocarbons in tyre pyrolysis oil. Fuel 1995, 74, 736–742. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.M.A.; Gaber, A.E.A.M. TiO2-photocatalytic oxidation of selected heterocyclic sulfur compounds. J. Photochem. Photobiol. A Chem. 1998, 114, 213–218. [Google Scholar] [CrossRef]
- Vargas, R.; Núñez, O. Photocatalytic degradation of oil industry hydrocarbons models at laboratory and at pilot-plant scale. Sol. Energy 2010, 84, 345–351. [Google Scholar] [CrossRef]
- Clennan, E.L. Persulfoxide: Key intermediate in reactions of singlet oxygen with sulfides. Acc. Chem. Res. 2001, 34, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.J.; Gu, C.L.; Kacher, M.L.; Foote, C. Chemistry of singlet oxygen. 45. Mechanism of photooxidation of sulfides. J. Am. Chem. Soc. 1983, 105, 4717–4721. [Google Scholar] [CrossRef]
- Mckee, M.L. A theoretical study of unimolecular reactions of dimethyl persulfoxide. J. Am. Chem. Soc. 1998, 120, 3963–3969. [Google Scholar] [CrossRef]
- Fox, M.A.; Abdel-Wahab, A.A. Selectivity in the TiO2-mediated photocatalytic oxidation of thioethers. Tetrahedron Lett. 1990, 31, 4533–4536. [Google Scholar] [CrossRef]
- Ali, M.F.; Al-Malki, A.; Ahmed, S. Chemical desulfurization of petroleum fractions for ultra-low sulfur fuels. Fuel Proc. Technol. 2009, 90, 536–544. [Google Scholar] [CrossRef]
- Ku, Y.; Hsieh, C.B. Photocatalytic decomposition of 2,4-diclorophenol in aqueous TiO2 suspensions. Water Res. 1992, 26, 1451–1456. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Sivasankar, B. Bioseparations: Principles and Techniques; Prentice-Hall of India: New Delhi, India, 2005; p. 203. [Google Scholar]
- Moeini-Nombel, L.; Matsuzawa, S. Effect of solvents and a substituent group on photooxidation of fluorine. J. Photochem. Photobiol. A Chem. 1998, 119, 15–23. [Google Scholar] [CrossRef]
- Hu, Y.; Yuan, C. Low-temperature preparation of photocatalytic TiO2 thin films on polymer substrates by direct deposition from anatase sol. J. Mater. Sci. Technol. 2006, 22, 239–244. [Google Scholar] [CrossRef]
- Legrini, O.; Oliveros, E.A.; Braun, M. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Chiou, C.H.; Wu, C.Y.; Juang, R.S. Influence of operating parameters on photocatalytic degradation of phenol in UV/TiO2 process. Chem. Eng. J. 2008, 139, 322–329. [Google Scholar] [CrossRef]
- Barakat, M.A.; Tseng, J.M.; Huang, C.P. Hydrogen peroxide-assisted photocatalytic oxidation of phenolic compounds. Appl. Catal. B: Environ. 2005, 59, 99–104. [Google Scholar] [CrossRef]
- Sauer, T.; Cesconeto Neto, G.; José, H.J.; Moreira, R.F.P.M. Kinetics of photocatalytic degradation of reactive dyes in a TiO2 slurry reactor. J. Photochem. Photobiol. A Chem. 2002, 149, 147–154. [Google Scholar] [CrossRef]
- Larrubia, M.A.; Alejandre, A.G.; Ramìrez, J.; Busca, G. A FT-IR study of the adsorption of indole, carbazole, benzothiophene, dibenzothiophene and 4,6-dibezothiophene over solid adsorbents and catalysts. Appl. Catal. A 2002, 224, 167–178. [Google Scholar] [CrossRef]
- Otsuki, S.; Nonaka, T.; Takashima, N.; Qian, W.; Ishihara, A.; Imai, T.; Kabe, T. Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction. Energy Fuel 2000, 14, 1232–1239. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Hirai, T.; Komasawa, I. A deep desulfurization process for light oil by photochemical reaction in an organic two-phase liquid-liquid extraction system. Ind. Eng. Chem. Res. 1998, 37, 203–211. [Google Scholar] [CrossRef]
- Department of Alternative Energy, Development and Efficiency (DEDE), Ministry of Energy. Oil and Thailand. 2009. Available online: http://www.dede.go.th/dede/images/stories/stat_dede/OilThailand2009.pdf (accessed on 27 August 2011).
- Abu Dhabi National Oil Company (ADNOC). Available online: http://www.adnoc.ae/content.aspx?newid=265&mid=265 (accessed on 26 August 2011).
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Trongkaew, P.; Utistham, T.; Reubroycharoen, P.; Hinchiranan, N. Photocatalytic Desulfurization of Waste Tire Pyrolysis Oil. Energies 2011, 4, 1880-1896. https://doi.org/10.3390/en4111880
Trongkaew P, Utistham T, Reubroycharoen P, Hinchiranan N. Photocatalytic Desulfurization of Waste Tire Pyrolysis Oil. Energies. 2011; 4(11):1880-1896. https://doi.org/10.3390/en4111880
Chicago/Turabian StyleTrongkaew, Phakakrong, Thanes Utistham, Prasert Reubroycharoen, and Napida Hinchiranan. 2011. "Photocatalytic Desulfurization of Waste Tire Pyrolysis Oil" Energies 4, no. 11: 1880-1896. https://doi.org/10.3390/en4111880




