Evaluation of Various Solid Biomass Fuels Using Thermal Analysis and Gas Emission Tests
Abstract
:1. Introduction
2. Incidents in Japan
2.1. Some Examples of Biomass Fuel Incidents in Japan
2.1.1. RDF Explosions

| RDF (Refuse Derived Fuel) | RPF (Refuse Paper & Plastic Fuel) | |
|---|---|---|
| Raw materials | Home waste | Industry waste |
| Heat of combustion, kJ / kg | 12,600~16,800 | 25,000~42,000 |
| Water content | Large, about 8% | Small |

2.1.2. RPF Fires
2.1.3. Wood Chip Fires
3. Cause Investigation and Mechanism of Heat Generation
- (1)
- Faint heat generation between room temperature and about 60 °C;
- (2)
- Combustion of fatty acid esters in materials;
- (3)
- Combustion of cellulose or other organic materials.


Absorption of Water and Chemical Reaction

4. Experimental
4.1. Examples of Biomass Fuel
| Fuel | Summary | Heat of Combustion, kJ/kg |
|---|---|---|
| RDF | Various domestic garbage materials exist (Figure 6) | 16,800~21,800 * 12,600~16,800 ** [10] |
| RPF | Consists of paper and plastic | 25,200~33,600 * 25,000~42,000 ** [10] |
| Wood-pellet (White) | Inner part of wood | 19,700 * |
| Wood-pellet (Bark) | Outer part of wood, oily material exists | 18,650 * |
| Chicken dung | Water content: about 40% | 8000 * |
| Cow dung | Water content: less than 20% | 6300 ** |
| Coal-wood mixture | Water content: about 10% | 21,500 * |
| Sewage sludge derived fuel | Various types are proposed | 14,880~23,400 * |
| C-RPF | Char-Refuse Paper & Plastic Fuel | 25,000 ** [10] |
| Fish meal | Water content 9.4% | 23,500 ** [13] |
| Soy sauce residue | No water | 22,000 ** [13] |
| Coal | Bituminous coal, standard material for evaluation [9] | 29,800 * |
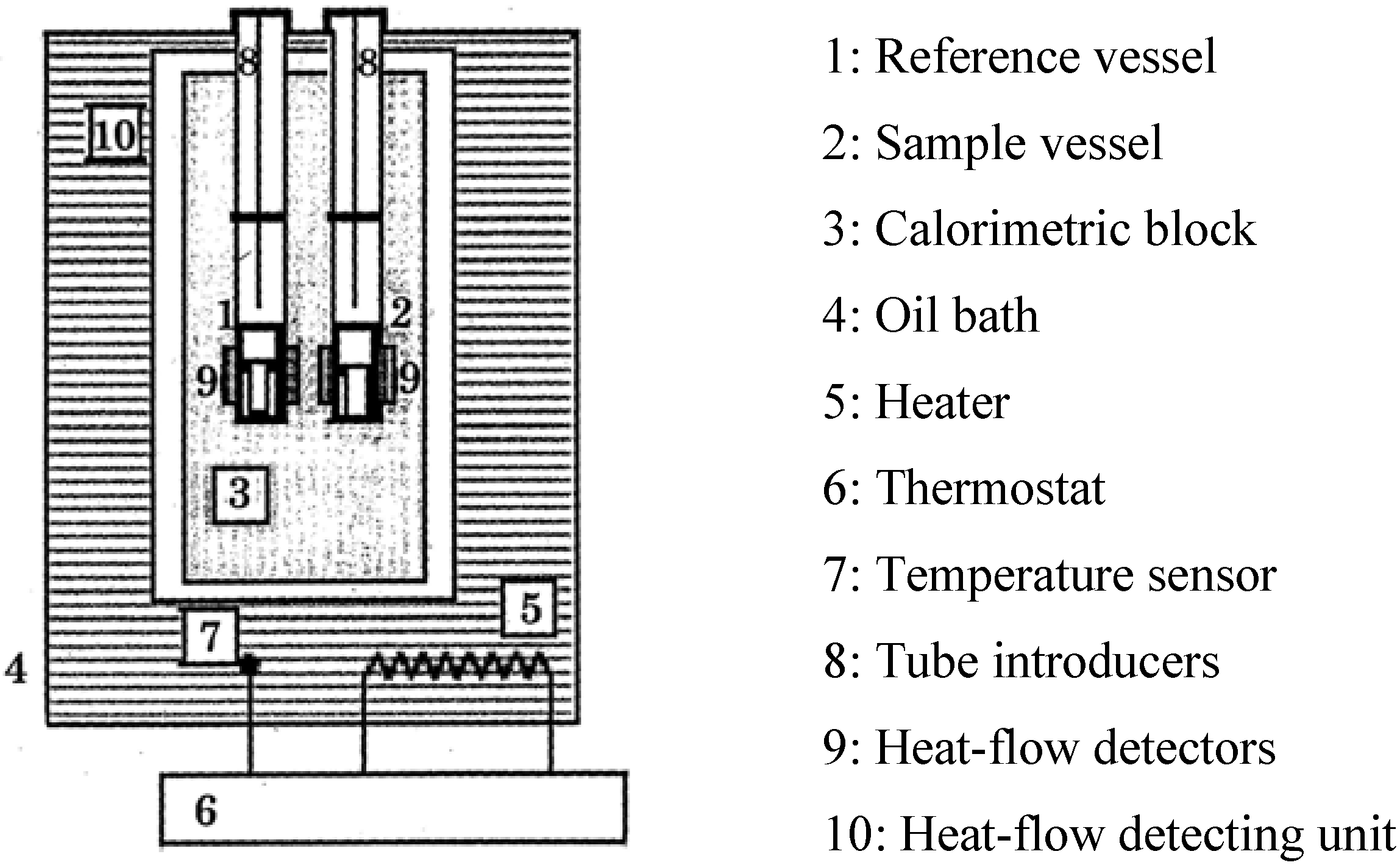
4.2. The High Sensitive Calorimeter, TAM, MS80 and C80
4.3. Gas Emission Tests
5. Methods and Results of Evaluation of Biomass Fuels
5.1. Test Methods in the UN Recommendations
5.2. Evaluation with Thermal Methods and Gas Emission Test
- (i)
- TG-DTA: The thermal analysis, TG-DTA, is widely used for studying thermal properties of solid or liquid materials. In our evaluation process it was used for the screening tests of thermal properties with small amount of materials. Wood chips were measured in the TG-DTA, and results are shown in Figure 8. They did not produce any large heat generation until 200 °C. However there was not enough data to decide that wood chips did not produce small heat generation which was related with causing fires, so we should ascertain heat generation with the highly sensitive calorimeters.
- (ii)
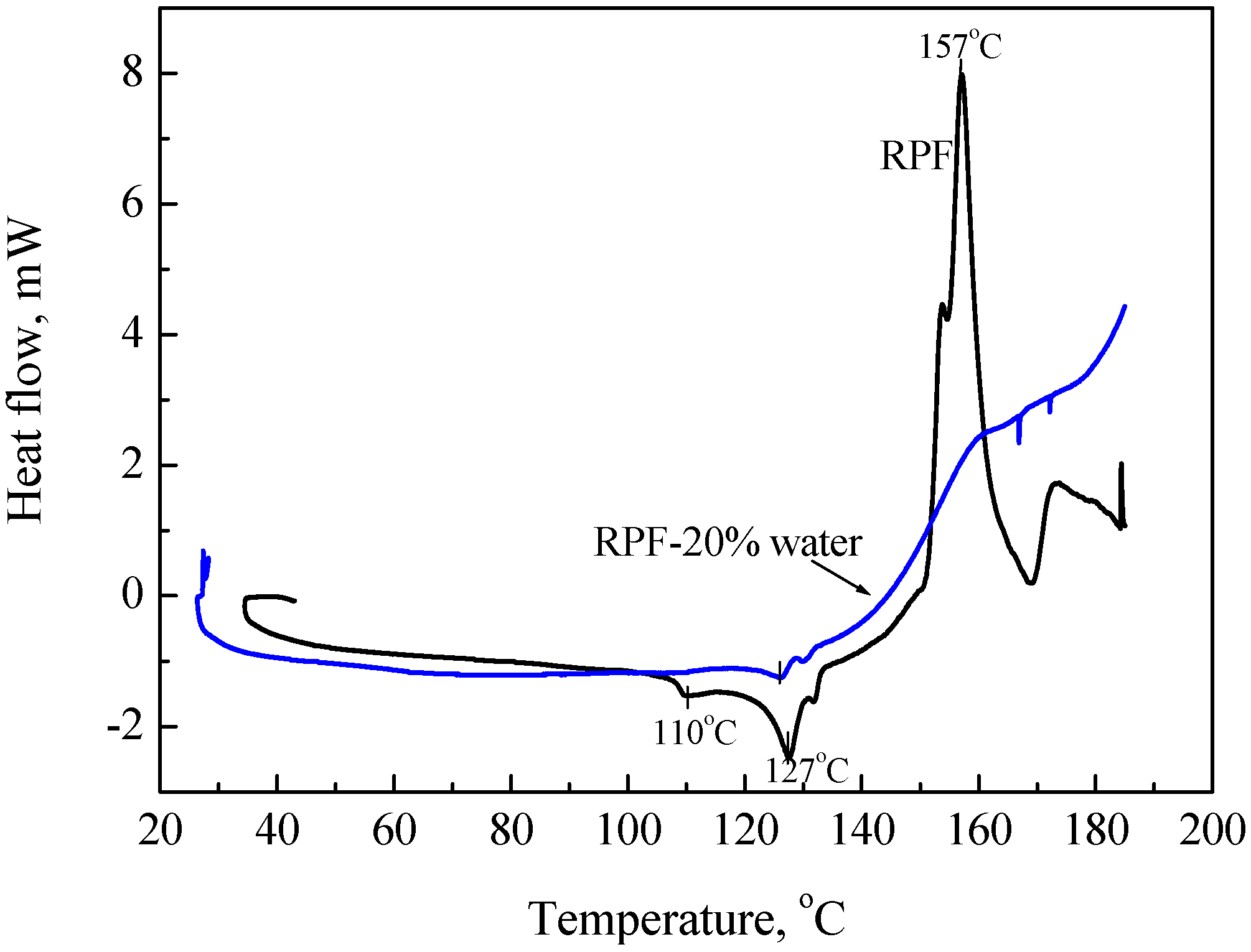
- Highly sensitive calorimeters: Because the DTA and DSC do not have high sensitivity, we used high sensitivity calorimeters, C80, MS80 or TAM. Sample amounts of measurement of C80 and MS80 are about one gram, smaller than that of TAM. Heat generation can be measured in the C80 from room temperature up to 300 °C, at a heating rate of 0.1 °C/min, and in the MS80 from room temperature up to 180 °C, at a heating rate of 0.02 °C/min [9]. The TAM is a much more sensitive calorimeter than the MS80. It is used under isothermal conditions. Figure 5 shows results of RDF with/without water. When water was added to wood chips, heat generation was observed after several hours.
- (iii)
- Adiabatic tester, SIT and critical height in a huge pile: To verify ignition with small heat generation, we detected the heat with highly sensitive calorimeters. We used for this purpose the adiabatic testers, SIT and/or ARC. Figure 9 shows SIT test results for RPF. When RPF was held at higher than 160 °C, its temperature started increasing after several hours.




6. Summary of Evaluation Results
| Rank | Heat Generation* | Without Water | With 20% Water |
|---|---|---|---|
| A | >50 J/g | Chicken dung, Sewage sludge derived fuel A, RDF | |
| B | 10~50 J/g | Chicken dung, RDF, Sewage sludge derived fuel A | Wood-pellet (Bark, White) |
| C | 5~10 J/g | Coal (Bituminous coal), Wood-pellet (Bark, White), Sewage sludge derived fuel B, Coal/wood mixture fuel , Cellulose A | Coal (Bituminous coal), Cellulose A, B, Coal/wood mixture fuel, Sewage sludge derived fuel B |
| D | <5 J/g | RPF, C-RPF, Cellulose B | RPF, C-RPF |
7. Conclusions
References
- Li, X.R.; Lim, W.-S.; Iwata, Y.; Koseki, H. Thermal characterisitics and their relevance to spontaneous ignition of refuse plastics/paper. J. Loss Prev. Process Ind. 2009, 22, 1–6. [Google Scholar] [CrossRef]
- Li, X.R.; Lim, W.-S.; Iwata, Y.; Koseki, H. Thermal behavior of sewage sludge derived fuels. Therm. Sci. 2008, 12, 137–148. [Google Scholar] [CrossRef]
- Shimizu, Y.; Wakakura, M.; Arai, M. Heat accumulations and fire accidents of waste piles. J. Loss Prev. Process Ind. 2009, 22, 86–90. [Google Scholar] [CrossRef]
- Moqbel, S.; Reinhart, D.; Chen, R.-H. Factors influencing spontaneous combustion of solid waste. Waste Manag. 2010, 30, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Persson, H. Silo fires require special tactics and equipment. Ind. Fire J. 2010, 81, 32–35. [Google Scholar]
- Matumaga, A.; Yasuhara, A.; Shimizu, Y.; Wakakura, M.; Shibamoto, T. Investigation on the spontaneous combustion of refuse-derived fuels during storage using a chemiluminescence technique. Waste Manag. Res. 2008, 26, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-R.; Koseki, H.; Iwata, Y. Risk assessment on processing facility of raw organic garbage. J. Hazard. Mater. 2008, 154, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-R.; Lim, W.-S.; Iwata, Y.; Koseki, H. Safety evaluation of sewage-sludge-derived fuels by comparison with other fuels. Fire Mater. 2009, 33, 187–200. [Google Scholar] [CrossRef]
- Li, X.-R.; Koseki, H.; Iwata, Y. A study on spontaneous ignition of bituminous coal. Therm. Sci. 2009, 13, 105–112. [Google Scholar] [CrossRef]
- Homepage of Japan RPF Association. Available online: http://www.jrpf.gr.jp/rpf-5.html (accessed on 10 April 2011).
- Murasawa, N. Chiba Institute of Science, Choshi, Japan, March 2010.
- Fu, Z.-M.; Li, X.-R.; Koseki, H. Heat generation of refuse derived fuel with water. J. Loss Prev. Process Ind. 2005, 18, 27–33. [Google Scholar] [CrossRef]
- Li, X.-R.; Koseki, H.; Momota, M. Evaluation of danger from fermentation-induced spontaneous ignition of wood chips. J. Hazard. Mater. 2006, A135, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.-M.; Koseki, H.; Iwata, Y. Investigation on spontaneous ignition of two kind of organic materials with water. Thermochim. Acta 2006, 440, 68–74. [Google Scholar] [CrossRef]
- United Nations. Recommendations on the Transport of Dangerous Goods: Manual of Tests and Criteria, 4th ed.; United Nations: New York, NY, USA, 2003. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Koseki, H. Evaluation of Various Solid Biomass Fuels Using Thermal Analysis and Gas Emission Tests. Energies 2011, 4, 616-627. https://doi.org/10.3390/en4040616
Koseki H. Evaluation of Various Solid Biomass Fuels Using Thermal Analysis and Gas Emission Tests. Energies. 2011; 4(4):616-627. https://doi.org/10.3390/en4040616
Chicago/Turabian StyleKoseki, Hiroshi. 2011. "Evaluation of Various Solid Biomass Fuels Using Thermal Analysis and Gas Emission Tests" Energies 4, no. 4: 616-627. https://doi.org/10.3390/en4040616




