Selective Preparation of Furfural from Xylose over Sulfonic Acid Functionalized Mesoporous Sba-15 Materials
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD

2.2. FTIR

2.3. TG

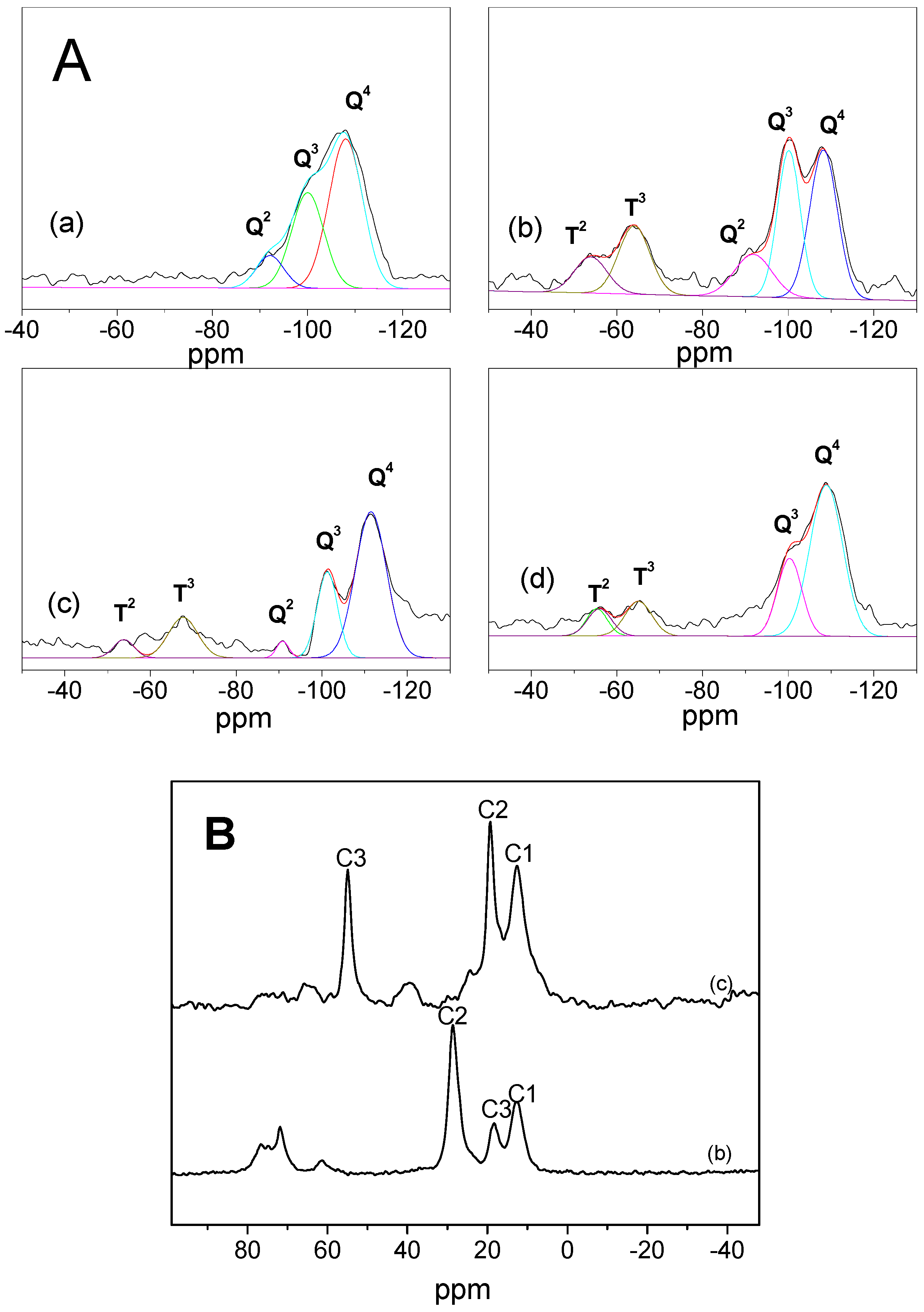
2.4. 29Si-NMR
| Catalyst | Conversion a (%) | Selectivity a (%) | T (%) | Q (%) | Q2 b (%) | Q3 b (%) | Q4 b (%) |
|---|---|---|---|---|---|---|---|
| None | 37.2 | 12.9 | - | - | - | - | - |
| SBA-15 | 38.9 | 13.5 | 0.0 | 100 | 9.6 | 35.5 | 54.9 |
| SBA-15-SH | 22.9 | 0.0 | 25.7 | 74.3 | 11.4 | 40.4 | 48.2 |
| SBA-15-SO3H(C) | 92.4 | 73.9 | 17.0 | 83.0 | 4.5 | 23.6 | 71.9 |
| SBA-15-SO3H(G) | 78.3 | 65.5 | 16.8 | 83.2 | 0.0 | 35.9 | 64.1 |
| H2SO4 (4%) | 92.3 | 63.5 | - | - | - | - | - |
2.5. 13C-NMR
2.6. Catalytic Performance
2.6.1. Catalytic Activity


2.6.2. Deactivation and Regeneration

| Catalyst | SBET (m2/g) | Vp (cm3/g) | DBJH (nm) | N (mmol/g) a | C (wt%) b |
|---|---|---|---|---|---|
| SBA-15 | 765.1 | 1.09 | 8.1 | - | - |
| SBA-15-SO3H(C) (fresh) | 746.7 | 1.26 | 6.6 | 1.49 | 0 |
| SBA-15-SO3H(C) (used) | 337.5 | 0.42 | 3.9, 5.7 | 1.48 | 33.5 |
| SBA-15-SO3H(C) (regeneration-H2O2) | 699.5 | 1.11 | 6.6 | 1.49 | 0.4 |
| SBA-15-SO3H(C) (regeneration-acetone) | 418.8 | 0.73 | 4.9, 7.8 | 1.49 | 28.2 |

3. Experimental Section
3.1. Preparation of the Catalysts
3.1.1. Synthesis of SBA-15
3.1.2. Synthesis of SBA-15-SH(C) Using the Co-Condensation Method
3.1.3. Synthesis of SBA-15-SO3H(C) using the Co-Condensation Method
3.1.4. Synthesis of SBA-15-SO3H(G) Using the Grafting Method
3.2. Characterization of the Catalysts
3.3. Catalytic Activity Tests
4. Conclusions
Acknowledgements
References
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Xiang, H.W.; Li, Y.W.; Jiao, H.; Wu, G.S.; Zhong, B.; Guo, G.Q. A new strategy for the efficient synthesis of 2-methylfuran and γ-butyrolactone. New J. Chem. 2003, 27, 208–210. [Google Scholar] [CrossRef]
- Horton, B. Green chemistry puts down roots. Nature 1999, 400, 797–799. [Google Scholar] [CrossRef]
- Anastas, P.T.; Zimmerman, J.B. Peer reviewed: design through the 12 principles of green engineering. Environ. Sci. Technol. 2003, 37, 94A–101A. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.H. Solid acids for green chemistry. Acc. Chem. Res. 2002, 35, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Moreau, C.; Belgacem, M.N.; Gandini, A. Recent catalytic advances in the chemistry of substituted furans from carbohydrates and in the ensuing polymers. Top. Catal. 2004, 27, 11–30. [Google Scholar] [CrossRef]
- Moreau, C.; Durand, R.; Peyron, D.; Duhamet, J.; Rivalier, P. Selective preparation of furfural from xylose over microporous solid acid catalysts. Ind. Crops Prod. 1998, 7, 95–99. [Google Scholar] [CrossRef]
- Dias, A.S.; Lima, S.; Carriazo, D.; Rives, V.; Pillinger, M.; Valente, A.A. Exfoliated titanate, niobate and titanoniobate nanosheets as solid acid catalysts for the liquid-phase dehydration of d-xylose into furfural. J. Catal. 2006, 244, 230–237. [Google Scholar] [CrossRef]
- Lima, S.; Pillinger, M.; Valente, A.A. Dehydration of d-xylose into furfural catalysed by solid acids derived from the layered zeolite Nu-6(1). Catal. Commun. 2008, 9, 2144–2148. [Google Scholar] [CrossRef]
- Lessard, J.; Morin, J.-F.; Wehrung, J.-F.; Magnin, D.; Chornet, E. High yield conversion of residual pentoses into furfural via zeolite catalysis and catalytic hydrogenation of furfural to 2-methylfuran. Top. Catal. 2010, 53, 1231–1234. [Google Scholar] [CrossRef]
- Wilson, K.; Clark, J.H. Solid acids and their use as environmentally friendly catalysts in organic synthesis. Pure Appl. Chem. 2004, 72, 1313–1319. [Google Scholar]
- Mercier, L.; Pinnavaia, T.J. Access in mesoporous materials: advantages of a uniform pore structure in the design of a heavy metal ion adsorbent for environmental remediation. Adv. Mater. 1997, 9, 500–503. [Google Scholar] [CrossRef]
- Dias, A.S.; Pillinger, M.; Valente, A.A. Dehydration of xylose into furfural over micro-mesoporous sulfonic acid catalysts. J. Catal. 2005, 229, 414–423. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Corma, A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 1997, 97, 2373–2419. [Google Scholar] [CrossRef] [PubMed]
- Jarupatrakorn, J.; Tilley, T.D. Silica-supported, single-site titanium catalysts for olefin epoxidation. A molecular precursor strategy for control of catalyst structure. J. Am. Chem. Soc. 2002, 124, 8380–8388. [Google Scholar] [CrossRef] [PubMed]
- Kureshy, R.I.; Ahmad, I.; Pathak, K.; Khan, N.H.; Abdi, S.H.R.; Jasra, R.V. Sulfonic acid functionalized mesoporous SBA-15 as an efficient and recyclable catalyst for the synthesis of chromenes from chromanols. Catal. Commun. 2009, 10, 572–575. [Google Scholar] [CrossRef]
- Shylesh, S.; Samuel, P.P.; Srilakshmi, C.; Parischa, R.; Singh, A.P. Sulfonic acid functionalized mesoporous silicas and organosilicas: synthesis, characterization and catalytic applications. J. Mol. Catal. A 2007, 274, 153–158. [Google Scholar] [CrossRef]
- Shylesh, S.; Sharma, S.; Mirajkar, S.P.; Singh, A.P. Silica functionalised sulphonic acid groups: synthesis, characterization and catalytic activity in acetalization and acetylation reactions. J. Mol. Catal. A 2004, 212, 219–228. [Google Scholar] [CrossRef]
- Hamoudi, S.; Royer, S.; Kaliaguine, S. Propyl- and arene-sulfonic acid functionalized periodic mesoporous organosilicas. Microporous Mesoporous Mater. 2004, 71, 17–25. [Google Scholar] [CrossRef]
- Chidambaram, M.; Venkatesan, C.; Singh, A.P. Organosilanesulfonic acid-functionalized Zr-TMS catalysts: synthesis, characterization and catalytic applications in condensation reactions. Appl. Catal. A 2006, 310, 79–90. [Google Scholar] [CrossRef]
- Wilson, K.; Lee, A.F.; Macquarrie, D.J.; Clark, J.H. Structure and reactivity of sol-gel sulphonic acid silicas. Appl. Catal. A 2002, 228, 127–133. [Google Scholar] [CrossRef]
- Engelhardt, G.; Jancke, H. Structure investigation of organosilicon polymers by silicon-29 NMR. Polym. Bull. 1981, 5, 577–584. [Google Scholar] [CrossRef]
- Lim, M.H.; Stein, A. Comparative studies of grafting and direct syntheses of inorganic-organic hybrid mesoporous materials. Chem. Mater. 1999, 11, 3285–3295. [Google Scholar] [CrossRef]
- Antal, M.J.J.; Leesomboon, T.; Mok, W.S.; Rochards, G.N. Mechanism of formation of 2-furaldehyde from d-xylose. Carbohydr. Res. 1991, 217, 71–85. [Google Scholar] [CrossRef]
- Lourvanij, K.; Rorrer, G.L. Reactions of aqueous glucose solutions over solid-acid Y-zeolite catalyst at 110–160 °C. Ind. Eng. Chem. Res. 1993, 32, 11–19. [Google Scholar] [CrossRef]
- Zeitsch, K.J. The Chemistry and technology of furfural and its many by-products. In Sugar Series; Elsevier: Amsterdam, The Netherlands, 2000; pp. 19–23. [Google Scholar]
- Benvenuti, F.; Carlini, C.; Patrono, P.; Galletti, A.M.R.; Sbrana, G.; Massucci, M.A.; Galli, P. Heterogeneous zirconium and titanium catalysts for the selective synthesis of 5-hydroxymethyl-2-furaldehyde from carbohydrates. Appl. Catal. A 2000, 193, 147–153. [Google Scholar] [CrossRef]
- Berrichi, Z.E.; Cherif, L.; Orsen, O.; Fraissard, J.; Tessonnier, J.P.; Vanhaecke, E.; Louis, B.; Ledoux, M.J.; Huu, C.P. Ga doped SBA-15 as an active and stable catalyst for Friedel-Crafts liquid-phase acylation. Appl. Catal. A 2006, 298, 194–202. [Google Scholar] [CrossRef]
- Richer, R.; Mercier, L. Direct synthesis of functionalized mesoporous silica by non-ionic alkylpolyethyleneoxide surfactant assembly. Chem. Commun. 1998, 16, 1775–1776. [Google Scholar] [CrossRef]
- Yang, L.M.; Wang, Y.J.; Luo, G.S.; Dai, Y.Y. Functionalization of SBA-15 mesoporous silica with thiol or sulfonic acid groups under the crystallization conditions. Microporous Mesoporous Mater. 2005, 84, 275–282. [Google Scholar] [CrossRef]
- Sako, T.; Taguchi, T.; Sugeta, T.; Nakazawa, N.; Okubo, T.; Hiaki, T.; Sato, M. Kinetic study of furfural formation accompanying supercritical carbon dioxide extraction. J. Chem. Eng. Jpn. 1992, 25, 372–377. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shi, X.; Wu, Y.; Yi, H.; Rui, G.; Li, P.; Yang, M.; Wang, G. Selective Preparation of Furfural from Xylose over Sulfonic Acid Functionalized Mesoporous Sba-15 Materials. Energies 2011, 4, 669-684. https://doi.org/10.3390/en4040669
Shi X, Wu Y, Yi H, Rui G, Li P, Yang M, Wang G. Selective Preparation of Furfural from Xylose over Sulfonic Acid Functionalized Mesoporous Sba-15 Materials. Energies. 2011; 4(4):669-684. https://doi.org/10.3390/en4040669
Chicago/Turabian StyleShi, Xuejun, Yulong Wu, Huaifeng Yi, Guo Rui, Panpan Li, Mingde Yang, and Gehua Wang. 2011. "Selective Preparation of Furfural from Xylose over Sulfonic Acid Functionalized Mesoporous Sba-15 Materials" Energies 4, no. 4: 669-684. https://doi.org/10.3390/en4040669




