Removal and Conversion of Tar in Syngas from Woody Biomass Gasification for Power Utilization Using Catalytic Hydrocracking
Abstract
:1. Introduction
2. Catalytic Hydrocracking






3. Experimental Setup
3.1. Fuel for Gasification

3.2. Facilities and Equipment
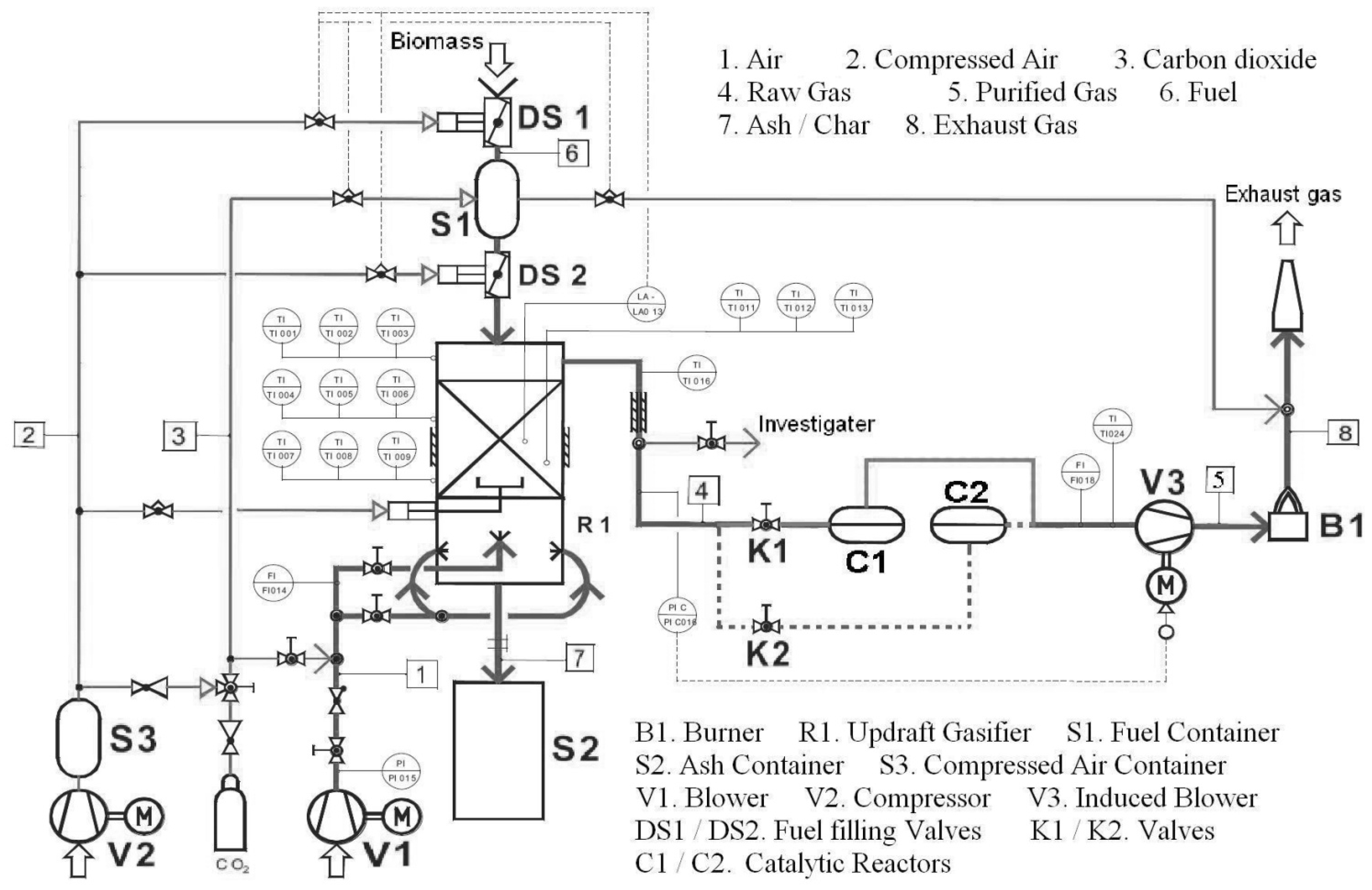
3.3. Catalyst and Catalytic Reactor

4. Sampling and Analysis
4.1. Sampling of Tar in Syngas
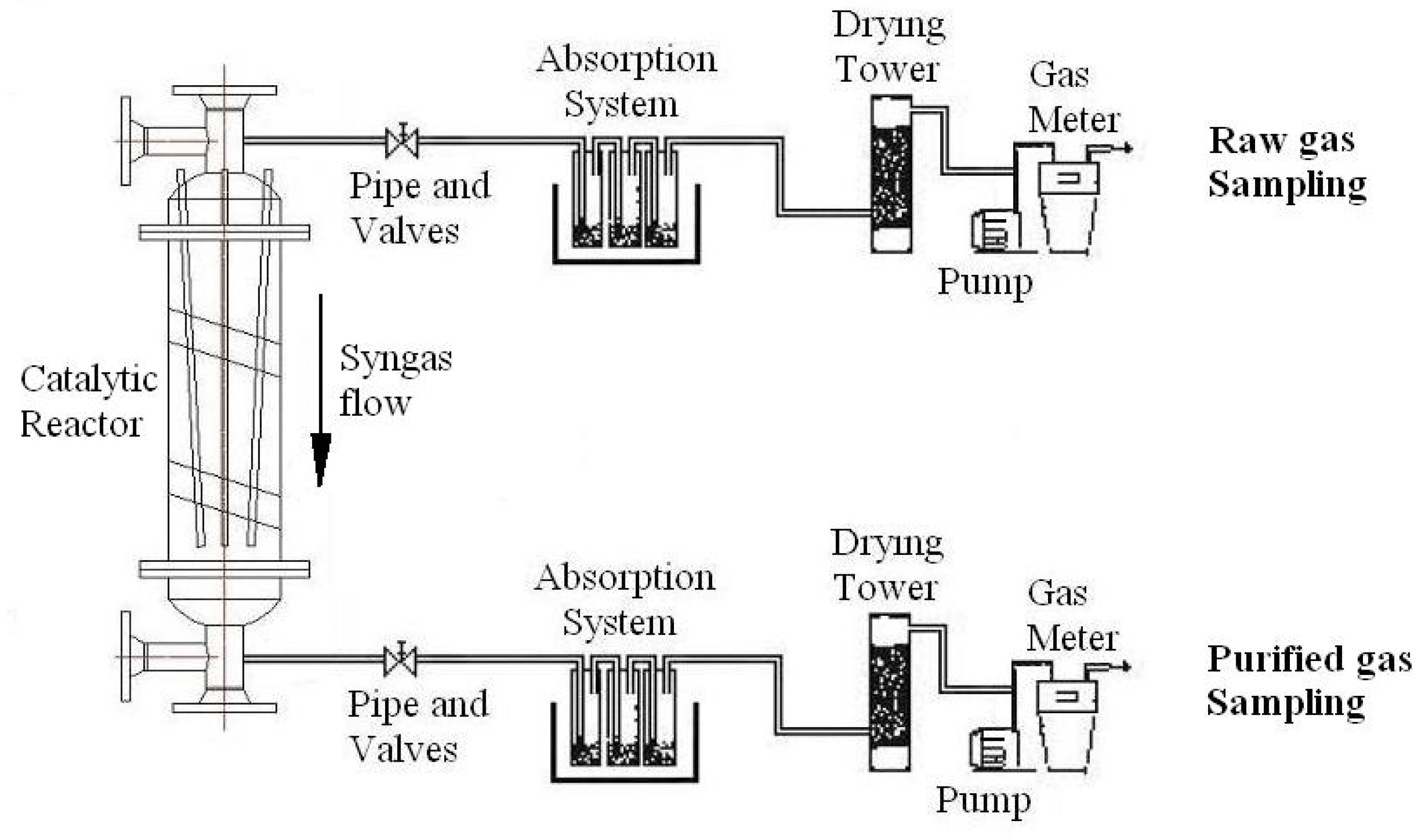
4.2. Analysis of Tar Samples
| Distillation Fractions | Molecular Weights | Boiling Points (°C) | Distillation Fractions | Molecular Weights | Boiling Points (°C) |
|---|---|---|---|---|---|
| D01 | <C7 | ≤98 | D13 | C19 | >317 |
| ≤330 | |||||
| D02 | C8 | >98 | D14 | C20 | >330 |
| ≤126 | ≤342.7 | ||||
| D03 | C9 | >126 | D15 | C21 | >342.7 |
| ≤151 | ≤356.5 | ||||
| D04 | C10 | >151 | D16 | C22 | >356.5 |
| ≤174 | ≤367 | ||||
| D05 | C11 | >174 | D17 | C23 | >367 |
| ≤196 | ≤380 | ||||
| D06 | C12 | >196 | D18 | C24 | >380 |
| ≤217 | ≤391.3 | ||||
| D07 | C13 | >217 | D19 | C25 | >391.3 |
| ≤234 | ≤401.9 | ||||
| D08 | C14 | >234 | D20 | C26 | >401.9 |
| ≤253 | ≤412.2 | ||||
| D09 | C15 | >253 | D21 | C27 | >412.2 |
| ≤270 | ≤442 | ||||
| D10 | C16 | >270 | D22 | C28 | >442 |
| ≤287 | ≤431.6 | ||||
| D11 | C17 | >287 | D23 | C29 | >431.6 |
| ≤302 | ≤440.8 | ||||
| D12 | C18 | >302 | |||
| ≤317 |
4.3. Tarry Impurities in IC Engines
5. Results and Discussion
5.1. Results for Different Temperatures at a Fixed Flow Rate of 20 Nm³/h
- CO 18–23%,
- CH4 3–12%,
- H2 8–10%,
- CO2 7–11%.
| Distillation fractions | Boiling Points (°C) | Tar concentration in both raw and purified gas (g/Nm3) | |||||
|---|---|---|---|---|---|---|---|
| 1st Experiment (500 °C) | 2nd Experiment (600 °C) | 3rd Experiment (700 °C) | |||||
| Raw gas | Purified gas | Raw gas | Purified gas | Raw gas | Purified gas | ||
| D01 | ≤98 | 6.134 | 5.146 | 7.891 | 4.301 | 7.516 | 1.581 |
| D02 | >98 | 1.3 | 0.42 | 2.712 | 0.618 | 4.407 | 0.292 |
| ≤126 | |||||||
| D03 | >126 | 0.547 | 0.171 | 2.035 | 0.498 | 2.912 | 0.583 |
| ≤151 | |||||||
| D04 | >151 | 0.523 | 0.223 | 1.084 | 0.338 | 1.708 | 0.273 |
| ≤174 | |||||||
| D05 | >174 | 0.534 | 0.118 | 1.155 | 0.196 | 1.018 | 0.175 |
| ≤196 | |||||||
| D06 | >196 | 0.258 | 0.253 | 1.000 | 0.126 | 1.116 | 0.131 |
| ≤217 | |||||||
| D07 | >217 | 0.453 | 0.153 | 1.071 | 0.167 | 0,886 | 0.049 |
| ≤234 | |||||||
| D08 | >234 | 0.144 | 0.102 | 0.552 | 0.058 | 0.860 | 0.048 |
| ≤253 | |||||||
| D09 | >253 | 0.089 | 0.040 | 0.348 | 0.021 | 0.194 | 0.032 |
| ≤270 | |||||||
| D10 | >270 | 1.118 | 0.878 | 0.473 | 0.415 | 0.311 | 0.014 |
| ≤287 | |||||||
| D11 | >287 | 0.004 | 0.006 | 0.078 | No trace | 0.137 | 0.004 |
| ≤302 | |||||||
| D12 | >302 | 0.009 | No trace | 0.031 | No trace | 0.089 | No trace |
| ≤317 | |||||||
| D13 | >317 | No trace | No trace | 0.019 | No trace | 0.018 | No trace |
| ≤330 | |||||||
| D14 | >330 | No trace | No trace | 0.017 | No trace | 0.004 | No trace |
| ≤342.7 | |||||||
| Total | - | 10.87 | 7.75 | 18.47 | 6.738 | 21.18 | 3.18 |
| Tar in gas engine D06–D23 | - | 2.075 | 1.432 | 3.598 | 0.787 | 3.615 | 0.278 |
| Conversion D06–D23 | - | 44.9% | 78.1% | 92.3% | |||
5.2. Variation of Flow Rate at Operation Temperature 700 °C
| Distillation fractions | Boiling Points (°C) | Tar concentration in both raw and purified gas (g/Nm3) | |||||
|---|---|---|---|---|---|---|---|
| 3rd Experiment (20 Nm3/h) | 4th Experiment (15 Nm3/h) | 5th Experiment (10 Nm3/h) | |||||
| Raw gas | Purified gas | Raw gas | Purified gas | Raw gas | Purified gas | ||
| D01 | ≤98 | 7.516 | 1.581 | 6.712 | 1.845 | 6.171 | 1.310 |
| D02 | >98 | 4.407 | 0.292 | 1.528 | 0.854 | 2.027 | 0.717 |
| ≤126 | |||||||
| D03 | >126 | 2.912 | 0.583 | 2.359 | 0.190 | 2.053 | 0.217 |
| ≤151 | |||||||
| D04 | >151 | 1.708 | 0.273 | 1.742 | 0.152 | 1.620 | 0.177 |
| ≤174 | |||||||
| D05 | >174 | 1.018 | 0.175 | 1.600 | 0.172 | 1.301 | 0.159 |
| ≤196 | |||||||
| D06 | >196 | 1.116 | 0.131 | 1.115 | 0.014 | 1.091 | 0.003 |
| ≤217 | |||||||
| D07 | >217 | 0,886 | 0.049 | 1.123 | 0.038 | 0.775 | 0.026 |
| ≤234 | |||||||
| D08 | >234 | 0.860 | 0.048 | 1.213 | 0.015 | 0.976 | No trace |
| ≤253 | |||||||
| D09 | >253 | 0.194 | 0.032 | 0.643 | No trace | 0.547 | No trace |
| ≤270 | |||||||
| D10 | >270 | 0.311 | 0.014 | 0.359 | No trace | 0.284 | No trace |
| ≤287 | |||||||
| D11 | >287 | 0.137 | 0.004 | 0.275 | No trace | 0.143 | No trace |
| ≤302 | |||||||
| D12 | >302 | 0.089 | No trace | 0.218 | No trace | 0.108 | No trace |
| ≤317 | |||||||
| D13 | >317 | 0.018 | No trace | 0.060 | No trace | 0.223 | No trace |
| ≤330 | |||||||
| D14 | >330 | 0.004 | No trace | 0.012 | No trace | 0.008 | No trace |
| ≤342.7 | |||||||
| D15 | >342.7 | No trace | No trace | 0.021 | No trace | No trace | No trace |
| ≤356.5 | |||||||
| Total | - | 21.18 | 3.18 | 18.98 | 3.28 | 16.86 | 2.61 |
| Tar in gas engine D06–D23 | - | 3.615 | 0.278 | 5.039 | 0.067 | 4.155 | 0.029 |
| Conversion D06–D23 | - | 92.3% | 98.6% | 99.3% | |||
5.3. Discussion of the Results

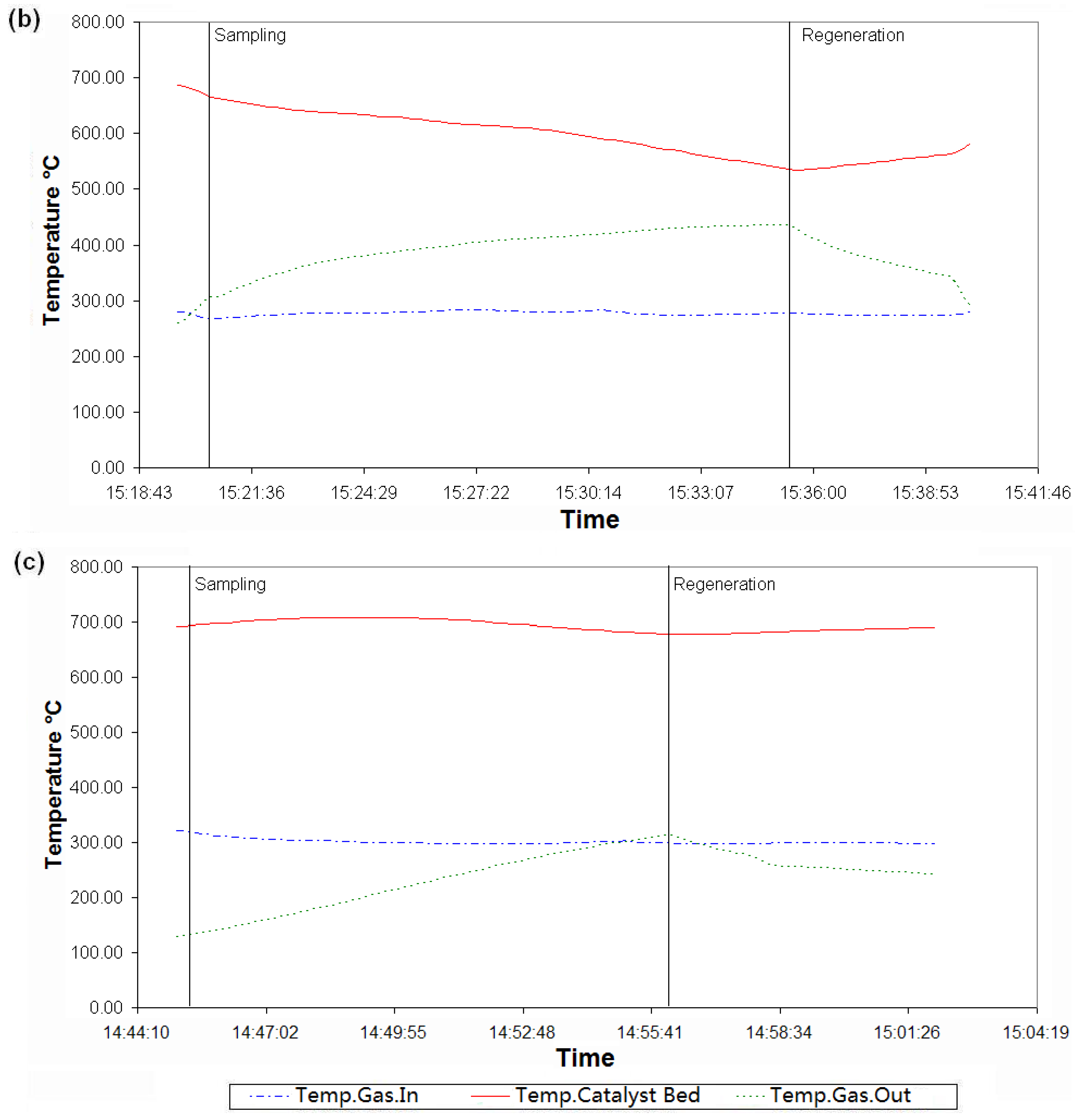
6. Conclusions
Acknowledgements
References
- Lin, Y.; Tanaka, S. Ethanol fermentation from biomass resources: Current state and prospects. Appl. Microbiol. Biot. 2006, 69, 627–642. [Google Scholar] [CrossRef]
- Caputo, A.C.; Palumbo, M.; Pelagagge, P.M.; Scacchia, F. Economics of biomass energy utilization in combustion and gasification plants: Effects of logistic variables. Biomass Bioenergy 2005, 28, 35–51. [Google Scholar] [CrossRef]
- Yoshioka, T.; Hirata, S.; Matsumura, Y.; Sakanishi, K. Woody biomass resources and conversion in Japan: The current situation and projections to 2010 and 2050. Biomass Bioenergy 2005, 29, 336–346. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L.; Jones, D.D.; Hanna, M.A. Contemporary issues in thermal gasification of biomass and its application to electricity and fuel production. Biomass Bioenergy 2008, 32, 573–581. [Google Scholar] [CrossRef]
- Cui, P.; Yan, Y.; Chuang, Z.; Hai, T. Study on the distribution and quantity of biomass residues resource in China. Biomass Bioenergy 2004, 27, 111–117. [Google Scholar] [CrossRef]
- Demirbes, M.; Balat, M. Recent advances on the production and utilization trends of bio-fuels: A global perspective. Energ. Convers. Manag. 2006, 47, 2371–2381. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Abdul, S.; Runqing, H.; Somashekar, H.; Racelis, D.; Rathnasiri, P.; Rungrawee, Y. An assessment of the potential for non-plantation biomass resources in selected Asian countries for 2010. Biomass Bioenergy 2005, 29, 153–166. [Google Scholar] [CrossRef]
- Wang, C.; Du, Z.; Pan, J.; Li, J.; Yang, Z. Direct conversion of biomass to bio-petroleum at low temperature. J. Anal. Appl. Pyrol. 2007, 78, 438–444. [Google Scholar] [CrossRef]
- Demirbes, A. Progress and recent trends in biofuels. Prog. Energ. Combust. 2007, 33, 1–18. [Google Scholar] [CrossRef]
- Asadulla, M.; Ito, S.; Kunimori, K.; Yamada, M.; Tomishige, K. Biomass gasification to hydrogen and syngas at low temperature: Novel catalytic system using fluidized-bed reactor. J. Catal. 2002, 208, 255–259. [Google Scholar] [CrossRef]
- Chen, G.; Andries, J.; Spliethoff, H.; Fang, M.; van de Enden, P.J. Biomass gasification integrated with pyrolysis in a circulating fluidized bed. Sol. Energy 2004, 76, 345–349. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical biomass gasification: A review of the current status of the technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Jenssen, F.J.J.G. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Erich, E. Decentralized Gasification of Biomass—Variant Processes to Produce Low-Tar Product Gases from Biomass. In Energy from Waste, 1st ed.; Thomé-Kozmiensky, K.J., Beckmann, M., Eds.; TK-Publishing Inc: Nietwerder, Germany, 2007; pp. 503–518. [Google Scholar]
- Senger, W.; Schöppe, G.; Erich, E. State of the gasification technology for the utilization of bio fuels in example of wood. Eur. J. Wood Prod. 1998, 56, 67–74. [Google Scholar] [CrossRef]
- Li, C.; Hirabayashi, D.; Suzuki, K. Steam reforming of biomass tar producing H2-rich gases over Ni/MgOx/CaO1−x catalyst. Bioresour. Technol. 2010, 101, 97–100. [Google Scholar] [CrossRef]
- Marutzky, R.; Seeger, K. Energy from Wood and Biomasses, 1st ed.; DRW Publishing Inc.: Stuttgart, Germany, 1999; pp. 20–112. [Google Scholar]
- Saxena, R.C.; Seal, D.; Kumer, S.; Goyer, H.B. Thermo-chemical routes for hydrogen rich gas from biomass: A review. Renew. Sustain. Energy Rev. 2008, 12, 1909–1927. [Google Scholar] [CrossRef]
- Bajohr, S.; Hoferer, J.; Reimert, R.; Schaub, G. Thermo-catalytic conversion of hydrocarbons from biomass pyrolysis and gasification. Proc. DGMK 2002, 2, 167–174. [Google Scholar]
- Jones, D.; Pujado, P. Handbook of Petroleum Processing, 1st ed.; Springer-Verlag: New York, NY, USA, 2006; pp. 30–144. [Google Scholar]
- Aznar, M.; Caballero, M.; Gil, J.; Martin, J.; Corella, J. Commerical steam reforming catalysts to improve biomass gasification with steam-oxygen mixtures. Ind. Eng. Chem. Res. 1998, 37, 2668–2680. [Google Scholar] [CrossRef]
- Gartner, B.; Kuchling, P.; Chudzinski, S. Catalytic cracking of long chain hydrocarbons in raw gas of wood coking. Proc. DGMK 2002, 2, 159–165. [Google Scholar]
- Mckendry, P. Energy production from biomass (Part 3): Gasification technologies. Bioresour. Technol. 2002, 83, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Jess, A.; Depner, H. Thermo-catalytic purification of raw gas from the gasification and coking of solid fuels. Chem. Eng. Technol. 1997, 69, 970–973. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, J.; Schmidt, K.G.; Bian, Z. Removal and Conversion of Tar in Syngas from Woody Biomass Gasification for Power Utilization Using Catalytic Hydrocracking. Energies 2011, 4, 1163-1177. https://doi.org/10.3390/en4081163
Huang J, Schmidt KG, Bian Z. Removal and Conversion of Tar in Syngas from Woody Biomass Gasification for Power Utilization Using Catalytic Hydrocracking. Energies. 2011; 4(8):1163-1177. https://doi.org/10.3390/en4081163
Chicago/Turabian StyleHuang, Jiu, Klaus Gerhard Schmidt, and Zhengfu Bian. 2011. "Removal and Conversion of Tar in Syngas from Woody Biomass Gasification for Power Utilization Using Catalytic Hydrocracking" Energies 4, no. 8: 1163-1177. https://doi.org/10.3390/en4081163




