Effect of CH4–Air Ratios on Gas Explosion Flame Microstructure and Propagation Behaviors
Abstract
:1. Introduction
2. Results and Discussion
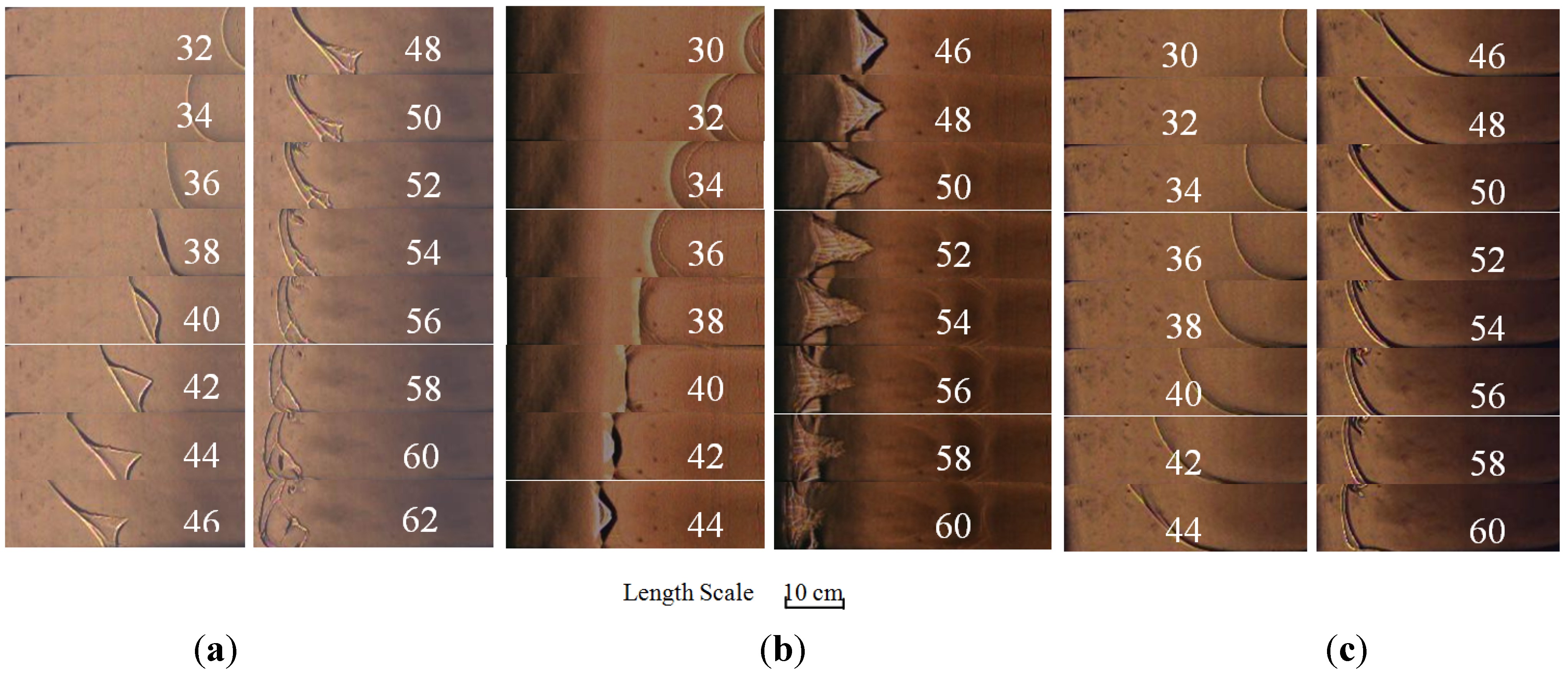
2.1. Flame Structure Characteristic Based on High Speed Schlieren Photographs
| equivalence ratio | volume concentration | (cm/s) | δ (cm) | ρu/ρb | Ma |
|---|---|---|---|---|---|
| 0.80 | 7.6% | 25.4 | 0.009 | 6.65 | 4.69 |
| 0.90 | 8.6% | 32.5 | 0.225 | 7.12 | 5.54 |
| 1.00 | 9.5% | 37.1 | 0.217 | 7.48 | 6.20 |
| 1.10 | 10.5% | 38.3 | 0.225 | 7.55 | 6.99 |
| 1.20 | 11.4% | 34.5 | 0.215 | 7.43 | 7.96 |
| 1.30 | 12.4% | 25.0 | 0.291 | 7.28 | 9.13 |
2.2. Flame Propagation Behavior on Different Equivalence Ratios
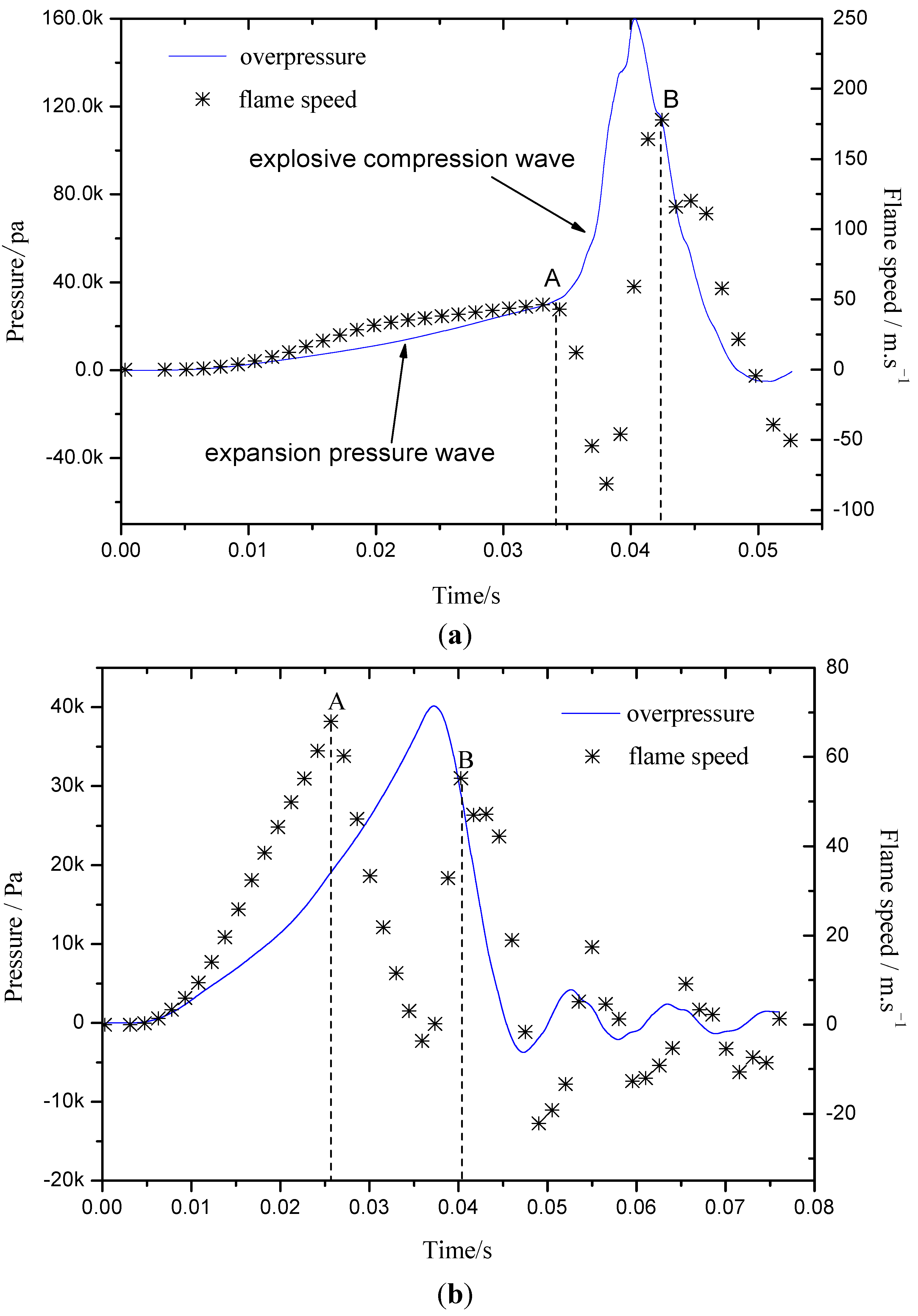

2.3. The Flame-Propagation Dynamics Characteristic on Different Flow Structure
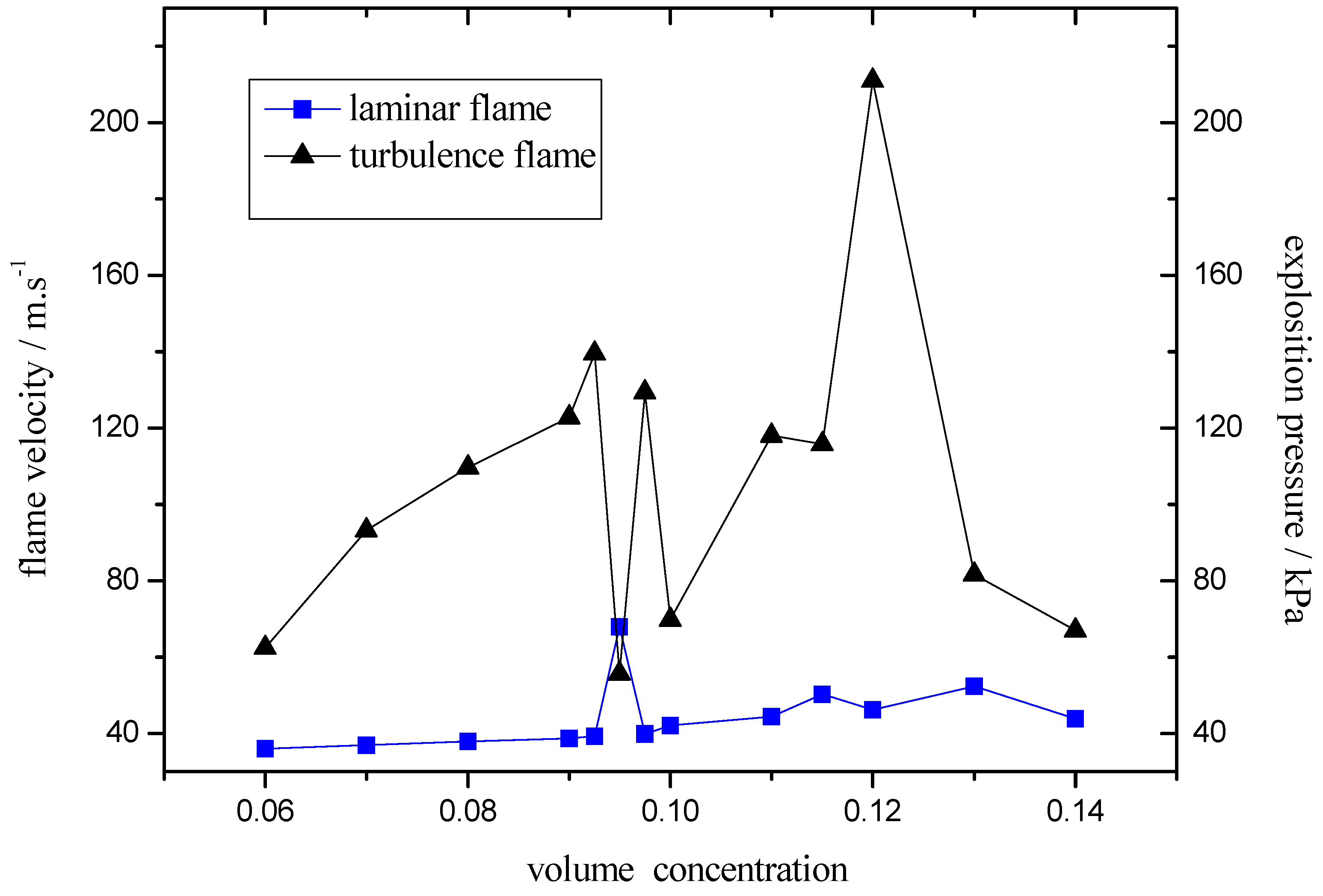
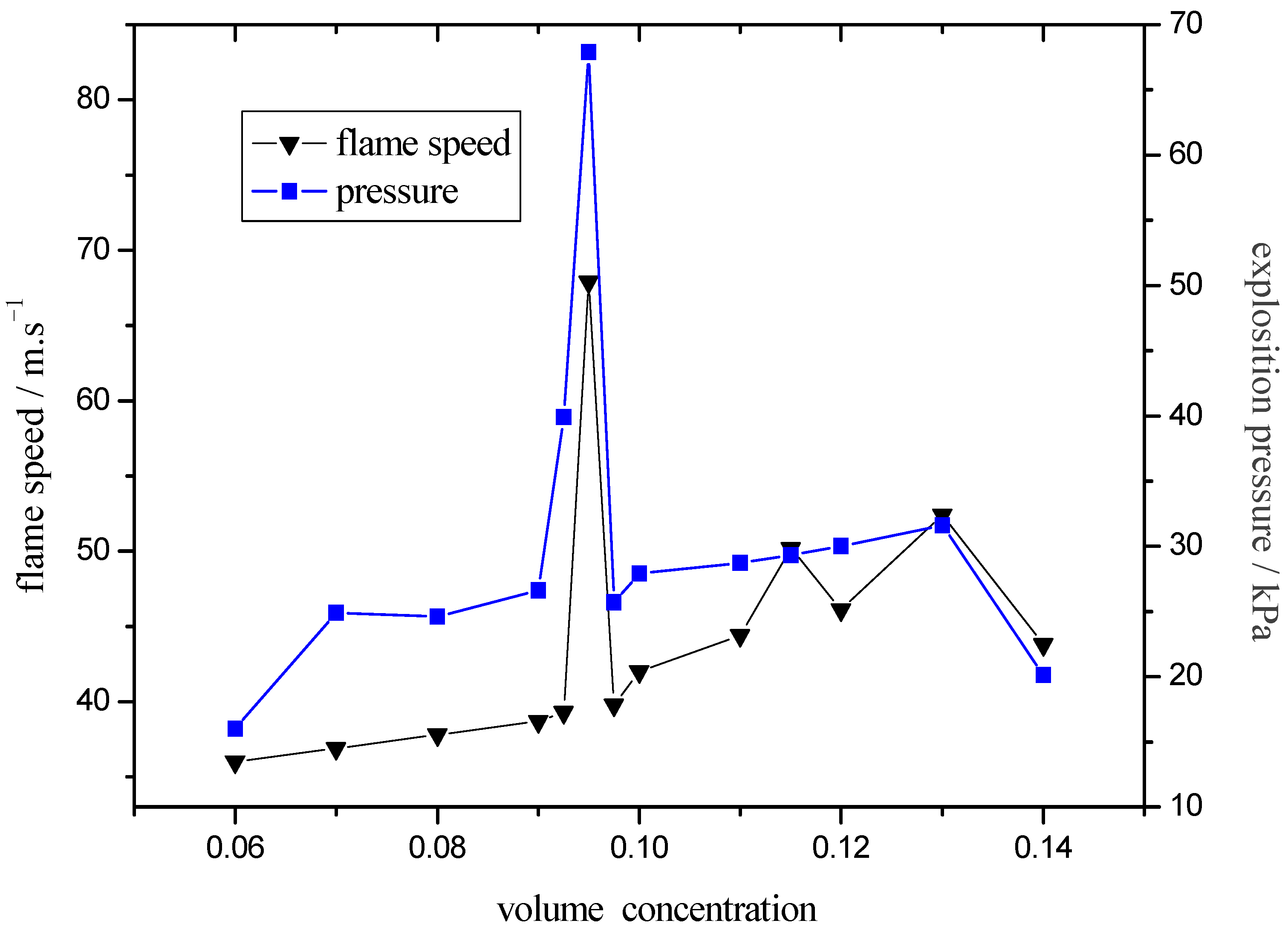

3. Experimental Section
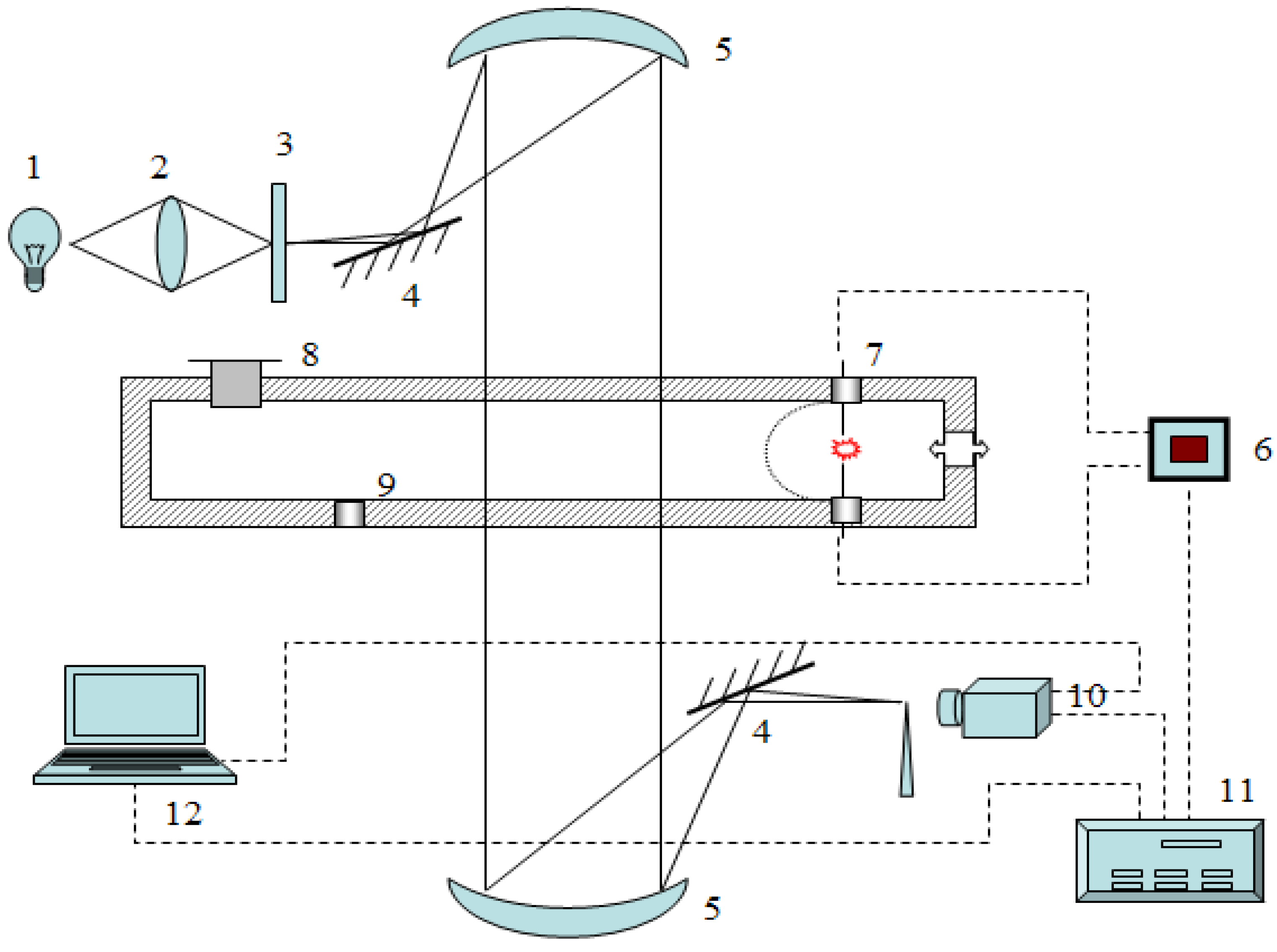
3.1. Experimental Apparatus
3.2. Experimental Procedure
- Methane volume concentration: 6%–14%;
- Ignition voltage: 30,000 V;
- Ignition time: 0.01 s;
- High speed camera meter frequency: 10,000 shooting frame/s;
- Data sampling frequency: 100 kHz.
4. Conclusions
- (1)
- The flame front structure transition was closely connected to the flame acceleration propagation process and flow character. The flame structure change was mainly due to the flow transition from laminar to turbulent.
- (2)
- The explosion flame was divided into laminar and turbulent stages. At different flame stages, the factors influencing flame propagation were different. The laminar flame propagation behavior was influenced mainly by gas inflation and pressure wave effects, while the turbulent flame speed was greatly dependent on turbulence intensity.
- (3)
- The turbulence intensity played an important role in peak value of explosive pressure and flame speed. On the case that it’s easier to form laminar-turbulent transition, the ideal explosive concentration would be easier to reach, which was the essential reason why the ideal explosive concentration differs under different test conditions.
Acknowledgments
References
- Masri, A.R.; Ibrahim, S.S.; Nehzat, N. Experimental study of premixed flame propagation over various solid obstructions. Exp. Therm. Fluid Sci. 2000, 21, 109–116. [Google Scholar] [CrossRef]
- Craig, T.; Johansen, G.C. Visualization of the unburned gas flow field ahead of an accelerating flame in an obstructed square channel. Combust. Flame 2009, 156, 405–416. [Google Scholar]
- Fairweather, M.; Hargrave, G.K.; Ibrahim, S.S. Studies of premixed flame propagation in explosion tubes. Combust. Flame 1999, 116, 504–518. [Google Scholar] [CrossRef]
- Alexiou, A.; Andrews, G.E.; Phylaktou, H. Side-vented gas explosion in a long vessel: The effect of vent position. J. Loss Prev. Process Ind. 1996, 5, 351–356. [Google Scholar] [CrossRef]
- Yang, Y.; He, X.Q. Fractal characteristics of flame front surface in premixed turbulent methane/air combustion. In Proceedings of 5th International Autumn Seminar on Propellants, Explosives and Pyrotecnics, Guilin, China, 15–18 October 2003.
- Yang, Y. The Flame Microstructure of Gas Explosion and the Mechanism of Flame Propagation in Tube [In Chinese]. Ph.D. Thesis, China University of Mining and Technology, Xuzhou, China, April 2003. [Google Scholar]
- Rubtsov, N.M.; Seplyarskii, B.S. On the nature of an upper concentration limit of flame propagation in an H2 + air mixture. Mendeleev Commun. 2009, 19, 227–229. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Lei, Y.; Wang, B.R.; Wang, Y.; Wei, M.G. Turbulence in laminar premixed V-flames. Sci. China 2003, 6, 574–580. [Google Scholar] [CrossRef]
- Oh, K.H.; Kim, H.; Kim, J.B. A study on the obstacle induced variation of the gas explosion characteristics. J. Loss Prev. Process Ind. 2001, 6, 597–602. [Google Scholar] [CrossRef]
- Patel, S.N.; Jarvis, S.; Ibrahim, S.S. An experimental and numerical investigation of premixed flame deflagration in a semiconfined explosion chamber. Proc. Combust. Inst. 2002, 29, 1849–1854. [Google Scholar] [CrossRef]
- Le, H.; Nayak, S.; Mannan, M.S. Upper flammability limits of hydrogen and light hydrocarbons in air at subatmospheric pressures. Ind. Eng. Chem. Res. 2012, 51, 9396–9402. [Google Scholar] [CrossRef]
- Xiao, H.H.; Makarov, D.; Sun, J.H.; Molkov, V. Experimental and numerical investigation of premixed flame propagation with distorted tulip shape in a closed duct. Combust. Flame 2012, 159, 1523–1538. [Google Scholar] [CrossRef]
- Clanet, C.; Searby, G. On the “tulip flame” phenomenon. Combust. Flame 1996, 105, 225–238. [Google Scholar] [CrossRef]
- Bychkov, V.; Akkerman, V.; Fru, G.; Petchenko, A.; Eriksson, L.E. Flame acceleration in the early stages of burning in tubes. Combust. Flame 2007, 150, 263–276. [Google Scholar] [CrossRef]
- Rankin, D.D.; Mecann, M.A. Overpressures from nondetonating, baffle-accelerated turbulent flames in tubes. Combust. Flame 2000, 120, 504–514. [Google Scholar] [CrossRef]
- Di Sarli, V.; Di Benedetto, A.; Russo, G. Large Eddy Simulation of transient premixed flame-vortex interactions in gas explosions. Chem. Eng. Sci. 2012, 71, 539–551. [Google Scholar] [CrossRef]
- Shelkin, K.I. Influence of tube non-uniformities on the detonation ignition and propagation in gases. Exp. Theor. Phys. 1940, 10, 823–827. [Google Scholar]
- Bychkov, V.; Valiev, D.; Eriksson, L.E. Physical mechanism of ultrafast flame acceleration. Phys. Rev. Lett. 2008, 101, 164501:1–164501:4. [Google Scholar] [CrossRef]
- Akkerman, V.; Bychkov, V.; Petchenko, A.; Eriksson, L.E. Accelerating flames in cylindrical tubes with nonslip at the walls. Combust. Flame 2006, 145, 206–219. [Google Scholar] [CrossRef]
- Bychkov, V.; Petchenko, A.; Akkerman, V.; Eriksson, L.E. Theory and modeling of accelerating flames in tubes. Phys. Rev. E 2005, 72, 046307:1–046307:10. [Google Scholar] [CrossRef]
- Park, D.J.; Green, A.R.; Lee, Y.S.; Chen, Y.C. Experimental studies on interactions between a freely propagating flame and single obstacles in a rectangular confinement. Combust. Flame 2007, 150, 27–39. [Google Scholar] [CrossRef]
- Di Sarli, V.; di Benedetto, A.; Russo, G. Using Large Eddy Simulation for understanding vented gas explosions in the presence of obstacles. J. Hazard. Mater. 2009, 169, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, M.P.; Armfield, S.W.; Masri, A.R.; Ibrahim, S.S. Large Eddy Simulation of a propagating turbulent premixed flame. Flow Turbul. Combust. 2003, 70, 1–19. [Google Scholar] [CrossRef]
- Di Sarli, V.; di Benedetto, A.; Russo, G.; Jarvis, S.; Long, E.J.; Hargrave, G.K. Large Eddy Simulation and PIV measurements of unsteady premixed flames accelerated by obstacles. Flow Turbul. Combust. 2009, 83, 227–250. [Google Scholar] [CrossRef]
- Mardani, A.; Tabejamaat, S.; Mohammadi, M.B. Numerical study of the effect of turbulence on rate of reactions in the MILD combustion regime. Combust. Theory Model. 2011, 15, 753–772. [Google Scholar] [CrossRef]
- Sadiki, A.; Maneshkarimi, M.R.; Chrigui, M. Towards an optimization of turbulence effects on heat and mass transfer in evaporating and reacting gas turbine sprays. In Proceedings of the 50th ASME Turbo-Expo, Reno, NV, USA, 6–9 June 2005.
- Salzano, E.; Marra, F.S.; Russo, G.; Lee, J.H.S. Numerical simulation of turbulent gas flames in tubes. J. Hazard. Mater. 2002, 95, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Oh, C.B. Flame structure and global flame response to the equivalence ratios of interacting partially premixed methane and hydrogen flames. Int. J. Hydrog. Energy 2012, 37, 7877–7888. [Google Scholar] [CrossRef]
- Barlow, R.S. Laser diagnostics and their interplay with computations to understand turbulent combustion. Proc. Combust. Inst. 2007, 31, 49–75. [Google Scholar] [CrossRef]
- Kersten, C.; Forster, H. Investigation of deflagrations and detonations in pipes and flame arresters by high-speed framing. J. Loss Prev. Process Ind. 2004, 17, 43–50. [Google Scholar] [CrossRef]
- Chen, X.F.; Sun, J.H.; Liu, Y. Microstructure of premixed propane/air flame in the transition from laminar to turbulent combustion. Chin. Sci. Bull. 2007, 52, 685–691. [Google Scholar] [CrossRef]
- Wu, M.H.; Burke, M.P.; Son, S.F.; Yettera, R.A. Flame acceleration and the transition to detonation of stoichiometric ethylene/oxygen in microscale tubes. Proc. Combust. Inst. 2007, 31, 2429–2436. [Google Scholar] [CrossRef]
- Torgny, E.; Carlsson, R.M. Combination of Schlieren and pulsed TV holography in the study of a high-speed flame jet. Opt. Lasers Eng. 2006, 44, 535–554. [Google Scholar]
- Kuzuu, K.; Ishii, K.; Kuwahara, K. Numerical simulation of premixed flame propagation in a closed tube. Fluid Dyn. Res. 1996, 3, 165–182. [Google Scholar] [CrossRef]
- McIntosh, A.C. The linearised response of the mass burning rate of a premixed flame to rapid pressure changes. Combust. Sci. Tech. 1993, 91, 329–346. [Google Scholar] [CrossRef]
- Strehlow, R. Combustion Fundamentals; McGraw-Hill: New York, NY, USA, 1984. [Google Scholar]
- Konga, B.N.; Fernandez, G.; Guillard, H.; Larrouturou, B. Numerical investigations of the tulip flames instability-comparisons with experimental results. Combust. Sci. Tech. 1992, 87, 69–89. [Google Scholar] [CrossRef]
- Guenoche, H. Nonsteady Flame Propagation; Oxford: New York, NY, USA, 1964. [Google Scholar]
- Patnaik, G.; Kailasanath, K. Numerical simulation of the extinguishment of downward propagating flames. In Proceedings of the 24th International Symposium on Combustion, Sydney, Australia, 5–10 July 1992.
- Bychkov, V.; Liberman, M.A. Dynamics and stability of premixed flames. Phys. Rep. 2000, 325, 116–237. [Google Scholar] [CrossRef]
- Modestov, M.; Bychkov, V.; Betti, R.; Eriksson, L.E. Bubble velocity in the nonlinear Rayleigh–Taylor instability at a deflagration front. Phys. Plasmas 2008, 15, 042703:1–042703:12. [Google Scholar]
- Bychkov, V.; Modestov, M.; Akkerman, V.; Eriksson, L.E. The Rayleigh–Taylor instability in inertial fusion, astrophysical plasma and flames. Plasma Phys. Control. Fusion 2007, 49, B513–B520. [Google Scholar] [CrossRef]
- Davis, S.G.; Quinard, J.; Searby, G. Markstein numbers in counterflow, methane- and propane-air flames: A computational study. Combust. Flame 2002, 130, 123–136. [Google Scholar] [CrossRef]
- Akkerman, V.; Bychkov, V. Velocity of weakly turbulent flames of finite thickness. Combust. Theory Model. 2005, 9, 323–351. [Google Scholar] [CrossRef]
- Xu, S.L.; Zhang, H.J.; Yue, P.T. Study on properties of pressure waves generated by steady flames in a duct. J. China Univ. Sci. Tech. 2000, 4, 387–392. [Google Scholar]
- Bychkov, V.; Liberman, M. Comment on “The influence of hydrodynamic instability on the structure of cellular flames”. Phys. Fluids 2002, 14, 2024–2025. [Google Scholar] [CrossRef]
- Petchenko, A.; Bychkov, V.; Akkerman, V. Flame-sound interaction in tubes with nonslip walls. Combust. Flame 2007, 149, 418–434. [Google Scholar] [CrossRef]
- Mcintosh, A.C. Pressure disturbances of different length scales interacting with conventional flames. Combust. Sci. Tech. 1991, 75, 287–309. [Google Scholar] [CrossRef]
- Bychkov, V.; Akkerman, V.; Valiev, D.; Law, C.K. Influence of gas compression on flame acceleration in channels with obstacles. Combust. Flame 2010, 157, 2008–2011. [Google Scholar] [CrossRef]
- Valiev, D.; Bychkov, V.; Akkerman, V.; Eriksson, L.E. Different stages of flame acceleration from slow burning to Chapman-Jouguet deflagration. Phys. Rev. E 2009, 80, 036317:1–036317:11. [Google Scholar] [CrossRef]
- Bychkov, V.; Akkerman, V.; Valiev, D.; Law, C.K. Role of compressibility in moderating flame acceleration in tubes. Phys. Rev. E 2010, 81, 026309:1–026309:9. [Google Scholar] [CrossRef]
- Wang, H.; Ge, L.M.; Deng, J. Comparison of explosion characteristics of ignitable gases in confined space. J. China Coal Soc. 2009, 2, 218–223. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, X.; Zhang, Y.; Zhang, Y. Effect of CH4–Air Ratios on Gas Explosion Flame Microstructure and Propagation Behaviors. Energies 2012, 5, 4132-4146. https://doi.org/10.3390/en5104132
Chen X, Zhang Y, Zhang Y. Effect of CH4–Air Ratios on Gas Explosion Flame Microstructure and Propagation Behaviors. Energies. 2012; 5(10):4132-4146. https://doi.org/10.3390/en5104132
Chicago/Turabian StyleChen, Xianfeng, Yin Zhang, and Ying Zhang. 2012. "Effect of CH4–Air Ratios on Gas Explosion Flame Microstructure and Propagation Behaviors" Energies 5, no. 10: 4132-4146. https://doi.org/10.3390/en5104132




