Adsorption of Thiophenic Compounds from Model Diesel Fuel Using Copper and Nickel Impregnated Activated Carbons
Abstract
:1. Introduction
2. Methodology
2.1. Material Preparation
| Carbon Sample | Step 1: Heat treatment (900 °C/ H2) | Step 2: Metal impregnation (incipient wetness) | Step 3: Heat treatment |
|---|---|---|---|
| ACFH | √ | - | - |
| ACFH-Cu2+ | √ | Cu(NO3)2 | 400 °C/ N2 |
| ACFH-Cu+ | √ | Cu(NO3)2 | 530 °C/ N2 |
| ACFH-Cu0 | √ | Cu(NO3)2 | 800 °C/ N2 |
| ACFH-Ni2+ | √ | Ni(NO3)2 | 550 °C/ N2 |
| ACFH-Ni0 | √ | Ni(NO3)2 | 800 °C/ H2 |
| ACH | √ | - | - |
| ACH-Cu+ | √ | Cu(NO3)2 | 530°C/ N2 |
2.2. Characterization
2.3. Adsorption Isotherms
3. Results and Discussion
3.1. Adsorbent Preparation and Characterization
3.1.1. Adsorbent Preparation
3.1.2. Characterization of Loaded Copper and Nickel Species
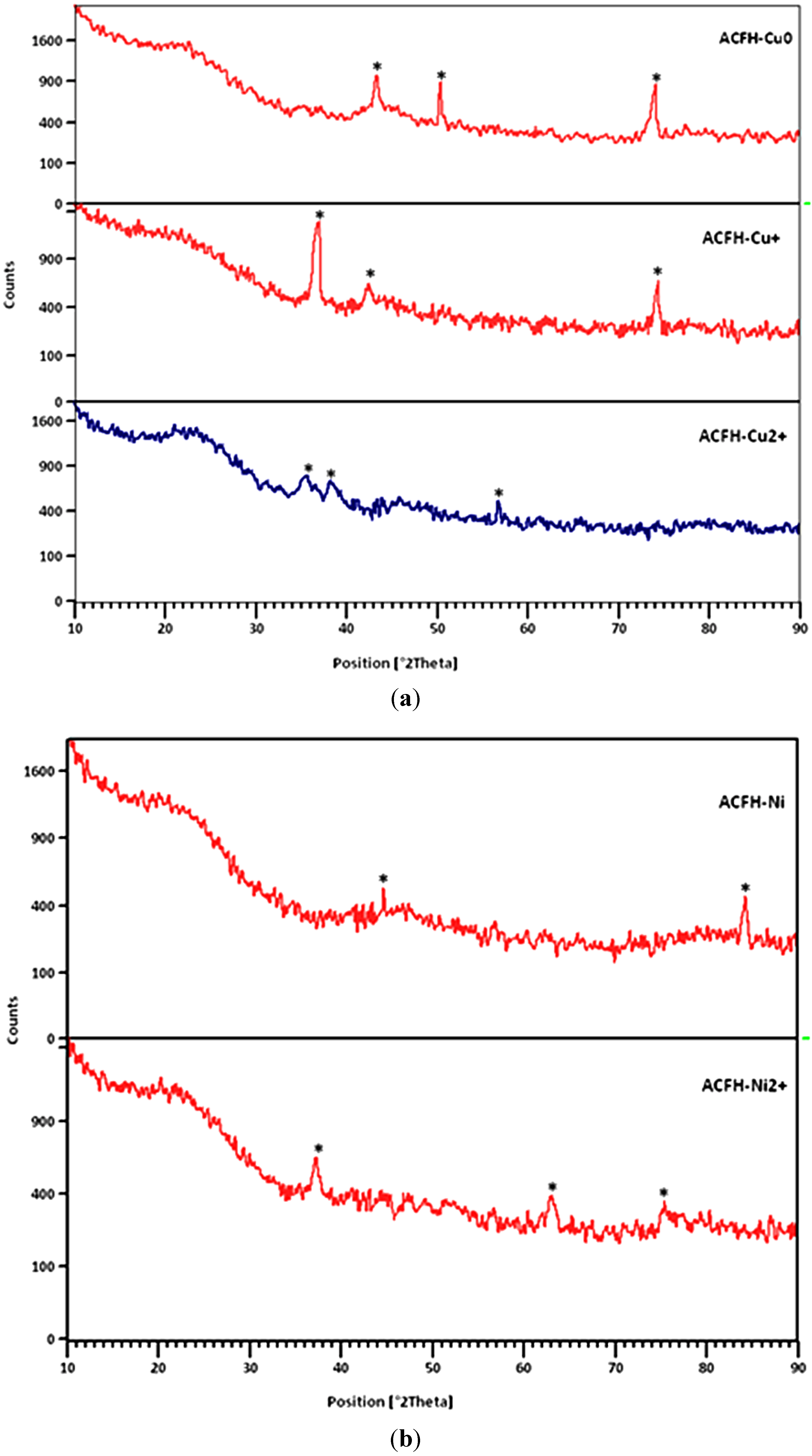
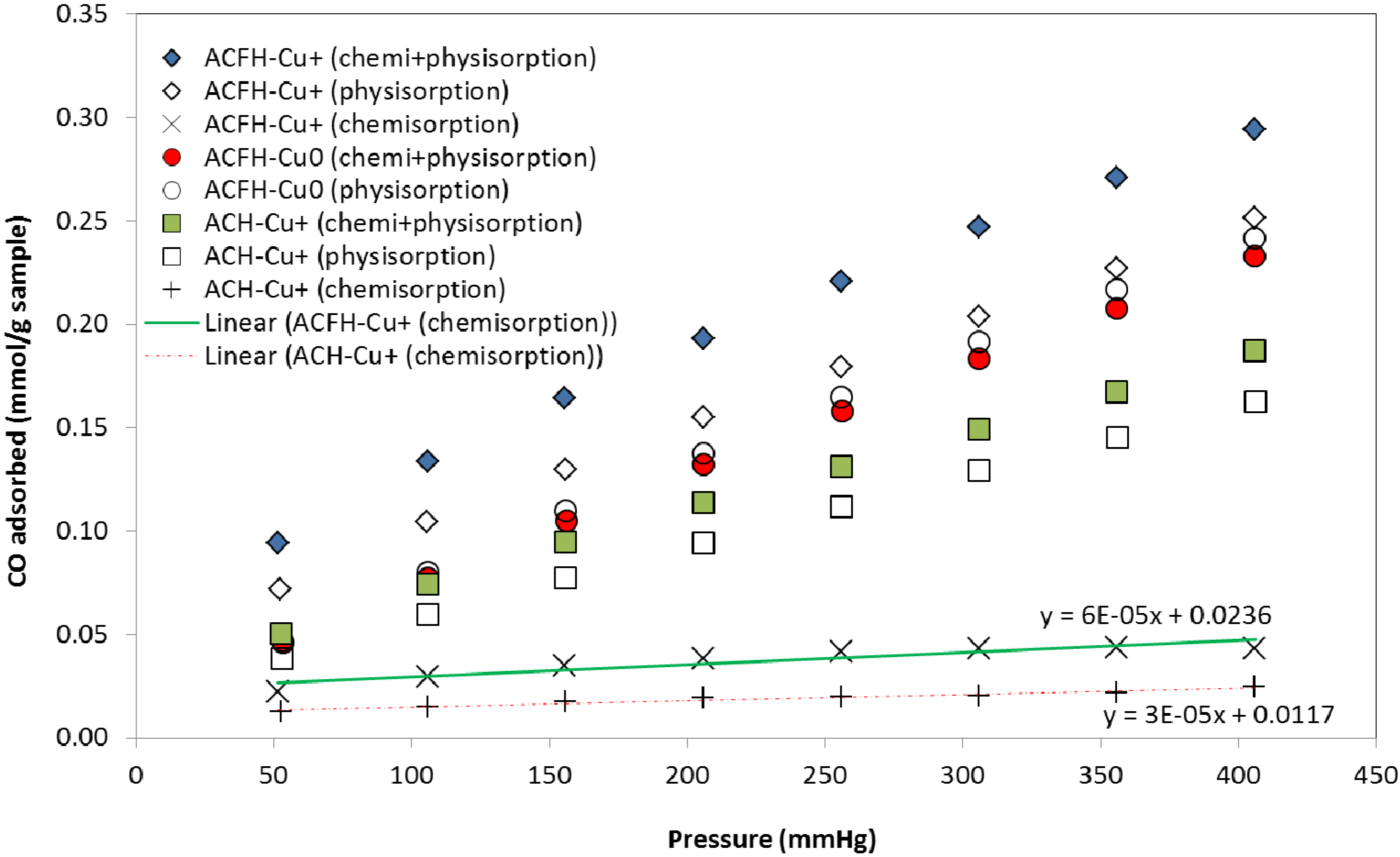
3.1.3. Porosity Characterization
| Sample | SBET m2/g | Vtotal cm3/g | Vmicro, DR cm3/g | Vmeso+macro cm3/g | DFT pore volume distribution, cm3/g (%) | ||
|---|---|---|---|---|---|---|---|
| <0.7 nm | 0.7–2 nm | >2 nm | |||||
| ACF | 979 | 0.378 | 0.376 | 0.002 | 0.205 (62.7) | 0.122 (37.3) | 0.000 (0.0) |
| ACFH | 1077 | 0.426 | 0.424 | 0.002 | 0.197 (52.8) | 0.176 (47.2) | 0.000 (0.0) |
| ACFH-Cu+2 | 1090 | 0.433 | 0.429 | 0.004 | 0.186 (50.8) | 0.180 (49.2) | 0.000 (0.0) |
| ACFH-Cu+ | 981 | 0.388 | 0.385 | 0.003 | 0.172 (50.6) | 0.167 (49.1) | 0.001 (0.3) |
| ACFH-Cu0 | 1327 | 0.526 | 0.514 | 0.012 | 0.282 (62.3) | 0.171 (37.7) | 0.000 (0.0) |
| ACFH-Ni+2 | 1075 | 0.430 | 0.419 | 0.011 | 0.184 (46.2) | 0.212 (53.3) | 0.002 (0.5) |
| ACFH-Ni0 | 1053 | 0.492 | 0.410 | 0.082 | 0.163 (39.8) | 0.191 (46.6) | 0.056 (13.7) |
| AC | 1365 | 0.894 | 0.495 | 0.399 | 0.100 (15.0) | 0.227 (34.0) | 0.341 (51.0) |
| ACH | 1341 | 0.805 | 0.511 | 0.294 | 0.078 (11.8) | 0.262 (39.8) | 0.319 (48.4) |
| ACH-Cu+ | 1245 | 0.800 | 0.452 | 0.348 | 0.088 (14.4) | 0.213 (34.9) | 0.309 0.7) |
3.2. Adsorption of Thiophenic Compounds



| Compound | Absolute Hardness (eV) | Soft/Hard, Acid/Base | Reference |
|---|---|---|---|
| Cu+ | 6.3 | Soft Acid | [51] |
| Cu2+ | 8.3 | Borderline Acid | [51] |
| Cu0 | 3.25 | Neutral | [51] |
| Ni2+ | 8.5 | Borderline Acid | [51] |
| Ni0 | 3.24 | Neutral | [51] |
| BT | 5.602 | Soft Base | [22] |
| DBT | 5.267 | Soft Base | [22] |
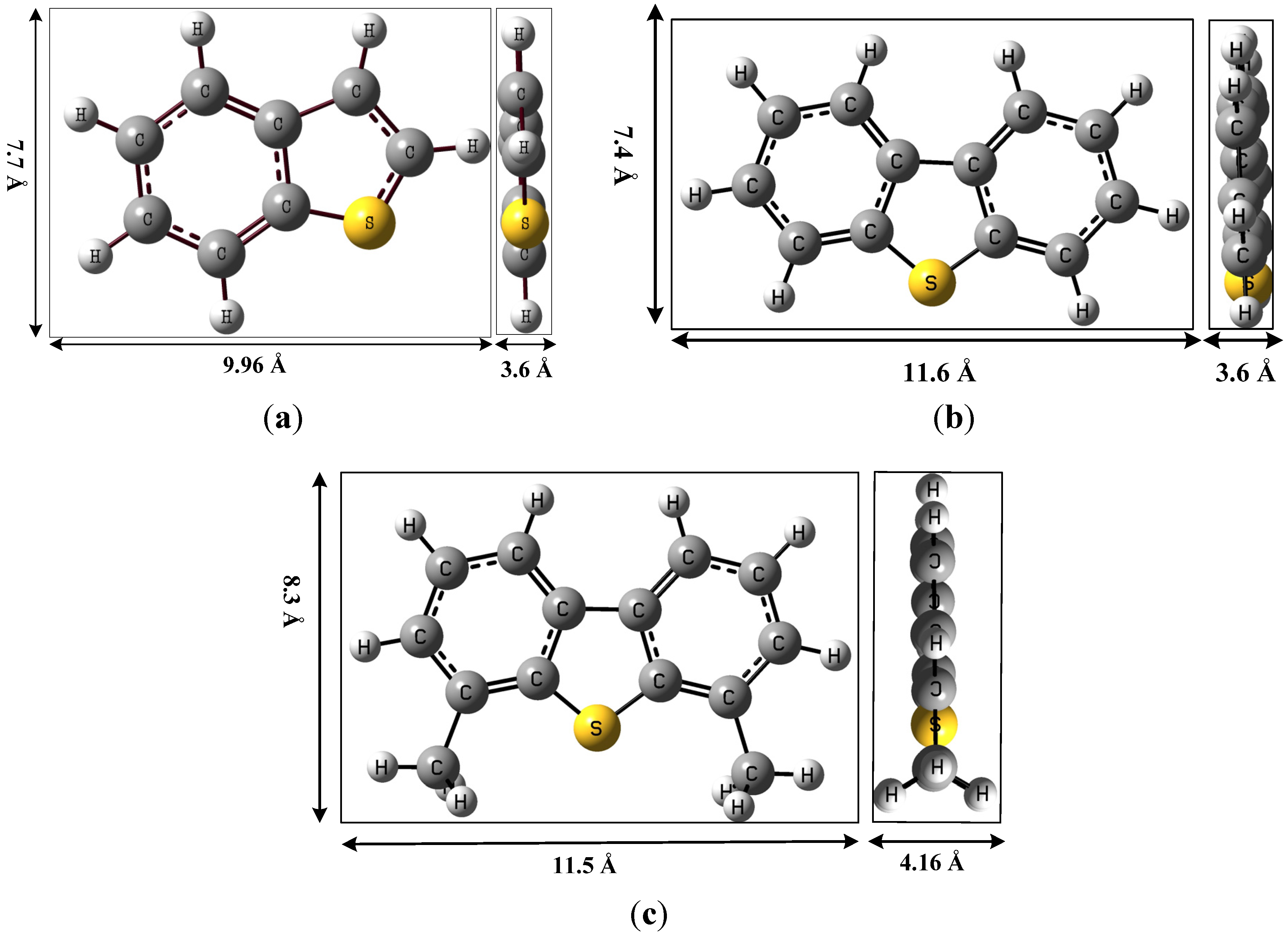
| TC | TC uptake * | Monolayer capacity of exposed Cu+ sites on ACFH-Cu+ ** | ||
|---|---|---|---|---|
| mg S/g ACFH | mg S/g ACFH-Cu+ | Difference *** (mg S/g ACFH-Cu+) | mg S/ g sample | |
| BT | 5.0 | 6.8 | 1.8 | 0.755 |
| DBT | 14.0 | 19.0 | 5.0 | 0.755 |
| 4,6-DMBT | 15.5 | 23.5 | 8.0 | 0.755 |
4. Summary and Conclusions
- Copper- or nickel-loaded adsorbents were prepared from hydrogen-treated activated carbon samples. Prepared adsorbents had the same total metal contents butwere selectively loaded with Ni, NiO, Cu, Cu2O, or CuO species. Metal-loaded samples and their precursors had similar porosities.
- Copper or nickel loading increased the uptake of thiophenic compounds (TC) from the model fuel up to 40%–53% (i.e., about 70% of the TC uptake by metal loaded carbons is due to TC adsorption on the carbon surface and the remaining 30% is due to the adsorption of TC on metal sites). This confirms that adsorption of TC is primarily governed by dispersion interactions in carbon micropores, but specific interactions between the loaded metal species and TC molecules further increase the TC uptake. Adsorbents loaded with Cu2O or NiO species showed the highest uptakes, due to more specific interactions, including π-complexation and acid-base interactions, between Cu+ or Ni2+ and TC molecules.
- A comparison of estimated maximum monolayer capacity of exposed Cu+ sites for TC adsorption with the experimental uptake data suggested two possibilities: (1) catalytic conversion of TC molecules to other compounds on the Cu+ sites, followed by adsorption of reaction products onto the carbon surface; and (2) multilayer accumulation of TC molecules on the Cu+ sites. The first possibility appears to be more likely.
- TC adsorption uptake of the majority of adsorbents followed the order of: 4,6-DMDBT > DBT > BT due to higher intensity of specific and non-specific interactions of larger TC molecules with adsorbents.
References
- Song, C.; Ma, X. New design approaches to ultra-clean diesel fuels by deep desulfurization and deep dearomatization. Appl. Catal. B Environ. 2003, 41, 207–238. [Google Scholar] [CrossRef]
- Houalla, M.; Broderick, D.H.; Sapre, A.V.; Nag, N.K.; de Beer, V.H.J.; Gates, B.C.; Kwart, H. Hydrodesulfurization of methyl-substituted dibenzothiophenes catalyzed by sulfided Co-Mo/γ-Al2O3. J. Catal. 1980, 61, 523–527. [Google Scholar] [CrossRef]
- Ma, X.; Sakanishi, K.; Mochida, I. Hydrodesulfurization reactivities of various sulfur compounds in diesel fuel. Ind. Eng. Chem. Res. 1994, 33, 218–222. [Google Scholar] [CrossRef]
- Ma, X.; Sakanishi, K.; Isoda, T.; Mochida, I. Hydrodesulfurization reactivities of narrow-cut fractions in a gas oil. Ind. Eng. Chem. Res. 1995, 34, 748–754. [Google Scholar] [CrossRef]
- Cristol, S.; Paul, J.F.; Payen, E.; Bougeard, D.; Hutschka, F.; Clémendot, S. DBT derivatives adsorption over molybdenum sulfide catalysts: A theoretical study. J. Catal. 2004, 224, 138–147. [Google Scholar] [CrossRef]
- Gislason, J. Pillips sulfur removal process nears commercialization. Oil Gas J. 2001, 99, 72–76. [Google Scholar]
- Kim, J.H.; Ma, X.; Zhou, A.; Song, C. Ultra-deep desulfurization and denitrogenation of diesel fuel by selective adsorption over three different adsorbents: A study on adsorptive selectivity and mechanism. Catal. Today 2006, 111, 74–83. [Google Scholar] [CrossRef]
- Jiang, M.; Ng, F.T.T. Adsorption of benzothiophene on Y zeolites investigated by infrared spectroscopy and flow calorimetry. Catal. Today 2006, 116, 530–536. [Google Scholar] [CrossRef]
- Jiang, M.; Ng, F.T.T.; Rahman, A.; Patel, V. Flow calorimetric and thermal gravimetric study of adsorption of thiophenic sulfur compounds on NaY zeolite. Thermochim. Acta 2005, 434, 27–36. [Google Scholar] [CrossRef]
- Ng, F.T.T.; Rahman, A.; Ohasi, T.; Jiang, M. A study of the adsorption of thiophenic sulfur compounds using flow calorimetry. Appl. Catal. B Environ. 2005, 56, 127–136. [Google Scholar] [CrossRef]
- Meng, C.; Fang, Y.; Jin, L.; Hu, H. Deep desulfurization of model gasoline by selective adsorption on Ag+/Al-MSU-S. Catal. Today 2010, 149, 138–142. [Google Scholar] [CrossRef]
- Wang, J.; Xu, F.; Xie, W.; Mei, Z.; Zhang, Q.; Cai, J.; Cai, W. The enhanced adsorption of dibenzothiophene onto cerium/nickel-exchanged zeolite Y. J. Hazard. Mater. 2009, 163, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.K.; Gunda, K.; Rehbein, P.; Ng, F.T.T. Flow calorimetry and adsorption study of dibenzothiophene, quinoline and naphthalene over modified Y zeolites. App. Catal. B Environ. 2010, 94, 225–233. [Google Scholar] [CrossRef]
- Ma, X.; Sprague, M.; Song, C. Deep desulfurization of gasoline by selective adsorption over nickel-based adsorbent for fuel cell applications. Ind. Eng. Chem. Res. 2005, 44, 5768–5775. [Google Scholar] [CrossRef]
- Rheinberg, O.V.; Lucka, K.; Köhne, H.; Schade, T.; Andersson, J.T. Selective removal of sulphur in liquid fuels for fuel cell applications. Fuel 2008, 87, 2988–2996. [Google Scholar] [CrossRef]
- Park, J.G.; Ko, C.H.; Yi, K.B.; Park, J.H.; Han, S.S.; Cho, S.H.; Kim, J.N. Reactive adsorption of sulfur compounds in diesel on nickel supported on mesoporous silica. Appl. Catal. B Environ. 2008, 81, 244–250. [Google Scholar] [CrossRef]
- Ma, X.; Sun, L.; Song, C. A new approach to deep desulfurization of gasoline, diesel fuel and jet fuel by selective adsorption for ultra-clean fuels and for fuel cell applications. Catal. Today 2002, 77, 107–116. [Google Scholar] [CrossRef]
- Komarneni, M.; Kadossov, E.; Justin, J.; Lu, M.; Burghaus, U. Adsorption of thiophene on silica-supported Mo clusters. Surf. Sci. 2010, 604, 1221–1229. [Google Scholar] [CrossRef]
- Jayne, D.; Zhang, Y.; Haji, S.; Erkey, C. Dynamics of removal of organosulfur compounds from diesel by adsorption on carbon aerogels for fuel cell applications. Int. J. Hydrogen Energy 2005, 30, 1287–1293. [Google Scholar] [CrossRef]
- Ania, C.O.; Bandosz, T.J. Metal-loaded polystyrene-based activated carbons as dibenzothiophene removal media via reactive adsorption. Carbon 2006, 44, 2404–2412. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Xia, Q.; Xi, H.; Wang, H. Desorption activation energy of dibenzothiophene on the activated carbons modified by different metal salt solutions. Chem. Eng. J. 2007, 132, 233–239. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Z.; Liu, B.; Xia, Q.; Yu, M. Adsorption of benzothiophene and dibenzothiophene on ion-impregnated activated carbons and ion-exchanged Y zeolites. Energy Fuels 2008, 22, 3858–3863. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Selective adsorption of dibenzothiophenes on activated carbons with Ag, Co, and Ni species deposited on their surfaces. Energy Fuels 2009, 23, 3737–3744. [Google Scholar] [CrossRef]
- Hernandez, S.P.; Fino, D.; Russo, N. High performance sorbents for diesel oil desulfurization. Chem. Eng. Sci. 2010, 65, 603–609. [Google Scholar] [CrossRef]
- Xiao, J.; Bian, G.; Zhang, W.; Li, Z. Adsorption of dibenzothiophene on Ag/Cu/Fe-supported activated carbons prepared by ultrasonic-assisted impregnation. J. Chem. Eng. Data 2010, 55, 5818–5823. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Adsorption of dibenzothiophenes on activated carbons with copper and iron deposited on their surfaces. Fuel Process. Technol. 2010, 91, 693–701. [Google Scholar] [CrossRef]
- Zhou, A.; Ma, X.; Song, C. Effects of oxidative modification of carbon surface on the adsorption of sulfur compounds in diesel fuel. Appl. Catal. B Environ. 2009, 87, 190–199. [Google Scholar] [CrossRef]
- Ferraz, M.C.M.A.; Monteiro, J.L.C. Structure of impregnated active carbons produced with almond shells influence of impregnation methodology. Fuel 2000, 79, 645–650. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Adsorption of dibenzothiophenes on nanoporous carbons: Identification of specific adsorption sites governing capacity and selectivity. Energy Fuels 2010, 24, 3352–3360. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Investigation of the enhancing effects of sulfur and/or oxygen functional groups of nanoporous carbons on adsorption of dibenzothiophenes. Carbon 2011, 49, 1216–1224. [Google Scholar] [CrossRef]
- Ania, C.O.; Parra, J.B.; Arenillas, A.; Rubiera, F.; Bandosz, T.J.; Pis, J.J. On the mechanism of reactive adsorption of dibenzothiophene on organic waste derived carbons. Appl. Surf. Sci. 2007, 253, 5899–5903. [Google Scholar] [CrossRef]
- Farag, H. Selective adsorption of refractory sulfur species on active carbons and carbon based CoMo catalyst. J. Colloid. Interf. Sci. 2007, 307, 1–8. [Google Scholar] [CrossRef]
- Vilarrasa-García, E.; Infantes-Molina, A.; Moreno-Tost, R.M.; Rodríguez-Castellón, E.; Jiménez-López, A.; Cavalcante, C.L.; Azevedo, D.C.S. Thiophene adsorption on microporous activated carbons impregnated with PdCl2. Energy Fuels 2010, 24, 3436–3442. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Deliyanni, E.; Seredych, M.; Bandosz, T.J. Interactions of 4,6-Dimethyldibenzothiophene with the surface of activated carbons. Langmuir 2009, 25, 9302–9312. [Google Scholar] [CrossRef] [PubMed]
- Seredych, M.; Lison, J.; Jans, U.; Bandosz, T.J. Textural and chemical factors affecting adsorption capacity of activated carbon in highly efficient desulfurization of diesel fuel. Carbon 2009, 47, 2491–2500. [Google Scholar] [CrossRef]
- Hu, Z.; Srinivasan, M.P.; Ni, P. Novel activation process for preparing highly microporous and mesoporous activated carbons. Carbon 2001, 39, 877–886. [Google Scholar] [CrossRef]
- Alhamed, Y.A.; Bamufleh, H.S. Sulfur removal from model diesel fuel using granular activated carbon from dates’ stones activated by ZnCl2. Fuel 2009, 88, 87–94. [Google Scholar] [CrossRef]
- Jia, Y.F.; Thomas, K.M. Adsorption of Cadmium Ions on Oxygen Surface Sites in Activated Carbon. Langmuir 2000, 16, 1114–1122. [Google Scholar] [CrossRef]
- De Souza, W.F.; Guimaraẽs, I.R.; Guerreiro, M.C.; Oliveira, L.C.A. Catalytic oxidation of sulfur and nitrogen compounds from diesel fuel. Appl. Catal. A Gen. 2009, 360, 205–209. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfaõ, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Morozov, I.V.; Znamenkov, K.O.; Korenev, Y.M.; Shlyakhtin, O.A. Thermal decomposition of Cu(NO3)2·3H2O at reduced pressures. Thermochim. Acta 2003, 403, 173–179. [Google Scholar] [CrossRef]
- Mu, J.; Perlmutter, D.O. Thermal decomposition of metal nitrates and their hydrates. Thermochim. Acta 1982, 56, 253–260. [Google Scholar] [CrossRef]
- Firmansyah, D.A.; Kim, T.; Kim, S.; Sullivan, K.; Zachariah, M.R.; Lee, D. Crystalline phase reduction of cuprous oxide (Cu2O) nano particles accompanied by a morphology change during ethanol-assisted spray pyrolysis. Langmuir 2009, 25, 7063–7071. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Ebner, J.R.; Friedman, R.M.; Vannice, M.A. Determination of metal dispersion and surface composition in supported Cu–Pt catalysts. J. Catal. 2002, 208, 381–392. [Google Scholar] [CrossRef]
- Mckee, D.W. Chemistry and Physics of Carbon; Marcel Dekker: New York, NY, USA, 1981; Volume 16, pp. 2–112. [Google Scholar]
- Gao, X.; Liu, S.; Zhang, Y.; Luo, Z.; Cen, K. Physicochemical properties of metal-doped activated carbons and relationship with their performance in the removal of SO2 and NO. J. Hazard. Mater. 2011, 188, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, J.L.; Trimm, D.L. Gasification of carbon deposits on nickel catalysts. J. Catal. 1975, 40, 154–159. [Google Scholar] [CrossRef]
- Hernández-Maldonado, A.J.; Qi, G.; Yang, R.T. Desulfurization of commercial fuels by pi-complexation: Monolayer CuCl/gama-Al2O3. Appl. Catal. B Environ. 2005, 61, 212–218. [Google Scholar] [CrossRef]
- Hernández-Maldonado, A.J.; Yang, R.T. Desulfurization of transportation fuels by adsorption. Catal. Rev. 2004, 46, 111–150. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute Hardness: Companion Parameter to Absolute Electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, HSAB, Part I:Fundamental principl. J. Chem. Educ. 1968, 45, 581–587. [Google Scholar] [CrossRef]
- Fallah, R.N.; Azizian, S. Removal of thiophenic compounds from liquid fuel by different modified activated carbon cloths. Fuel Process. Technol. 2012, 93, 45–52. [Google Scholar] [CrossRef]
© 2012 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Moosavi, E.S.; Dastgheib, S.A.; Karimzadeh, R. Adsorption of Thiophenic Compounds from Model Diesel Fuel Using Copper and Nickel Impregnated Activated Carbons. Energies 2012, 5, 4233-4250. https://doi.org/10.3390/en5104233
Moosavi ES, Dastgheib SA, Karimzadeh R. Adsorption of Thiophenic Compounds from Model Diesel Fuel Using Copper and Nickel Impregnated Activated Carbons. Energies. 2012; 5(10):4233-4250. https://doi.org/10.3390/en5104233
Chicago/Turabian StyleMoosavi, Elham S., Seyed A. Dastgheib, and Ramin Karimzadeh. 2012. "Adsorption of Thiophenic Compounds from Model Diesel Fuel Using Copper and Nickel Impregnated Activated Carbons" Energies 5, no. 10: 4233-4250. https://doi.org/10.3390/en5104233




