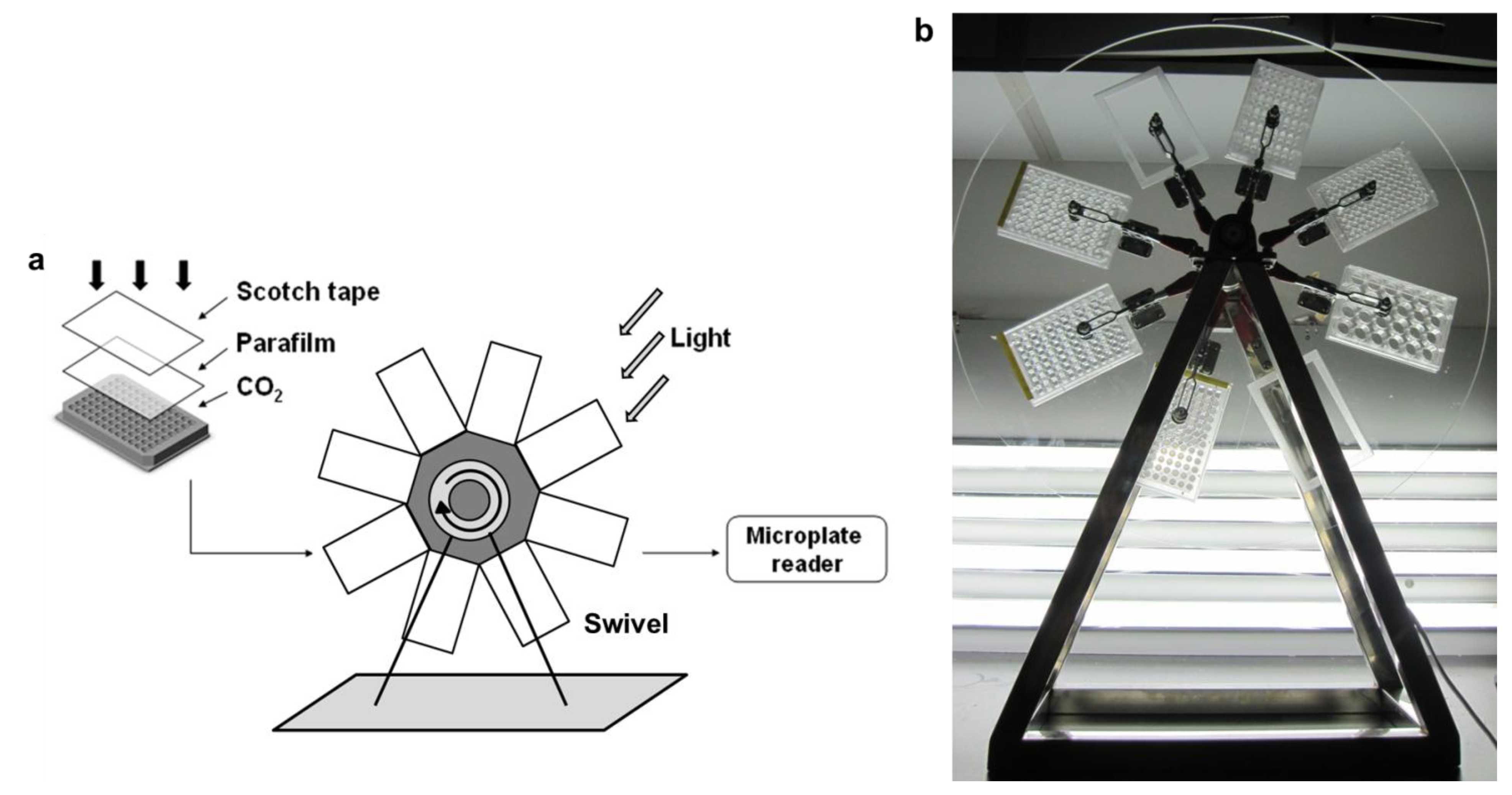
3.1.1. Consistency of Parallel Samples in M96SS
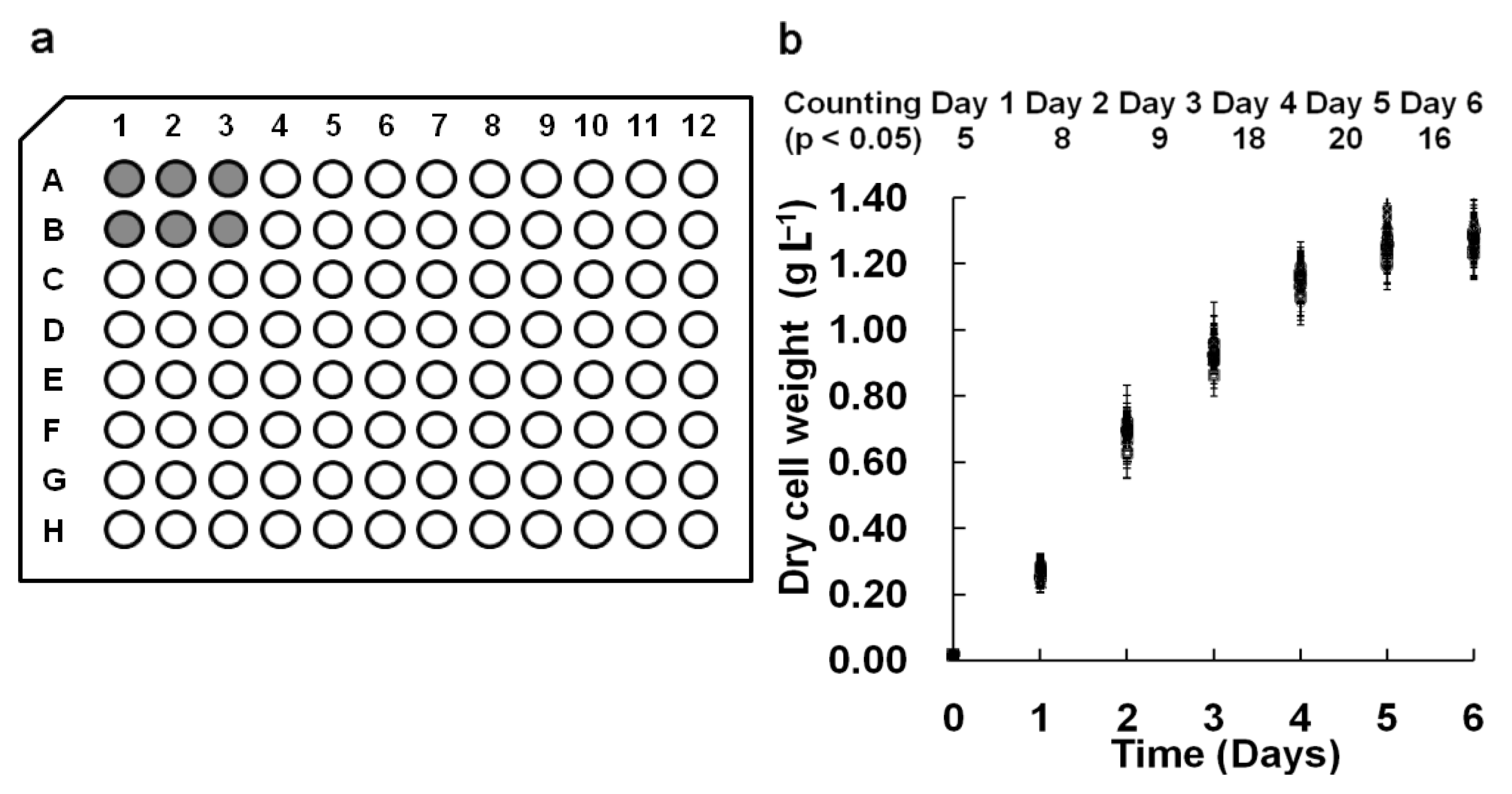
In our M96SS, each microplate had the capacity to test 16 operating conditions (six replicates per sample).
Chlorella pyrenoidosa SJTU-2 was cultivated in each well of two microplates under 0.8 g L
−1 d
−1 CO
2. The cell density of each sample under the same operating condition is shown in
Figure 2(b). To analyze the significant difference between each of the 32 parallel samples, hundreds (C2 32 = 496) of
t-tests were performed daily. The number of samples with
p < 0.05 was counted (
Figure 2b). The result indicated that most parallel samples in M96SS have no significant differences.
In some cases, microalgae cultivated in the corner wells of a microplate appeared to have a higher cell density, possibly due to excessive illumination. Wells at the center received parallel lighting only from the bottom, while those at the corner received additional illumination from the sidewall. This might cause a cell density value for these samples. In most cases, such deviations were within the acceptable range.
3.1.2. Agreement between M96SS and Traditional Flask Culture
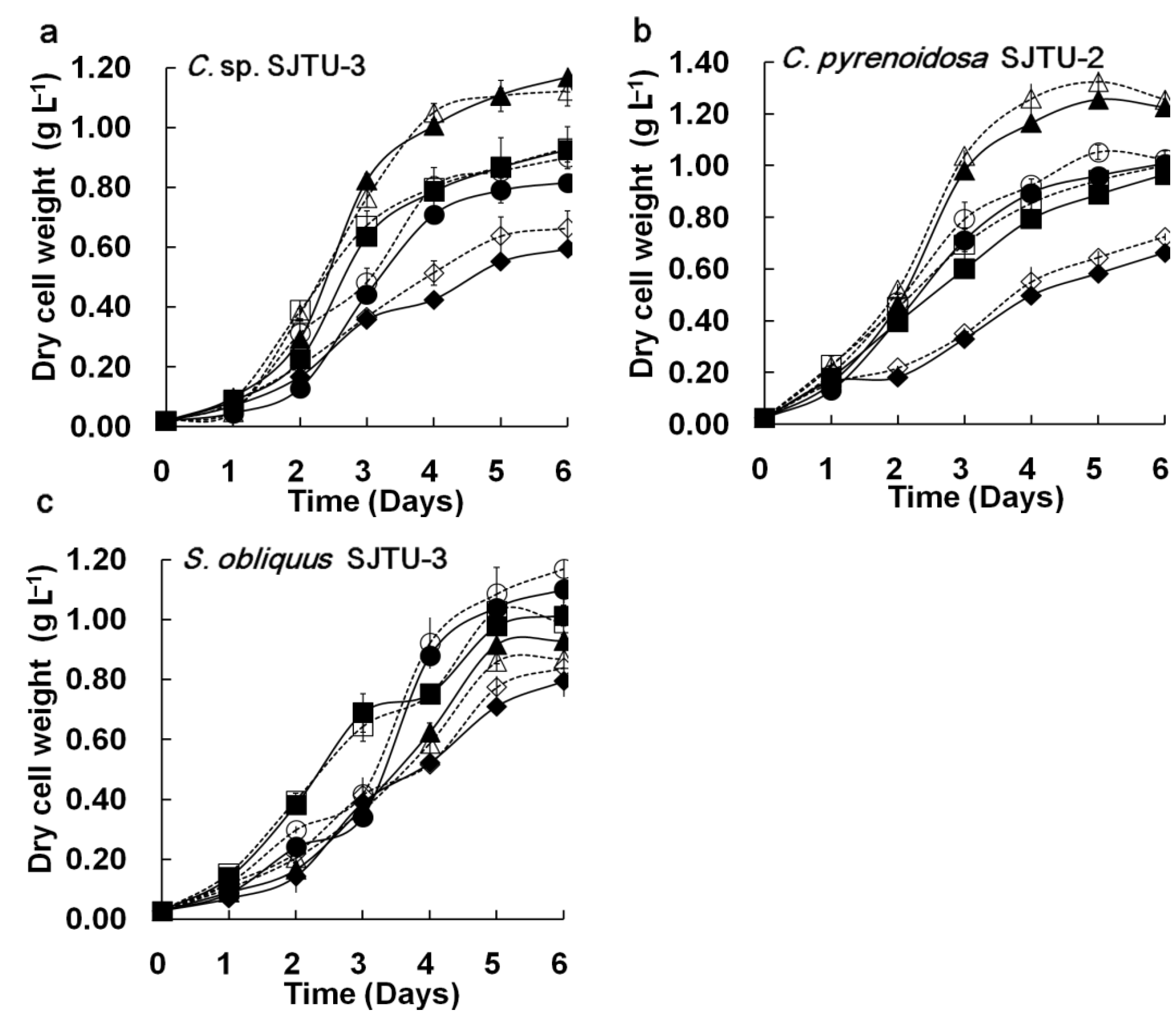
Figure 3 shows the comparison of cell growth, residual nitrate, and culture pH of three microalgae in the M96SS and flask culture. Cell growth is the main parameter for evaluating the potential for CO
2 fixation of a specific algal strain [
16]. As shown in
Figure 3, each of the microalgae exhibited similar growth trends (in response to different CO
2 supply conditions) in the M96SS and flask culture [
Figure 3(a–c)]. The
t-test did not reveal significant differences in cell growth between M96SS and flask culture (
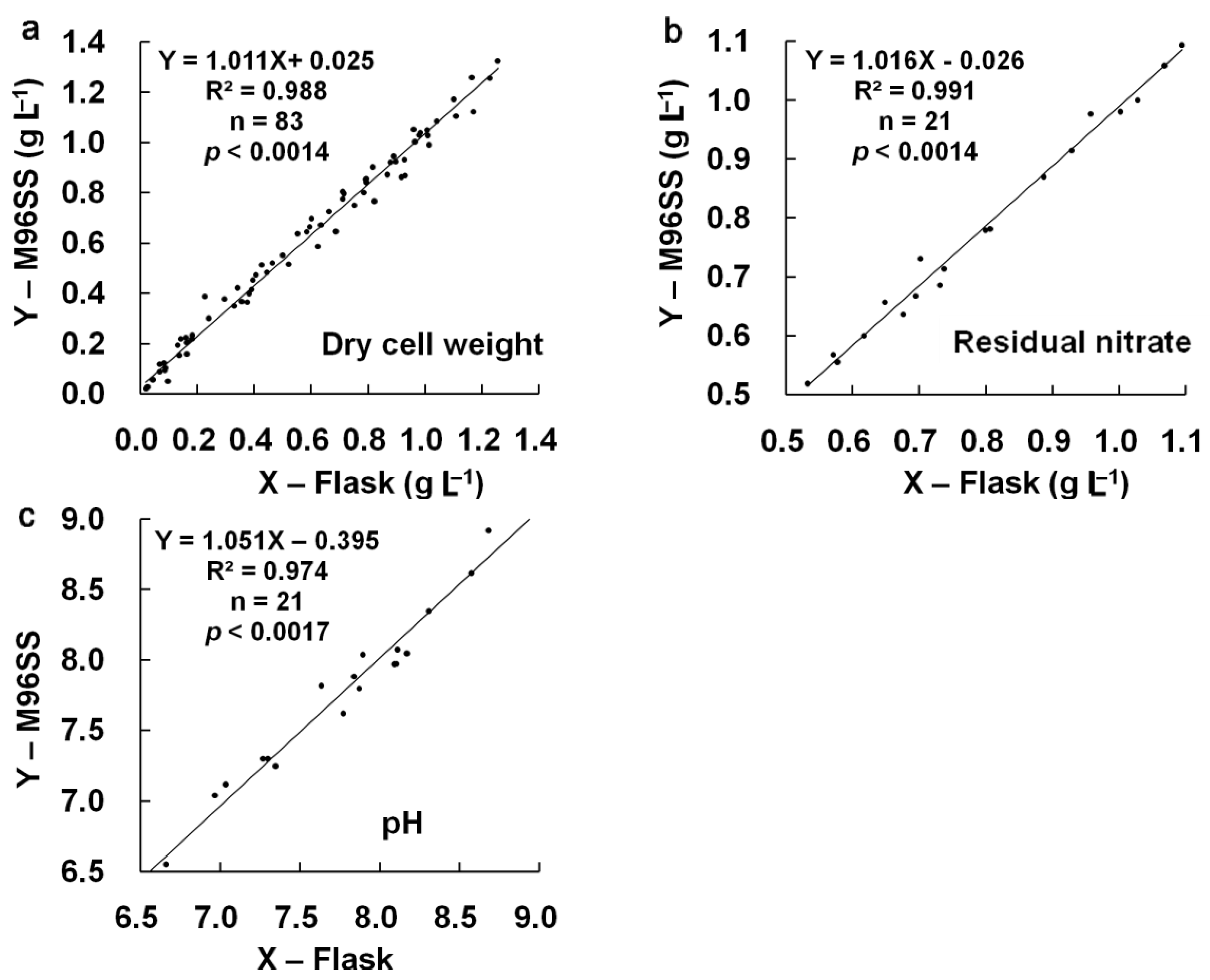
p = 0.605). Linear regression analysis [
Figure 4(a)] also confirmed the agreement between M96SS and flask culture (
R2: 0.988 at
p < 0.0014). The results also provided information regarding the effects of CO
2 on microalgal growth.
Chlorella sp. SJTU-3 [
Figure 3(a)] and
Chlorella pyrenoidosa SJTU-2 [
Figure 3(b)] had the best performance at 0.8 g L
−1 d
−1 CO
2. The dry cell weights of
Chlorella sp. SJTU-3 and
Chlorella pyrenoidosa SJTU-2 after 6 days were 1.168 ± 0.053 g L
−1 and 1.256 ± 0.032 g L
−1 (average values between microplate and flask). For
Scenedesmus obliquus SJTU-3, cell growth at 0.4 g L
−1 d
−1 CO
2 was much better before day 3 than that at other CO
2 supply conditions [
Figure 3(c)]. When dry cell weight was above 0.4 g L
−1 after day 3, 1.2 g L
−1 d
−1 CO
2 seemed to be more conducive to cell growth. A similar phenomenon was reported elsewhere [
17]. Microalgae at a higher cell density are more tolerant of high CO
2 levels.
The presence of nitrogen, the type of nitrogen source, and a correct range of pH value are important factors for microalgae [
18,
19]. In microalgal cultivation, nitrogen source concentration and pH value are usually important variables owing to the nitrogen uptake and CO
2 consumption by microalga growth. Therefore, by comparing these two parameters in M96SS and flasks under the same operating conditions, the agreement between the cultures in both systems can be further validated. The comparison of residual nitrate and pH in M96SS and flask cultures are shown in
Figure 3(d,e). Residual nitrate decreased daily during cultivation. Medium pH sharply declined at day 2, possibly because the low biomass at the beginning of the culture could not fully utilize dissolved CO
2. The major trend of pH variation increased with increasing biomass. The results of the
t-test (
p = 0.833, 0.989) and linear regression analysis [
Figure 4(b,c)] showed good agreement between both systems for these two parameters (
R2: 0.991, 0.974 at
p < 0.0014, 0.0017, respectively). These results further confirmed the similarity of results for M96SS and traditional flask culture.
Figure 3.
Comparison of microalgal dynamic growth characteristics in M96SS versus flask culture. The dotted line with hollow symbols represents cultivation in the M96SS. The Solid line with solid symbols represents cultivation in flasks. (a–c) Cell growth of 3 microalgae under different CO2 supply conditions. For symbols in (a–c), diamond: 0.2 g L−1 d−1 CO2; square: 0.4 g L−1 d−1 CO2; triangle: 0.8 g L−1 d−1 CO2, cycle: 1.2 g L−1 d−1 CO2. The hollow symbols represent the data in M96SS. Solid symbols represent the data in flasks; (d,e) Residual nitrate and pH value of the culture media of 3 microalgae under their optimal CO2 supply conditions. For symbols in (d,e), diamond: C. sp. SJTU-3 in 0.8 g L−1 d−1 CO2; triangle: C. pyrenoidosa SJTU-2 in 0.8 g L−1 d−1 CO2; cycle: S. obliquus SJTU-3 in 0.4 g L−1 d−1 CO2. The hollow symbols represent the data in M96SS. Solid symbols represent the data in flasks. The culture was illuminated at 180 μM m−2 s−1 and incubated at 25 ± 1 °C. Each cultivation was performed in triplicate.
Figure 3.
Comparison of microalgal dynamic growth characteristics in M96SS versus flask culture. The dotted line with hollow symbols represents cultivation in the M96SS. The Solid line with solid symbols represents cultivation in flasks. (a–c) Cell growth of 3 microalgae under different CO2 supply conditions. For symbols in (a–c), diamond: 0.2 g L−1 d−1 CO2; square: 0.4 g L−1 d−1 CO2; triangle: 0.8 g L−1 d−1 CO2, cycle: 1.2 g L−1 d−1 CO2. The hollow symbols represent the data in M96SS. Solid symbols represent the data in flasks; (d,e) Residual nitrate and pH value of the culture media of 3 microalgae under their optimal CO2 supply conditions. For symbols in (d,e), diamond: C. sp. SJTU-3 in 0.8 g L−1 d−1 CO2; triangle: C. pyrenoidosa SJTU-2 in 0.8 g L−1 d−1 CO2; cycle: S. obliquus SJTU-3 in 0.4 g L−1 d−1 CO2. The hollow symbols represent the data in M96SS. Solid symbols represent the data in flasks. The culture was illuminated at 180 μM m−2 s−1 and incubated at 25 ± 1 °C. Each cultivation was performed in triplicate.
![Energies 05 04372 g003a]()
![Energies 05 04372 g003b]()
Figure 4.
Linear regression analysis of cell growth, residual nitrate, and pH in M96SS versus flask culture.
Figure 4.
Linear regression analysis of cell growth, residual nitrate, and pH in M96SS versus flask culture.
There are several likely reasons for the agreement between M96SS and flask cultures. First, both systems had utilized 1/3 of the vessel capacity for working volume. Under the same CO
2 supply conditions, CO
2 supply per unit culture volume was equivalent. According to Henry’s law, the CO
2 gas-liquid distribution would be nearly the same in both systems [
20]. Second, the effect of variation in CO
2 transfer on microalgal growth could be neglected. The mass transfer rate of CO
2 into the liquid media was much faster than the microalgal cell growth rate [
21]. Thus even if the overall volumetric mass transfer coefficient (kLa) was low, the demand of CO
2 for photosynthesis by microalgal growth can be satisfied [
22]. Third, for microalgal cultivation, the medium pH value is directly affected by the amount of dissolved CO
2. To a certain extent, the agreement of pH profile in M96SS and flasks can also indicate a similar profile of dissolved CO
2 in both systems.
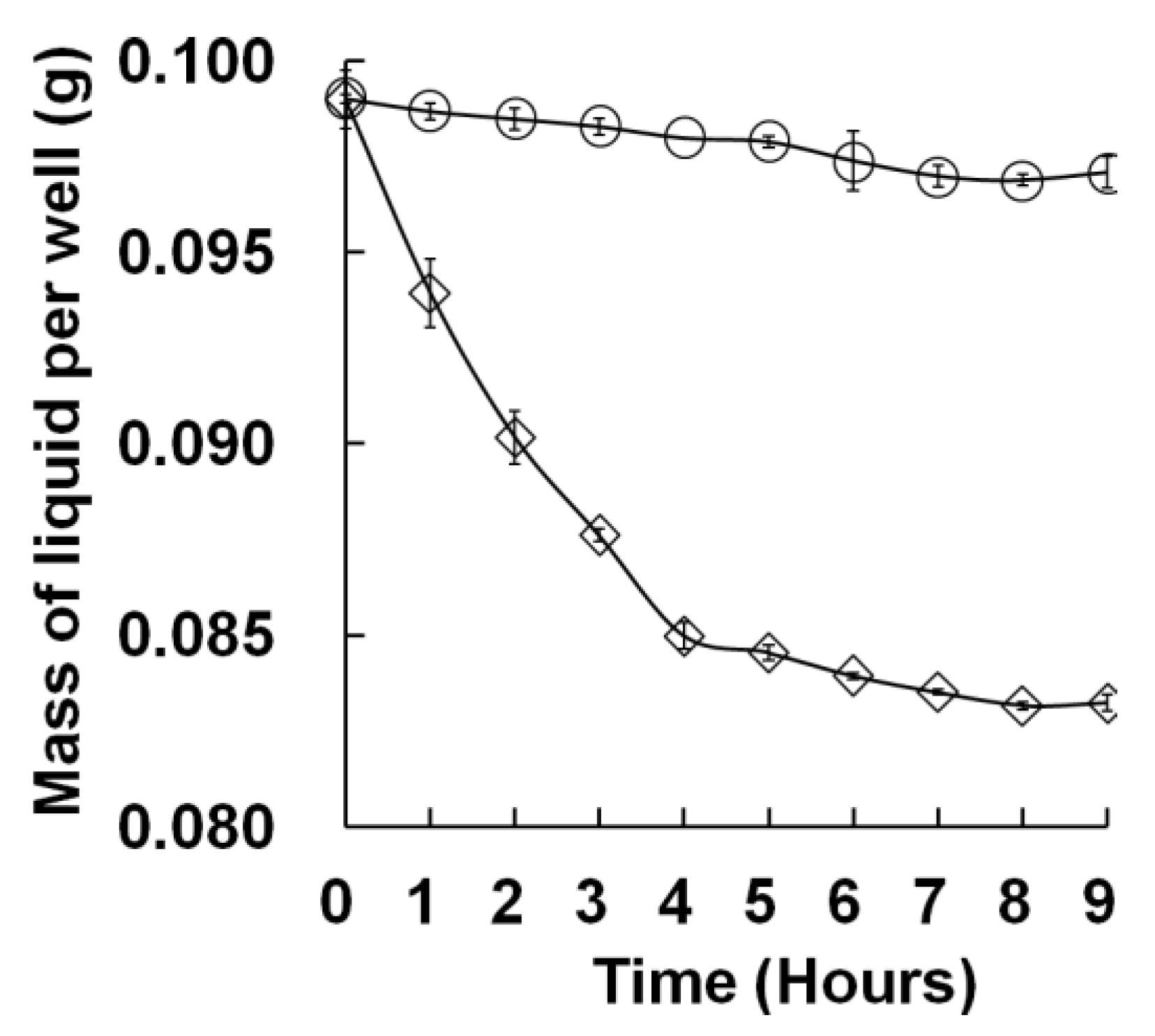
Leaking CO
2 was the main cause of experimental error in this study. If not well controlled, the reproducibility of M96SS
versus traditional flasks culture would be weakened. This problem was also reflected by the larger standard deviation of samples located at the edge of the microplate [
Figure 2(b)]. CO
2 leakage was easy to prevent in flask cultures. Before flushing CO
2, the opening of each flask was heated with a spirit lamp so that after flushing with CO
2, the Scotch
® tape tightly sealed the flask very quickly. In the M96SS, the Parafilm
® was tightly pressed with a rigid plastic frame to seal the microplate after CO
2 replenishment. Before cultivation, each well of a microplate should be examined to ensure it is completely sealed. The hydrophobic Parafilm
® efficiently prevents leakage of liquid medium, but the film is not completely airtight. Another layer of Scotch
® tape was attached above the Parafilm
® to prevent CO
2 leakage in the M96SS.