Experimental Research on the Mechanical Properties of Methane Hydrate-Ice Mixtures
Abstract
:1. Introduction
2. Experimental Apparatus and Test Conditions
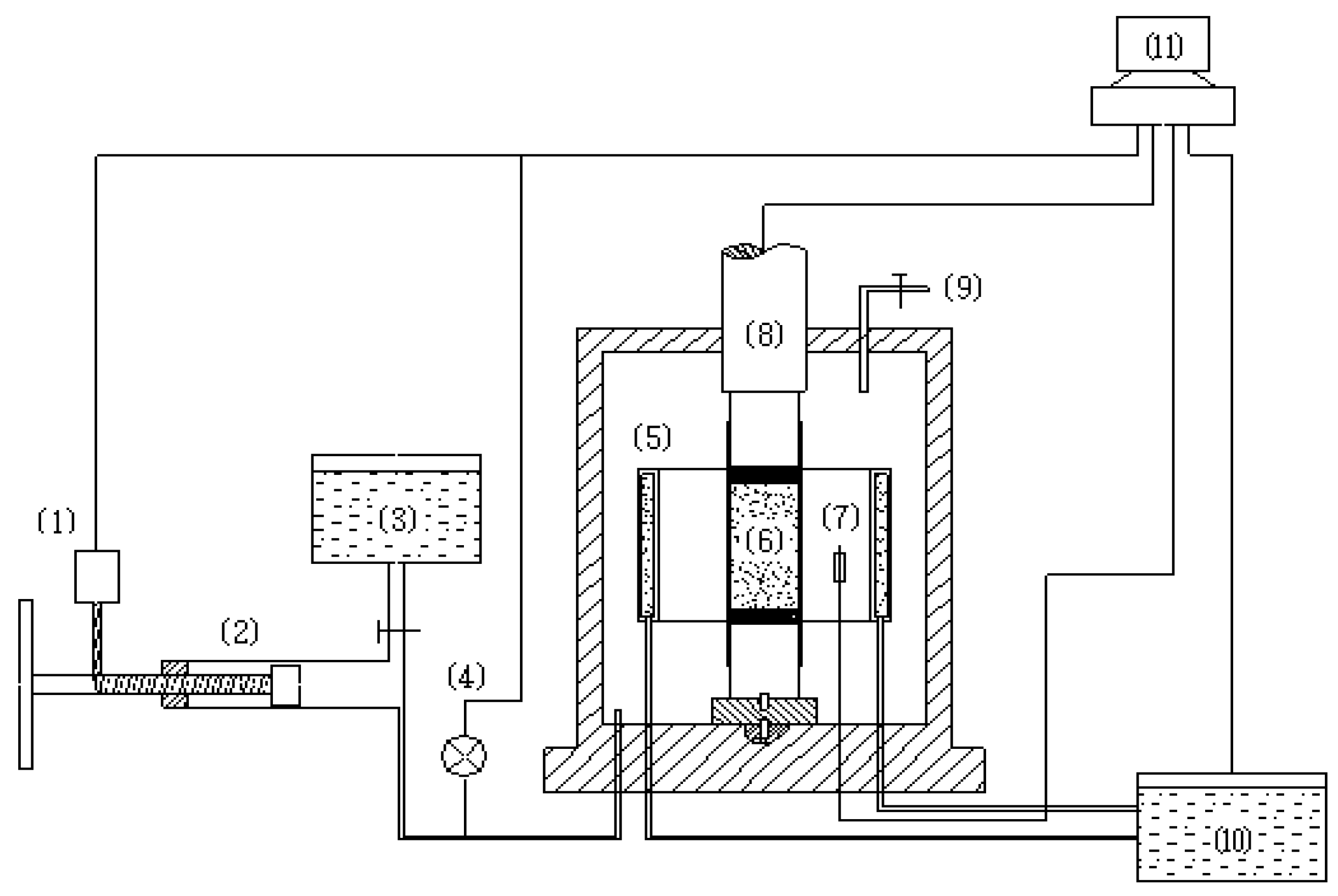
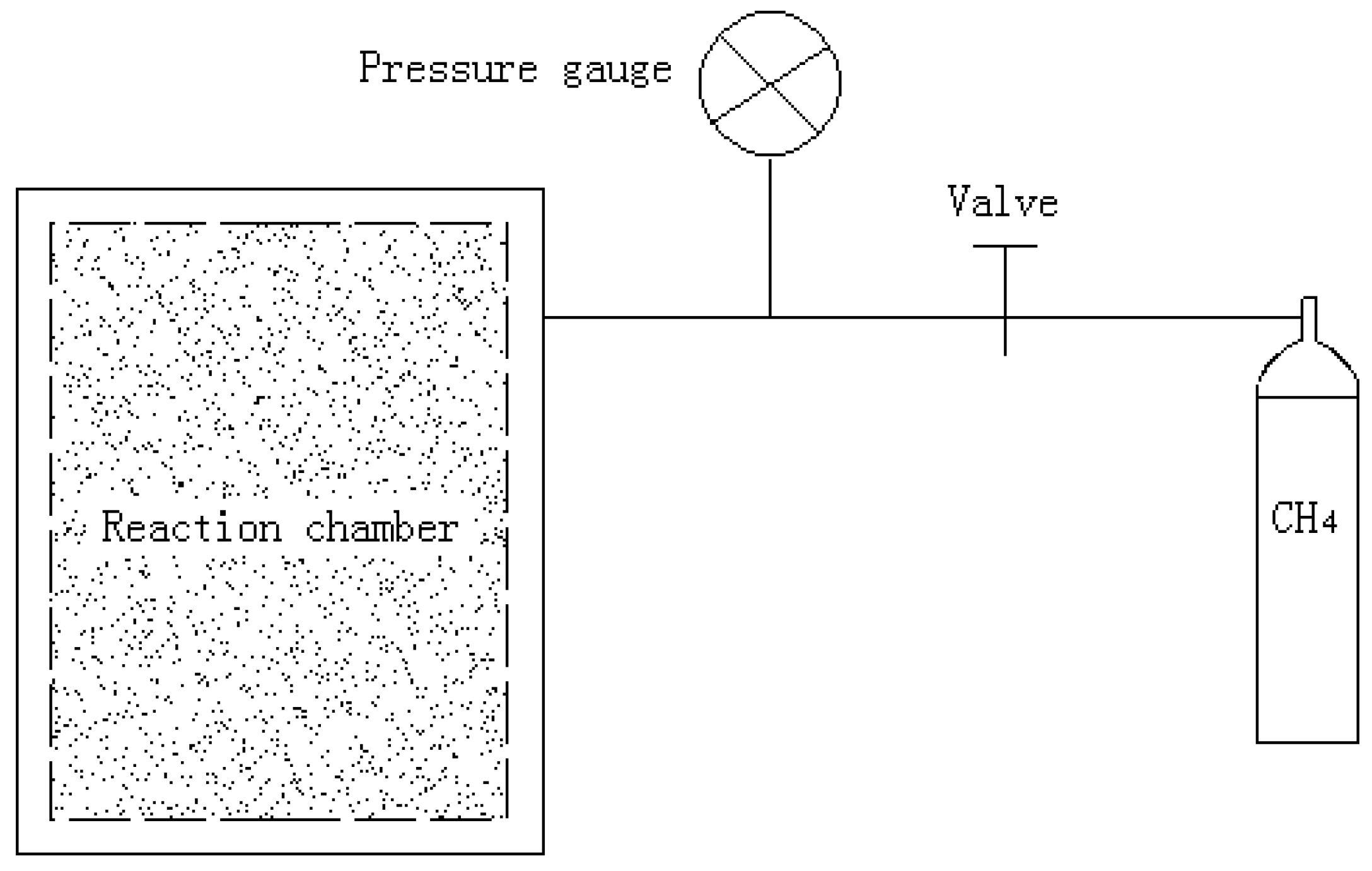
| Temperature | Strain rate | Methane hydrate content | Confining pressure (MPa) |
|---|---|---|---|
| −10 °C | 1.33%/min | 0% | 2.5, 5, 10 |
| 10% | 2.5, 5, 10 | ||
| 20% | 2.5, 5, 10 | ||
| 30% | 2.5, 5, 10 |
3. Results and Discussion
3.1. Stress-Strain Curves of Methane Hydrate-Ice Mixture
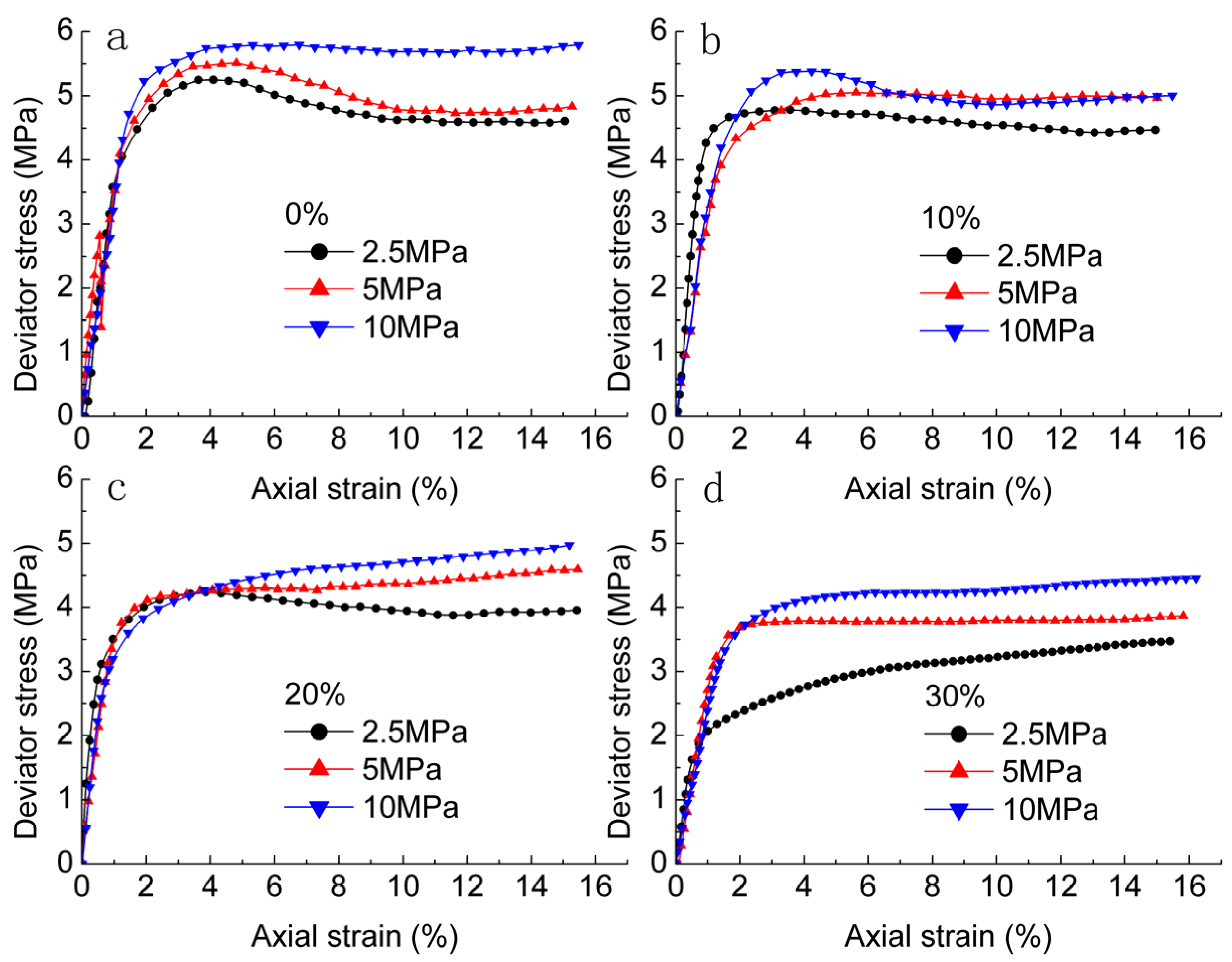
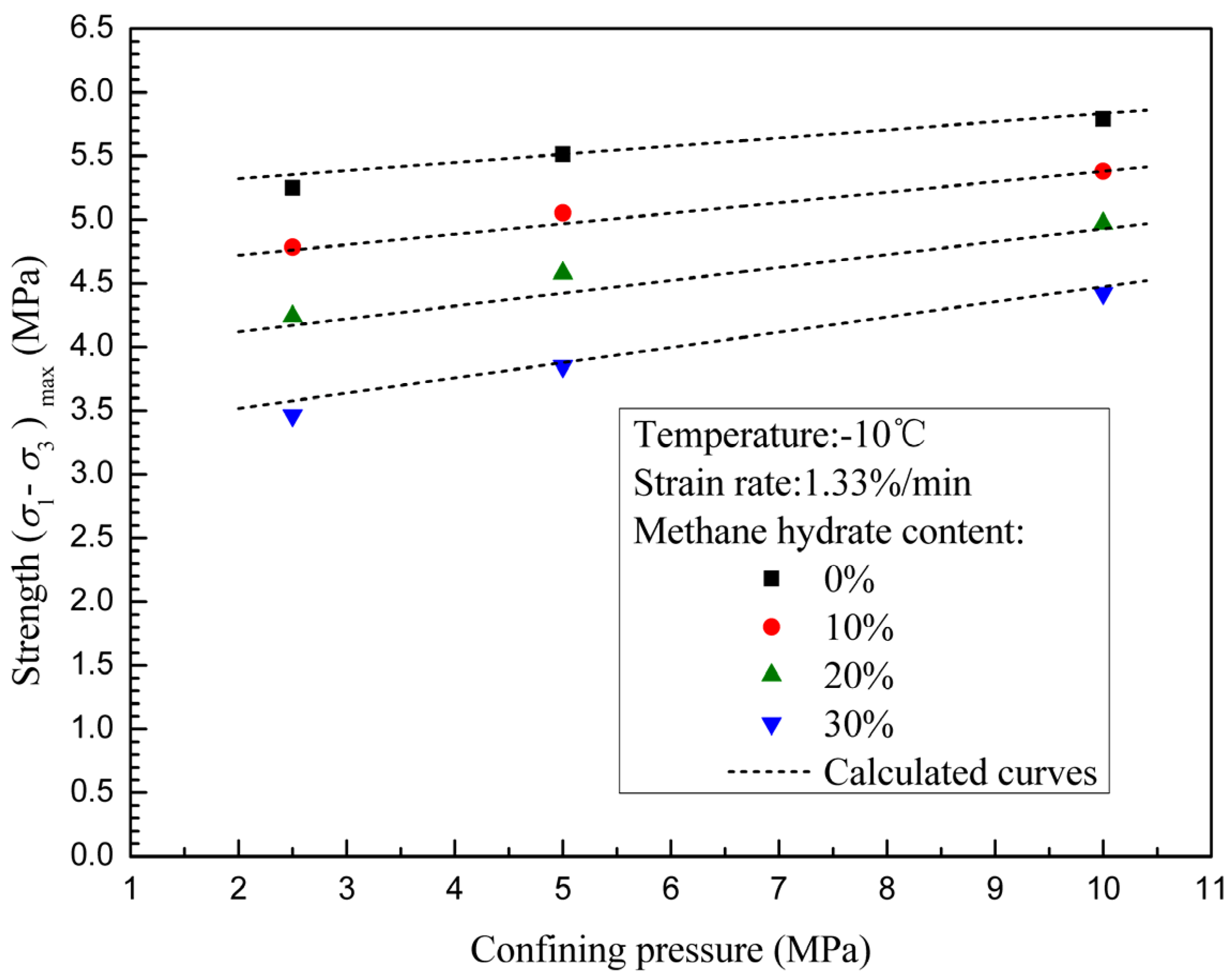
3.2. Effects of Confining Pressure and Methane Hydrate Content on Failure Strength
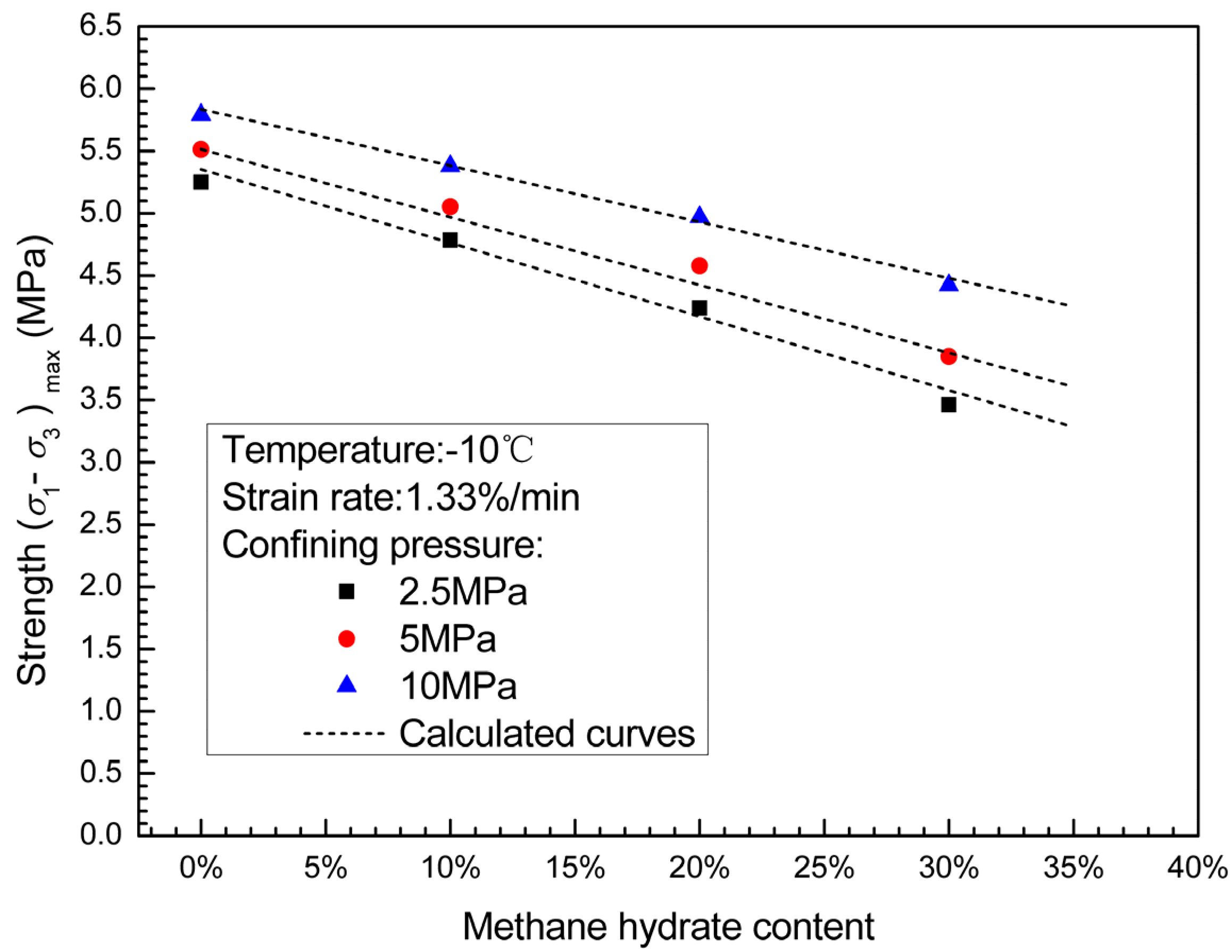
3.3. Failure Strength and Shear Strength
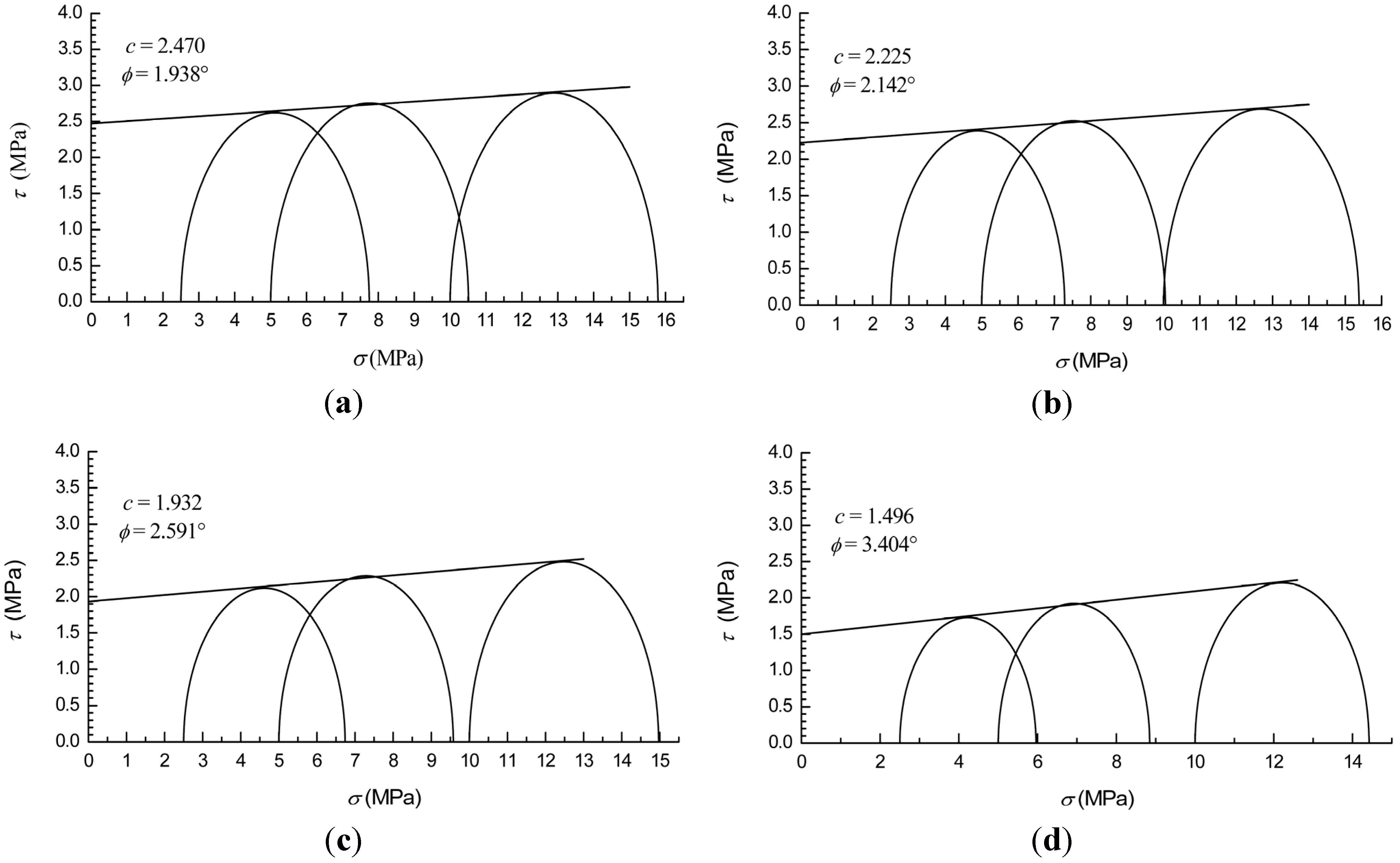
| Methane Hydrate Content | A | B (MPa) | c (MPa) | (°) |
|---|---|---|---|---|
| 0% | 1.070 | 5.110 | 2.47 | 1.938 |
| 10% | 1.078 | 4.620 | 2.225 | 2.142 |
| 20% | 1.095 | 4.043 | 1.932 | 2.591 |
| 30% | 1.126 | 3.175 | 1.496 | 3.404 |
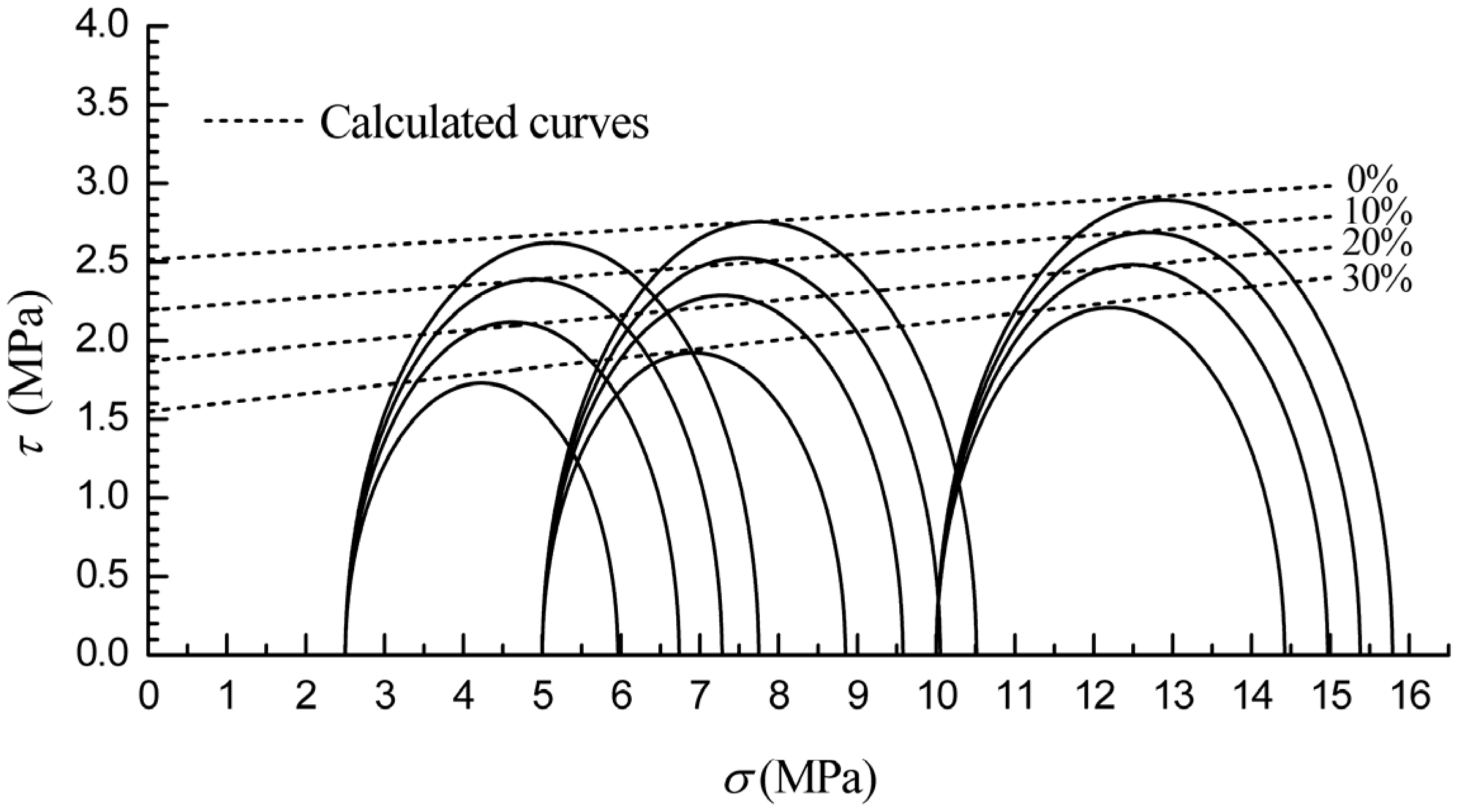
4. Conclusions
- (1)
- The deformation characteristic is strongly affected by confining pressure and methane hydrate content. Strain hardening in the stress-strain relationship became stronger with increase in confining pressure and methane hydrate content.
- (2)
- The failure strengths of methane hydrate-ice mixture specimens are significantly increased with confining pressure when confining pressure is less than 10 MPa. Confining pressure increasingly impacts on failure strength with increasing methane hydrate content, which means that the internal friction angle increases with methane hydrate content.
- (3)
- The failure strengths of methane hydrate-ice mixture specimens decreases with methane hydrate content, and shows a more significant dependence on methane hydrate content at higher confining pressure.
- (4)
- The cohesion of methane hydrate-ice mixture specimens decreases with methane hydrate content, and the strength of ice specimens are higher than that of methane hydrate-ice mixture specimens.
Acknowledgments
Nomenclature
cohesion (MPa) | |
coefficient of determination | |
temperature (°C) | |
strain rate (%/min) | |
normal stress at failure plane (MPa) | |
major principal stress at failure (MPa) | |
secondary principal stress (MPa) | |
minor principal stress or confining pressure (MPa) | |
maximum deviator stress or failure strength (MPa) | |
shear stress at failure (MPa) | |
internal friction angle (°) | |
methane hydrate content |
References
- Ripmeester, J.A.; Ratcliffe, C.I. Low-temperature cross-polarization/magic angle spinning 13C NMR of solid methane hydrates: Structure, cage occupancy, and hydration number. J. Phys. Chem. 1988, 92, 337–339. [Google Scholar] [CrossRef]
- Ripmeester, J.A.; Tse, J.S.; Ratcliffe, C.I.; Powell, B.M. A new clathrate hydrate structure. Nature 1987, 325, 135–136. [Google Scholar] [CrossRef]
- Sloan, E.D. Gas hydrates: Review of physical/chemical properties. Energy Fuels 1998, 12, 191–196. [Google Scholar] [CrossRef]
- Suess, E.; Bohrmann, G.; Greinert, J.; Lausch, E. Flammable ice. Sci. Am. 1999, 281, 76–83. [Google Scholar] [CrossRef]
- MacDonald, I.R.; Guinasso, N.L.; Sassen, R.; Brooks, J.M.; Lee, L.; Scott, K.T. Gas hydrate that breaches the sea floor on the continental slope of the Gulf of Mexico. Geology 1994, 22, 699–702. [Google Scholar] [CrossRef]
- Hoffmann, R. Old gas, new gas. Am. Sci. 2006, 94, 16–18. [Google Scholar] [CrossRef]
- Kvenvolden, K.A.; Ginsburg, G.D.; Soloviev, V.A. Worldwide distribution of subaquatic gas hydrates. Geo-Mar. Lett. 1993, 13, 32–40. [Google Scholar] [CrossRef]
- Kvenvolden, K.A. Methane hydrate—A major reservoir of carbon in the shallow geosphere? Chem. Geol. 1988, 71, 41–51. [Google Scholar] [CrossRef]
- Collett, T.S. Energy resource potential of natural gas hydrates. AAPG Bull. 2002, 86, 1971–1992. [Google Scholar]
- Goodmanm, A. In situ Gas Hydrates: Past Experience and Exploitation Concepts. In Proceedings of the International Gas Research Conference, Chicago, IL, USA, 9–12 June 1980; pp. 376–391.
- Winters, W.J.; Pecher, I.A.; Waite, W.F.; Mason, D.H. Physical properties and rock physics models of sediment containing natural and laboratory-formed methane gas hydrate. Am. Miner. 2004, 89, 1221–1227. [Google Scholar]
- Winters, W.J.; Waite, W.F.; Mason, D.H.; Gilbert, L.Y.; Pecher, I.A. Methane gas hydrate effect on sediment acoustic and strength properties. J. Petrol. Sci. Eng. 2007, 56, 127–135. [Google Scholar] [CrossRef]
- Hyodo, M.; Nakata, Y.; Yoshimoto, N.; Ebinuma, T. Basic research on the mechanical behavior of methane hydrate-sediments mixture. Soils Found. 2005, 45, 75–85. [Google Scholar] [CrossRef]
- Miyazaki, K.; Masui, A.; Tenma, N.; Ogata, Y.; Aoki, K. Study on mechanical behavior for methane hydrate sediment based on constant strain-rate test and unloading-reloading test under triaxial compression. Int. J. Offshore Polar Eng. 2010, 20, 61–67. [Google Scholar]
- Miyazaki, K.; Masui, A.; Sakamoto, Y.; Aoki, K.; Tenma, N.; Ogata, Y.; Yamaguchi, T. Triaxial compressive properties of artificial methane-hydrate-bearing sediment. J. Geophys. Res. 2011, 116. [Google Scholar] [CrossRef]
- Miyazaki, K.; Tenma, N.; Aoki, K. Effects of confining pressure on mechanical properties of artificial methane-hydrate-bearing sediment in triaxial compression test. Int. J. Offshore Polar Eng. 2011, 21, 148–154. [Google Scholar]
- Durham, W.B.; Kirby, S.H.; Stern, L.A.; Zhang, W. The strength and rheology of methane clathrate hydrate. J. Geophys. Res. 2003, 108. [Google Scholar] [CrossRef]
- Li, Y.H.; Song, Y.C.; Yu, F.; Liu, W.G.; Zhao, J.F. Experimental study on mechanical properties of gas hydrate-bearing sediments using kaolin clay. China Ocean Eng. 2011, 25, 113–122. [Google Scholar] [CrossRef]
- Song, Y.C.; Yu, F.; Li, Y.H.; Liu, W.G.; Zhao, J.F. Mechanical property of artificial methane hydrate under triaxial compression. J. Nat. Gas Chem. 2010, 19, 246–250. [Google Scholar] [CrossRef]
- Yu, F.; Song, Y.C.; Li, Y.H.; Liu, W.G.; Lam, W.H. Analysis of stress-strain behavior and constitutive relation of methane hydrate-bearing sediments with various porosity. Int. J. Offshore Polar Eng. 2011, 21, 316–322. [Google Scholar]
- Yu, F.; Song, Y.C.; Liu, W.G.; Li, Y.H.; Lam, W.H. Analyses of stress strain behavior and constitutive model of artificial methane hydrate. J. Petrol. Sci. Eng. 2011, 77, 183–188. [Google Scholar] [CrossRef]
- Li, Y.H.; Song, Y.C.; Yu, F.; Liu, W.G.; Wang, R. Effect of confining pressure on mechanical behavior of methane hydrate-bearing sediments. Petrol. Explor. Dev. 2011, 38, 637–640. [Google Scholar] [CrossRef]
- Haeckel, M.; Suess, E.; Wallmann, K.; Rickert, D. Rising methane gas bubbles form massive hydrate layers at the seafloor. Geochim. Cosmochim. Acta 2004, 68, 4335–4345. [Google Scholar] [CrossRef] [Green Version]
- Yuan, T.; Nahar, K.S.; Chand, R.; Hyndman, R.D.; Spence, G.D.; Chapman, N.R. Marine gas hydrates: Seismic observations of bottom-simulating reflectors off the West Coast of Canada and the East Coast of India. Geohorizons 1998, 3, 1–11. [Google Scholar]
- Bohrmann, G.; Kuhs, W.F.; Klapp, S.A.; Techmer, K.S.; Klein, H.; Murshed, M.; Abegg, F. Appearance and preservation of natural gas hydrate from Hydrate Ridge sampled during ODP Leg 204 drilling. Mar. Geol. 2007, 244, 1–14. [Google Scholar] [CrossRef]
- Kleinberg, R.L.; Flaum, C.; Straley, C.; Brewer, P.G.; Malby, G.E.; Peltzer, E.T.; Friederich, G.; Yesinowski, J.P. Seafloor nuclear magnetic resonance assay of methane hydrate in sediment and rock. J Geophys. Res. 2003, 108. [Google Scholar] [CrossRef]
- Kvenvolden, K.A.; Claypool, G.E.; Threlkeld, C.N.; Sloan, E.D. Geochemistry of a naturally occurring massive marine gas hydrate. Org. Geochem. 1984, 6, 703–713. [Google Scholar] [CrossRef]
- Vásárhelyi, B. Investigation of Crack Propagation with Different Confining Pressure on anysotropic Gneiss. In Rock Mechanics—A Challenge for Society; Särkkä, P., Eloranta, P., Eds.; Taylor & Francis: Lisse, The Netherlands, 2001; pp. 187–190. [Google Scholar]
- Lauke, B.; Schüller, T. Calculation of stress concentration caused by a coated particle in polymer matrix to determine adhesion strength at the interface. Compos. Sci. Technol. 2002, 62, 1965–1978. [Google Scholar] [CrossRef]
- Huang, M.S.; Li, Z.H. Influences of particle size and interface energy on the stress concentration induced by the oblate spheroidal particle and the void nucleation mechanism. Int. J. Solids Struct. 2006, 43, 4097–4115. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Y.; Song, Y.; Liu, W.; Yu, F. Experimental Research on the Mechanical Properties of Methane Hydrate-Ice Mixtures. Energies 2012, 5, 181-192. https://doi.org/10.3390/en5020181
Li Y, Song Y, Liu W, Yu F. Experimental Research on the Mechanical Properties of Methane Hydrate-Ice Mixtures. Energies. 2012; 5(2):181-192. https://doi.org/10.3390/en5020181
Chicago/Turabian StyleLi, Yanghui, Yongchen Song, Weiguo Liu, and Feng Yu. 2012. "Experimental Research on the Mechanical Properties of Methane Hydrate-Ice Mixtures" Energies 5, no. 2: 181-192. https://doi.org/10.3390/en5020181




