Optimization of Agitation and Aeration for Very High Gravity Ethanol Fermentation from Sweet Sorghum Juice by Saccharomyces cerevisiae Using an Orthogonal Array Design
Abstract
:1. Introduction
2. Experimental Section
2.1. Microorganism and Inoculum Preparation
2.2. Raw Material
2.3. Ethanol Production Medium
2.4. Preliminary Experiments
| Condition | Agitation rate (rpm) | Aeration rate (vvm) | Aeration timing (h) |
|---|---|---|---|
| 1 | 100 | - | - |
| 2 | 100 | 0.5 | 2 |
| 3 | 200 | - | - |
| 4 | 200 | 2.5 | 6 |
2.5. Orthogonal Experimental Design
2.6. Fermentation Conditions
| Run | A | B | Blank | C |
|---|---|---|---|---|
| Agitation rate (rpm) | Aeration rate (vvm) | Aeration timing (h) | ||
| 1 | 100 | 0.5 | 1 | 2 |
| 2 | 100 | 2.5 | 3 | 6 |
| 3 | 200 | 0.5 | 2 | 6 |
| 4 | 300 | 1.5 | 1 | 6 |
| 5 | 300 | 0.5 | 3 | 4 |
| 6 | 200 | 1.5 | 3 | 2 |
| 7 | 200 | 2.5 | 1 | 4 |
| 8 | 300 | 2.5 | 2 | 2 |
| 9 | 100 | 1.5 | 2 | 4 |
2.7. Analytical Methods
3. Results and Discussion
3.1. Preliminary Results
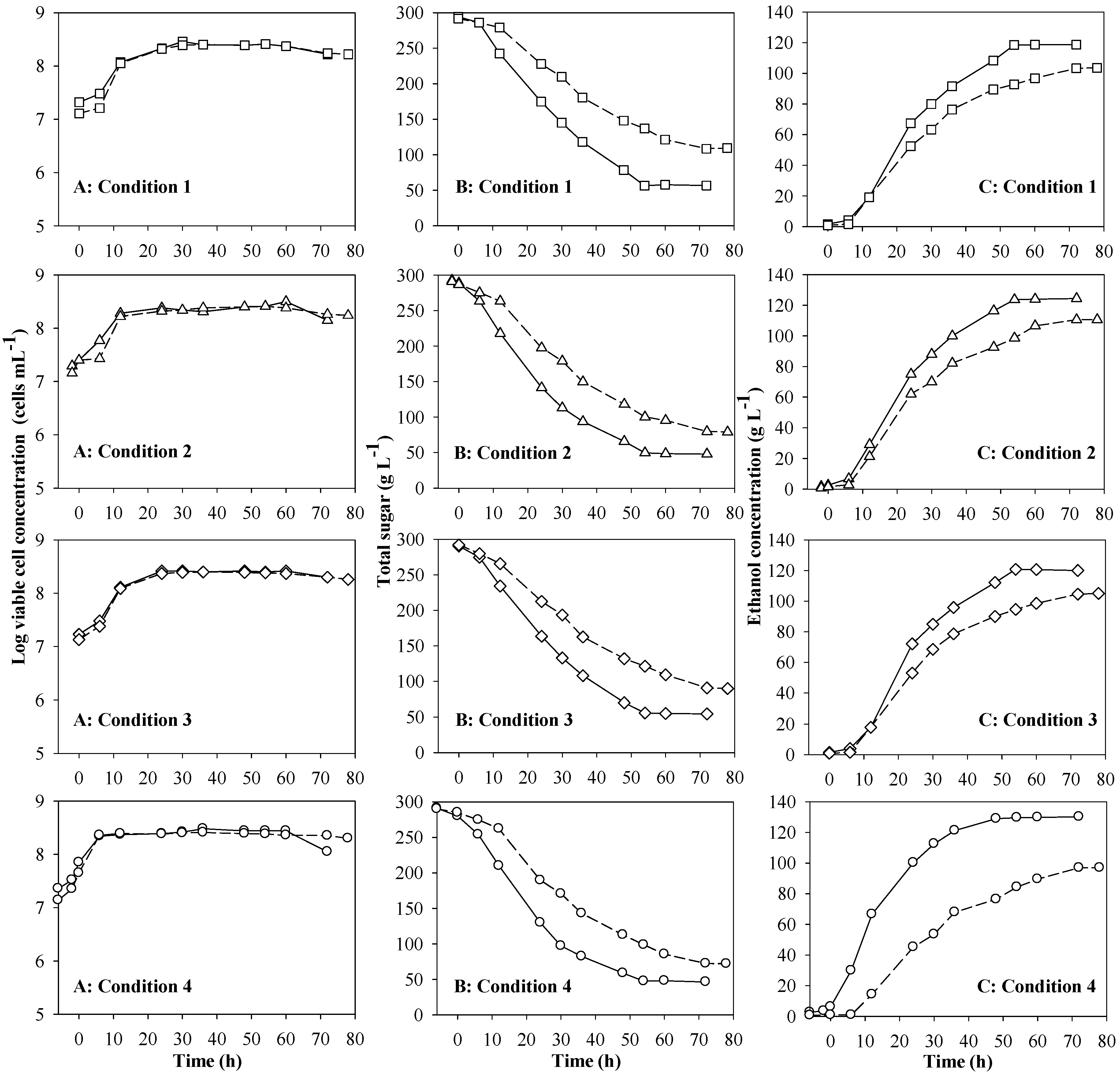
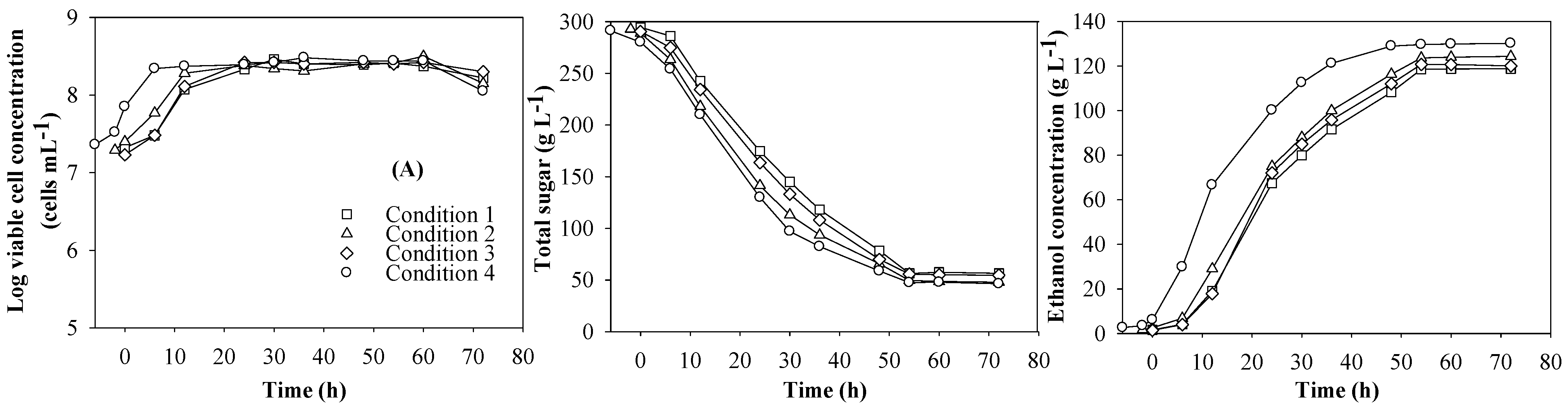
| Condition a | Sugar consumption (%) | P (g L−1) | Qp (g L−1h−1) | Yp/s (g g−1) |
|---|---|---|---|---|
| 1 | 80.84 ± 2.02 b,c | 119.44 ± 1.10 b | 2.21 ± 0.02 c | 0.51 ± 0.01 c,d |
| 2 | 83.02 ± 1.03 c | 121.33 ± 0.60 c | 1.96 ± 0.01 b | 0.47 ± 0.01 b |
| 3 | 80.08 ± 1.16 b | 118.02 ± 1.19 b | 2.19 ± 0.04 c | 0.50 ± 0.01 c |
| 4 | 82.29 ± 0.94 b,c | 128.98 ± 0.34 d | 2.39 ± 0.01 d | 0.52 ± 0.00 d |
3.2. The Orthogonal Experiment Results of Ethanol Fermentation

3.3. Impact of Multi-Factors on Ethanol Concentration
| Run a | P (g L−1) | Qp (g L−1h−1) | Yp/s (g g−1) |
|---|---|---|---|
| 1 | 121.33 ± 0.60 c | 1.96 ± 0.01 b | 0.47 ± 0.01 b,c |
| 2 | 117.30 ± 1.85 b | 2.17 ± 0.03 c | 0.48 ± 0.01 b,c |
| 3 | 126.64 ± 2.21 d | 2.35 ± 0.04 e | 0.49 ± 0.00 c,d |
| 4 | 121.36 ± 1.32 c | 2.25 ± 0.02 d | 0.47 ± 0.01 b,c |
| 5 | 129.22 ± 1.94 e,f | 2.23 ± 0.03 d | 0.51 ± 0.01 e |
| 6 | 127.03 ± 0.61 d,e | 2.27 ± 0.01 d | 0.48 ± 0.01 b,c |
| 7 | 131.02 ± 0.93 f | 2.52 ± 0.02 f | 0.50 ± 0.00 d,e |
| 8 | 129.27 ± 0.39 e,f | 2.59 ± 0.01 g | 0.47 ± 0.01 b,c |
| 9 | 121.28 ± 0.58 c | 2.33 ± 0.01 e | 0.46 ± 0.02 b |
| A | B | Blank | C | |
|---|---|---|---|---|
| Agitation rate | Aeration rate | Aeration timing | ||
| K1 | 719.82 | 754.38 | 747.42 | 755.28 |
| K2 | 769.38 | 739.32 | 754.38 | 763.02 |
| K3 | 759.72 | 755.16 | 747.12 | 730.62 |
| k1 | 119.97 | 125.73 | 124.57 | 125.88 |
| k2 | 128.23 | 123.22 | 125.73 | 127.17 |
| k3 | 126.62 | 125.86 | 124.52 | 121.77 |
| R | 8.26 | 2.64 | 1.21 | 5.40 |
| Q | A2 | B3 | C2 |
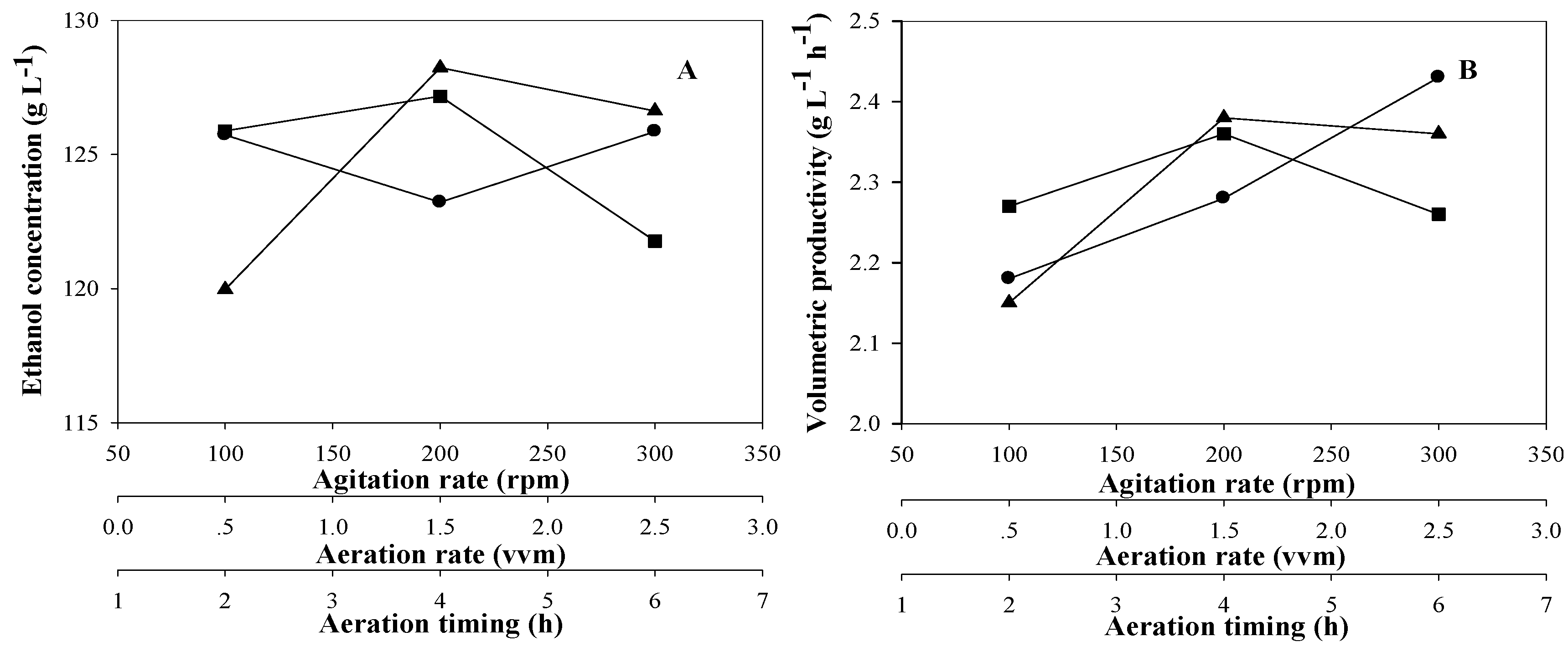
3.4. Impact of Multi-Factors on Ethanol Productivity
| A | B | Blank | C | |
|---|---|---|---|---|
| Agitation rate | Aeration rate | Aeration timing | ||
| K1 | 12.90 | 13.08 | 13.44 | 13.62 |
| K2 | 14.28 | 13.68 | 14.52 | 14.16 |
| K3 | 14.16 | 14.58 | 13.32 | 13.56 |
| k1 | 2.15 | 2.18 | 2.24 | 2.27 |
| k2 | 2.38 | 2.28 | 2.42 | 2.36 |
| k3 | 2.36 | 2.43 | 2.22 | 2.26 |
| R | 0.23 | 0.25 | 0.20 | 0.10 |
| Q | A2 | B3 | C2 |
3.5. Verification Experiments

| Condition | Size of Fermenter | Sugar Consumption (%) | P (g L−1) | Qp (g L−1 h−1) | Yp/s (g g−1) | T (h) |
|---|---|---|---|---|---|---|
| Optimum (A2B3C2) | 2-L | 89.9 ± 0.51 a | 132.82 ± 1.06 a | 2.55 ± 0.00 a | 0.50 ± 0.00 a | 52 |
| 5-L | 88.41 ± 1.57 a | 129.72 ± 2.03 a | 2.50 ± 0.04 a | 0.50 ± 0.00 a | 52 | |
| Control | 2-L | 80.08 ± 1.16 b | 118.02 ± 1.19 b | 2.19 ± 0.04 b | 0.50 ± 0.01 a,b | 54 |
| 5-L | 78.97 ± 1.75 b | 120.72 ± 1.37 b | 2.24 ± 0.03 b | 0.52 ± 0.01 b | 54 |
4. Conclusions
Acknowledgments
References
- Ward, O.P.; Singh, A. Bioethanol technology: Developments and perspective. Adv. Appl. Microbiol. 2002, 51, 53–80. [Google Scholar]
- Bai, F.W.; Anderson, W.A.; Moo-Young, M. Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol. Adv. 2008, 26, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, C.P.; Yadav, R.K.P.; Ohga, S. Agricultural waste residues as potential sources of bioethanol. Sci. World 2008, 6, 19–23. [Google Scholar]
- Bennett, A.S.; Anex, R.P. Production, transportation and milling costs of sweet sorghum as a feedstock for centrifuged bioethanol production in the upper Midwest. Bioresour. Technol. 2009, 100, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Jaisil, P.; Pakdee, P.; Pothisoong, T.; Lertprasert-rat, K. Production cost of sweet sorghum (Sorghum bicolor (L.) Moench) and syrup production for ethanol plant. J. Natl. Res. Counc. Thail. 2009, 148–156. [Google Scholar]
- Wu, X.; Staggenborg, S.; Propheter, J.L.; Rooney, W.L.; Yu, J.; Wang, D. Features of sweet sorghum juice and their performance in ethanol fermentation. Ind. Crop Prod. 2010, 31, 164–170. [Google Scholar] [CrossRef]
- Bayrock, D.P.; Ingledew, W.M. Application of multistage continuous fermentation for production of fuel alcohol by very-high-gravity fermentation technology. J. Ind. Microbiol. Biotechnol. 2001, 27, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Khongsay, N.; Laopaiboon, L.; Laopaiboon, P. Growth and batch ethanol fermentation of Saccharomyces cerevisiae on sweet sorghum stem juice under normal and very high gravity conditions. Biotechnology 2010, 9, 9–16. [Google Scholar] [CrossRef]
- Bai, F.W.; Chen, L.J.; Zhang, Z.; Anderson, W.A.; Moo-Young, M. Continuous ethanol production and evaluation of yeast cell lysis and viability loss under very high gravity medium conditions. J. Biotechnol. 2004, 110, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.C.; Hynes, S.H.; Jones, A.M.; Ingledew, W.M. Production of fuel alcohol from wheat by VHG technology: effect of sugar concentration and fermentation temperature. Appl. Biochem. Biotechnol. 1993, 43, 211–226. [Google Scholar] [CrossRef]
- Ozmichi, S.; Kargi, F. Ethanol fermentation of cheese whey powder solution by repeated fed-batch operation. Enzyme Microb. Technol. 2007, 41, 169–174. [Google Scholar] [CrossRef]
- Bafrncová, P.; Šmogrovičová, D.; Sláviková, I.; Pátková, J.; Dőmény, Z. Improvement of very high gravity ethanol fermentation by media supplementation using Saccharomyces cerevisiae. Biotechnol. Lett. 1999, 21, 337–341. [Google Scholar] [CrossRef]
- Watanabe, M.; Tamura, K.; Magbanua, J.P.; Takano, K.; Kitamoyo, K.; Kitagaki, H.; Akao, T.; Shimoi, H. Elevated expression of genes under the control of stress response element (STRE) and Msn2p in an ethanol tolerate sake yeast Kyokai No.11. J. Biosci. Bioeng. 2007, 104, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qureshi, N. How microbes tolerate ethanol and butanol. New Biotechnol. 2009, 26, 117–121. [Google Scholar] [CrossRef]
- Badotti, F.; Belloch, C.; Rosa, C.A.; Barrio, E.; Querol, A. Physiological and molecular characterization of Saccharomyces cerevisiae cachaça strains isolated from different geographic regions in Brazil. World J. Microbiol. Biotechnol. 2010, 26, 579–587. [Google Scholar] [CrossRef]
- Casey, G.P.; Magnus, C.A.; Ingledew, W.M. High-gravity brewing: effects of nutrition on yeast composition, fermentative ability, and alcohol production. Appl. Environ. Microbiol. 1984, 48, 639–646. [Google Scholar] [PubMed]
- Alfenore, S.; Cameleyre, X.; Benbadis, L.; Bideaux, C.; Uribelarrea, J-L.; Goma, G.; Molina-Jouve, C.; Guillouet, S.E. Aeration strategy: a need for very high ethanol performance in Saccharomyces cerevisiae fed-batch process. Appl. Microbiol. Biotechnol. 2004, 63, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Patrascu, E.; Rapeanu, G.; Hopulele, T. Current approaches to efficient biotechnological production of ethanol. Innovat. Rom. Food Biotechnol. 2009, 4, 1–11. [Google Scholar]
- Márquezt, T.; Millánt, C.; Salmon, J.M. Plasma membrane sterols are involved in yeast’s ability to adsorb polyphenolic compounds resulting from wine model solution browning. J. Agric. Food Chem. 2009, 57, 8026–8032. [Google Scholar] [CrossRef] [PubMed]
- Breisha, G.Z. Production of 16% ethanol from 35% sucrose. Biomass Bioenerg. 2010, 34, 1243–1249. [Google Scholar] [CrossRef]
- Jacquier, N.; Schneiter, R. Ypk1, the yeast orthologue of the human serum-and glucocorticoid-induced kinase, is required for efficient uptake of fatty acids. J. Cell Sci. 2010, 123, 2218–2227. [Google Scholar] [CrossRef] [PubMed]
- Landolfo, S.; Zara, G.; Zara, S.; Budroni, M.; Ciani, M.; Mannazzu, I. Oleic acid and ergosterol supplementation mitigates oxidative stress in wine strains of Saccharomyces cerevisiae. Int. J. Food Microbiol. 2010, 141, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Laopaiboon, L.; Nuanpeng, S.; Srinophakun, P.; Klanrit, P.; Laopaiboon, P. Ethanol production from sweet sorghum juice using very high gravity technology: Effects of carbon and nitrogen supplementations. Bioresour. Technol. 2009, 100, 4176–4182. [Google Scholar] [CrossRef] [PubMed]
- Nuanpeng, S.; Laopaiboon, L.; Srinophakun, P.; Klanrit, P.; Jaisil, P.; Laopaiboon, P. Ethanol production from sweet sorghum juice under very high gravity conditions: Batch, repeated-batch and scale up for fermentation. Electron. J. Biotechnol. 2011, 14, 1–12. [Google Scholar]
- Liu, Y.; Qi, T.; Shen, N.; Gan, M.; Jin, Y.; Zhao, H. Improvement of ethanol concentration and yield by initial aeration and agitation culture in very high gravity fermentation. Chin. J. Appl. Environ. Biol. 2009, 15, 563–567. [Google Scholar]
- Taguchi, G. System of Experimental Design; Krus International Press: New York, NY, USA, 1990. [Google Scholar]
- Ke, W.; Jiuyu, H.; Lei, W.; Huimin, L. Phase factor sequences algorithm in partial transmit sequence. Trans. Tianjin Univ. 2009, 15, 23–26. [Google Scholar] [CrossRef]
- Yang, L.J.; Qi, Y.M.; Dang, X.N. Design and optimization of technology and structure parameters for sheet metal drawing by orthogonal experiment. Adv. Mater. Res. 2011, 295, 1714–1717. [Google Scholar] [CrossRef]
- Park, S.H. Robust Design and Analysis for Quality Engineering; Chapman & Hall Press: London, UK, 1996. [Google Scholar]
- Farzaneh, A.; Ehteshamzadeh, M.; Mohammadi, M. Corrosion performance of the electroless Ni-P coatings prepared in different conditions and optimized by the Taguchi method. J. Appl. Electrochem. 2010, 41, 19–27. [Google Scholar] [CrossRef]
- Zoecklien, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Wine Analysis and Production; Chapman & Hall Press: New York, NY, USA, 1995. [Google Scholar]
- Mecozzi, M. Estimation of total carbohydrate amount in environmental samples by the phenol sulphuric acid method assisted by multivariate calibration. Chemom. Intel. Lab. Syst. 2005, 79, 84–90. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chien, W.S.; Duan, K.J.; Chang, P.R. Effect of aeration timing and interval during very-high-gravity ethanol fermentation. Process Biochem. 2011, 46, 1025–1028. [Google Scholar] [CrossRef]
- Hammond, J. Yeast growth and nutrition. In Brewing Yeast Fermentation Performance; Smart, K., Ed.; Oxford Brookes University Press: Oxford, UK, 2000. [Google Scholar]
- Stowe, R.E.; Mayer, R.P. Efficient screening of process variables. Ind. Eng. Chem. 1966, 56, 36–40. [Google Scholar] [CrossRef]
- Jangchud, A. Product optimization. In Statistics for Product Development and Application; Kasetsart University: Bangkok, Thailand, 2006; pp. 241–288. [Google Scholar]
- Liu, R.; Shen, F. Impacts of main factors on bioethanol fermentation from stalk juice of sweet sorghum by immobilized Saccharomyces cerevisiae (CICC 1308). Bioresour. Technol. 2008, 99, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Fornairon-Bonnefond, C.; Demaretz, V.; Rosenfeld, E.; Salmon, J.M. Oxygen addition and sterol synthesis in Saccharomyces cerevisiae during enological fermentation. J. Biosci. Bioeng. 2002, 93, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cortés, G.; Córdova-López, J.A.; Herrera-López, E.J.; Morán-Marroquín, G.A.; Valle-Rodríguez, J.O.V.; Díaz-Montaňo, D.M. Effect of pH, aeration and feeding non-sterilized agave juice in a continuous agave juice fermentation. J. Sci. Food Agric. 2010, 90, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Lin, Y.H.; Bai, F.W. Development of redox potential-controlled schemes for very-high-gravity ethanol fermentation. J. Biotechnol. 2011, 153, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Kuhbeck, F.; Muller, M.; Back, W.; Kurz, T.; Krottenthaler, M. Effect of hot trub and particle addition on fermentation performance of Saccharomyces cerevisiae. Enzyme Microb. Technol. 2007, 41, 711–720. [Google Scholar] [CrossRef]
- Briggs, D.E.; Boulton, C.A.; Brookes, P.A.; Stevens, R. Brewing: Science and Practice; Woodhead Press: Cambridge, UK, 2004. [Google Scholar]
- Andreasen, A.A.; Stier, T.J. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J. Cell Comp. Physiol. 1953, 43, 23–36. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Khongsay, N.; Laopaiboon, L.; Jaisil, P.; Laopaiboon, P. Optimization of Agitation and Aeration for Very High Gravity Ethanol Fermentation from Sweet Sorghum Juice by Saccharomyces cerevisiae Using an Orthogonal Array Design. Energies 2012, 5, 561-576. https://doi.org/10.3390/en5030561
Khongsay N, Laopaiboon L, Jaisil P, Laopaiboon P. Optimization of Agitation and Aeration for Very High Gravity Ethanol Fermentation from Sweet Sorghum Juice by Saccharomyces cerevisiae Using an Orthogonal Array Design. Energies. 2012; 5(3):561-576. https://doi.org/10.3390/en5030561
Chicago/Turabian StyleKhongsay, Naulchan, Lakkana Laopaiboon, Prasit Jaisil, and Pattana Laopaiboon. 2012. "Optimization of Agitation and Aeration for Very High Gravity Ethanol Fermentation from Sweet Sorghum Juice by Saccharomyces cerevisiae Using an Orthogonal Array Design" Energies 5, no. 3: 561-576. https://doi.org/10.3390/en5030561




