Direct Observation of THF Hydrate Formation in Porous Microstructure Using Magnetic Resonance Imaging
Abstract
:1. Introduction
2. Experimental Section
2.1. Apparatus and Materials

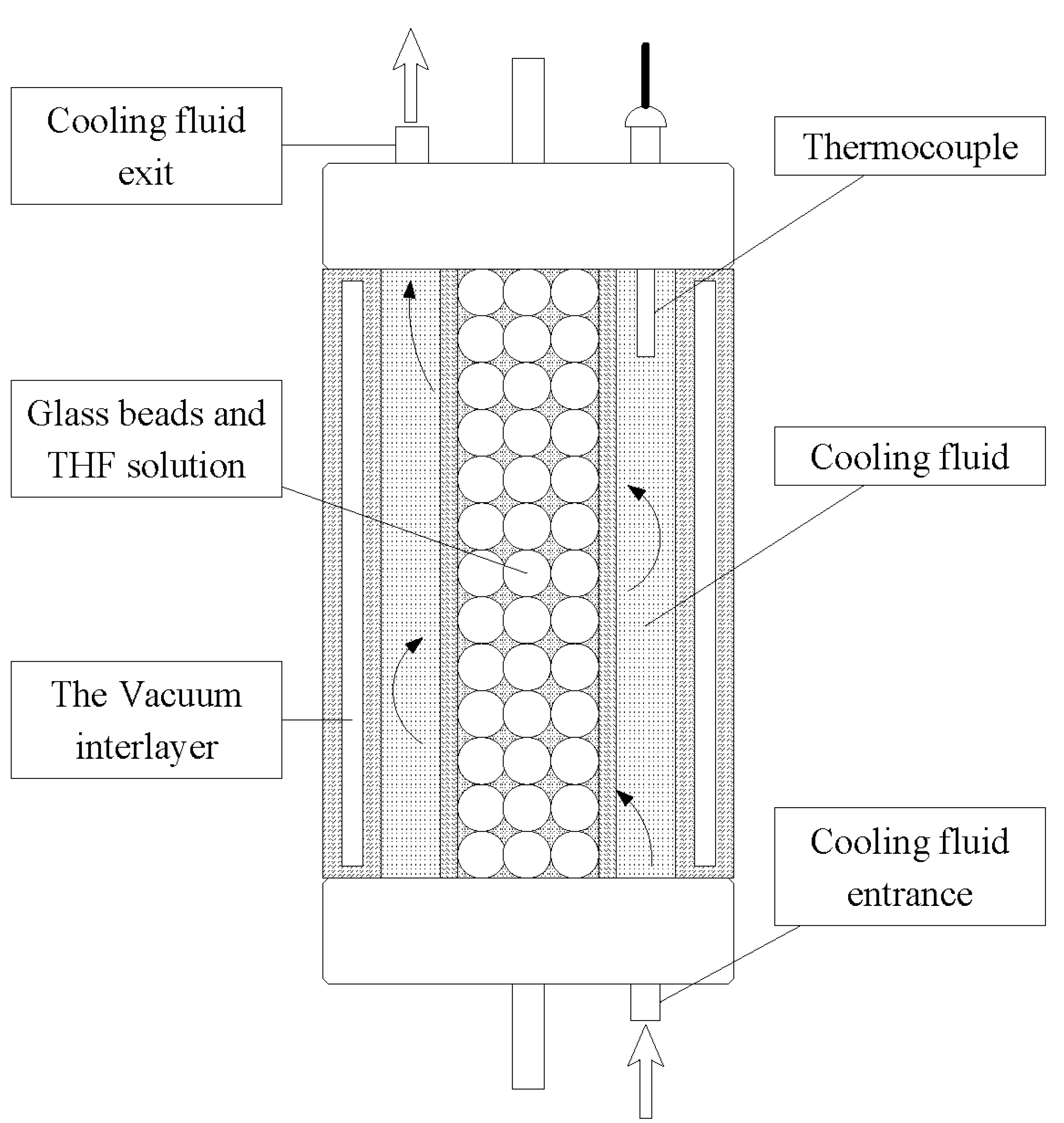
2.2. Procedure
3. Results and Discussion
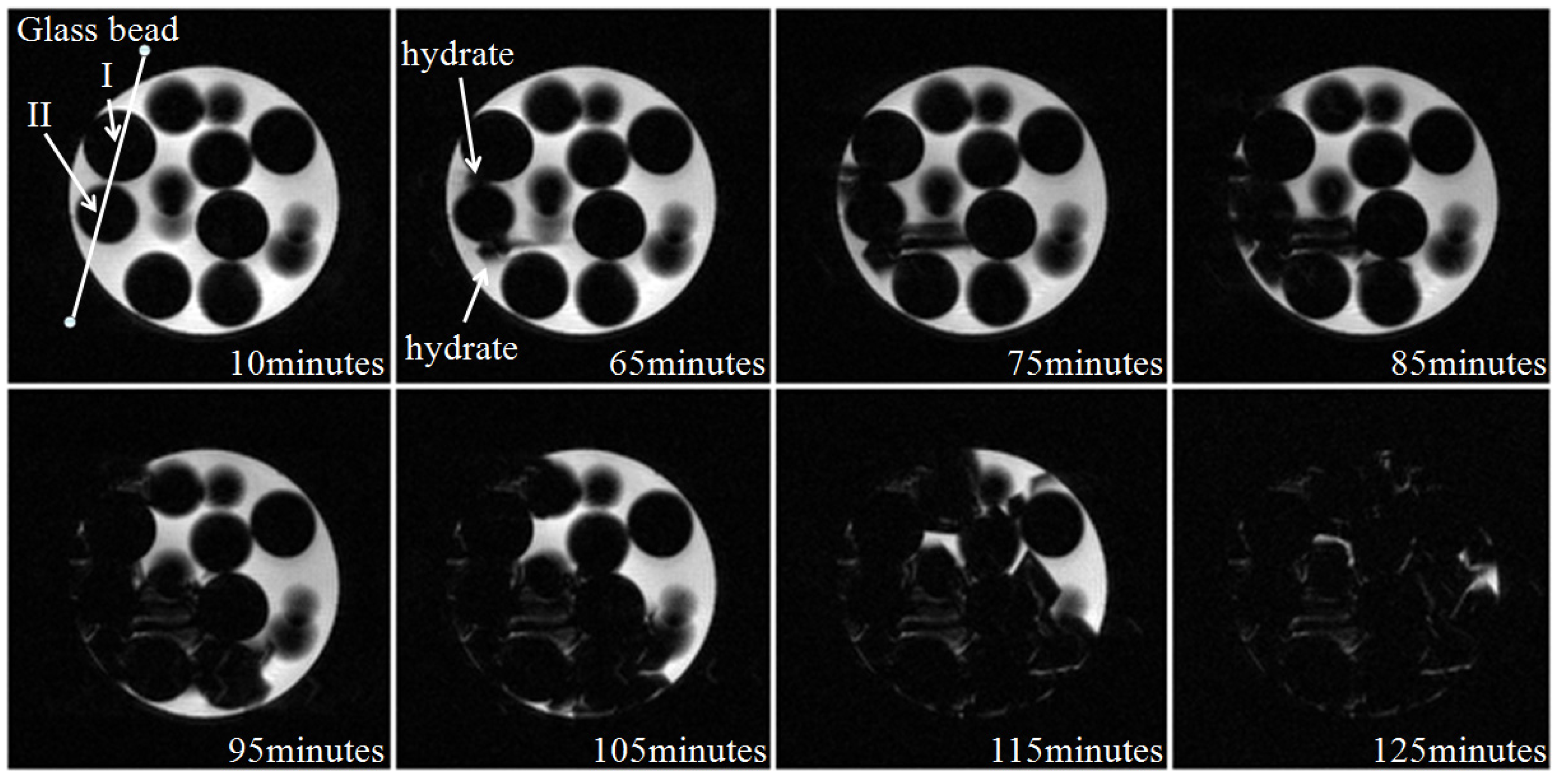
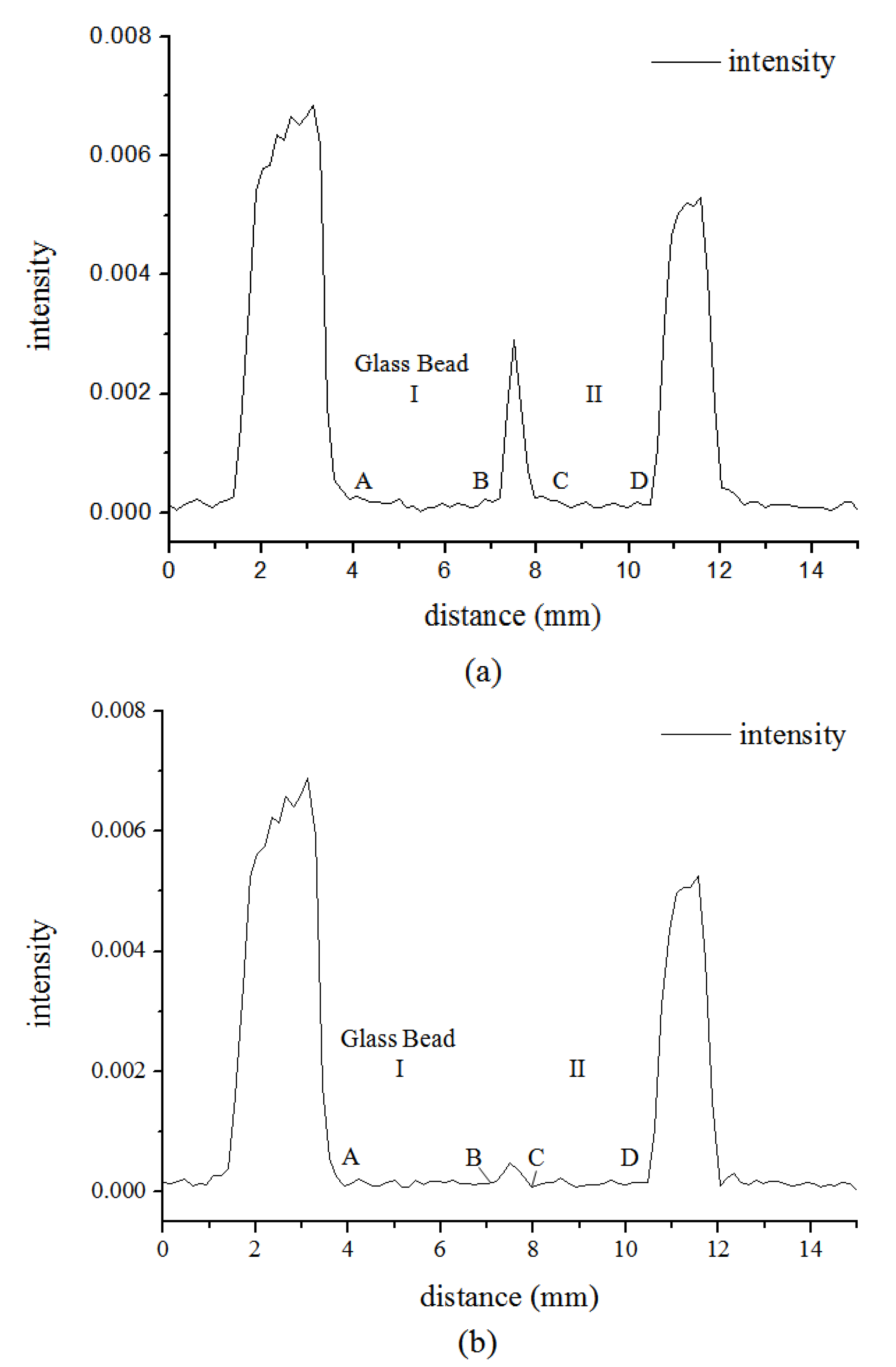
3.1. Microstructure of Hydrate in 19% THF Solution

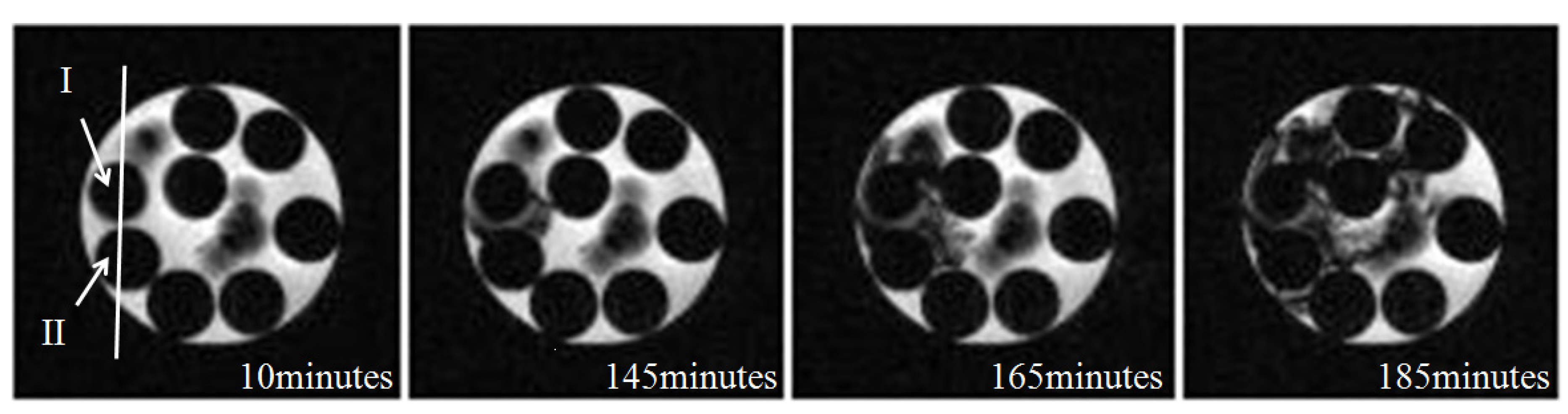
3.2. Microstructure of Hydrate in 11.4% and 5.7% THF Solution
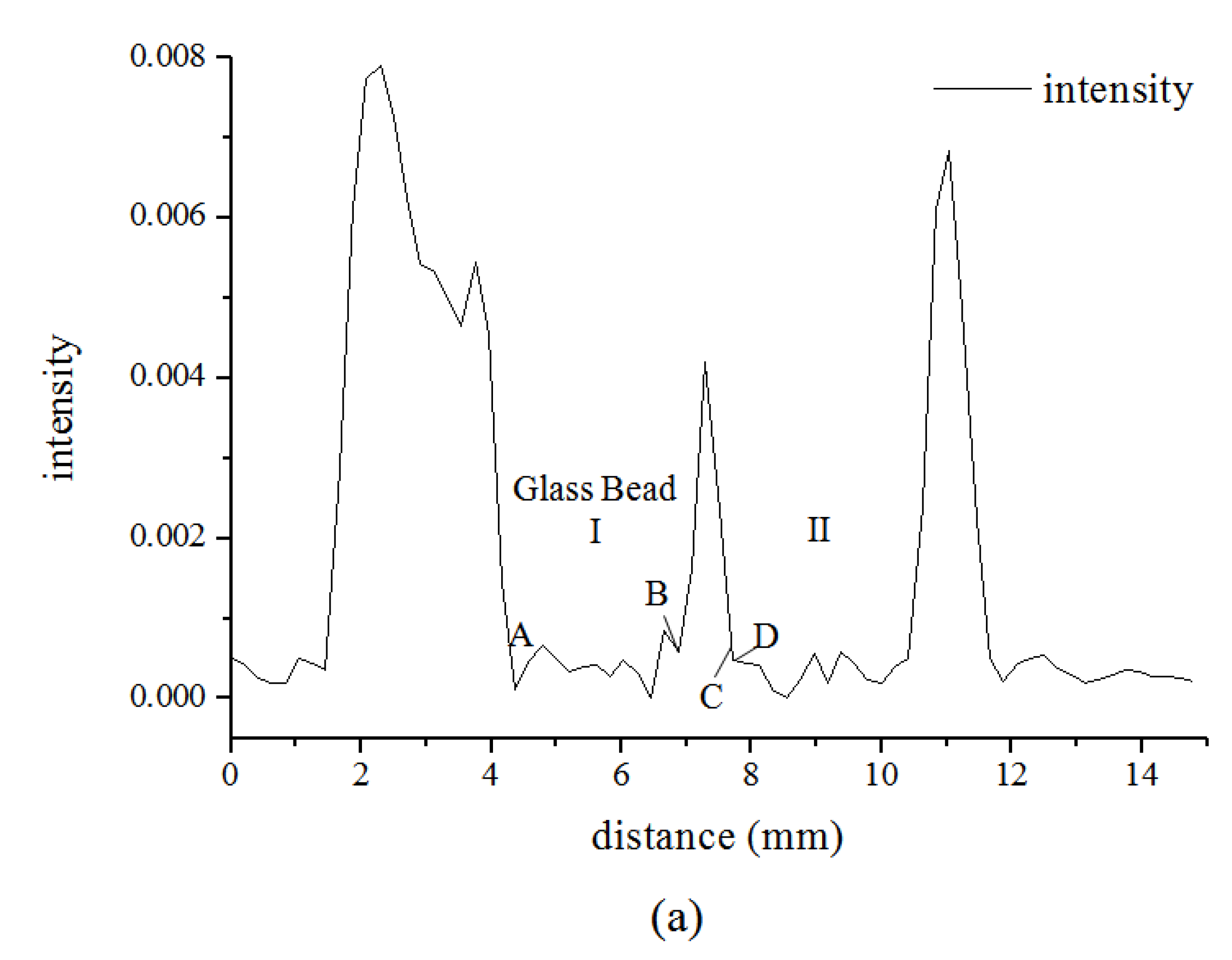
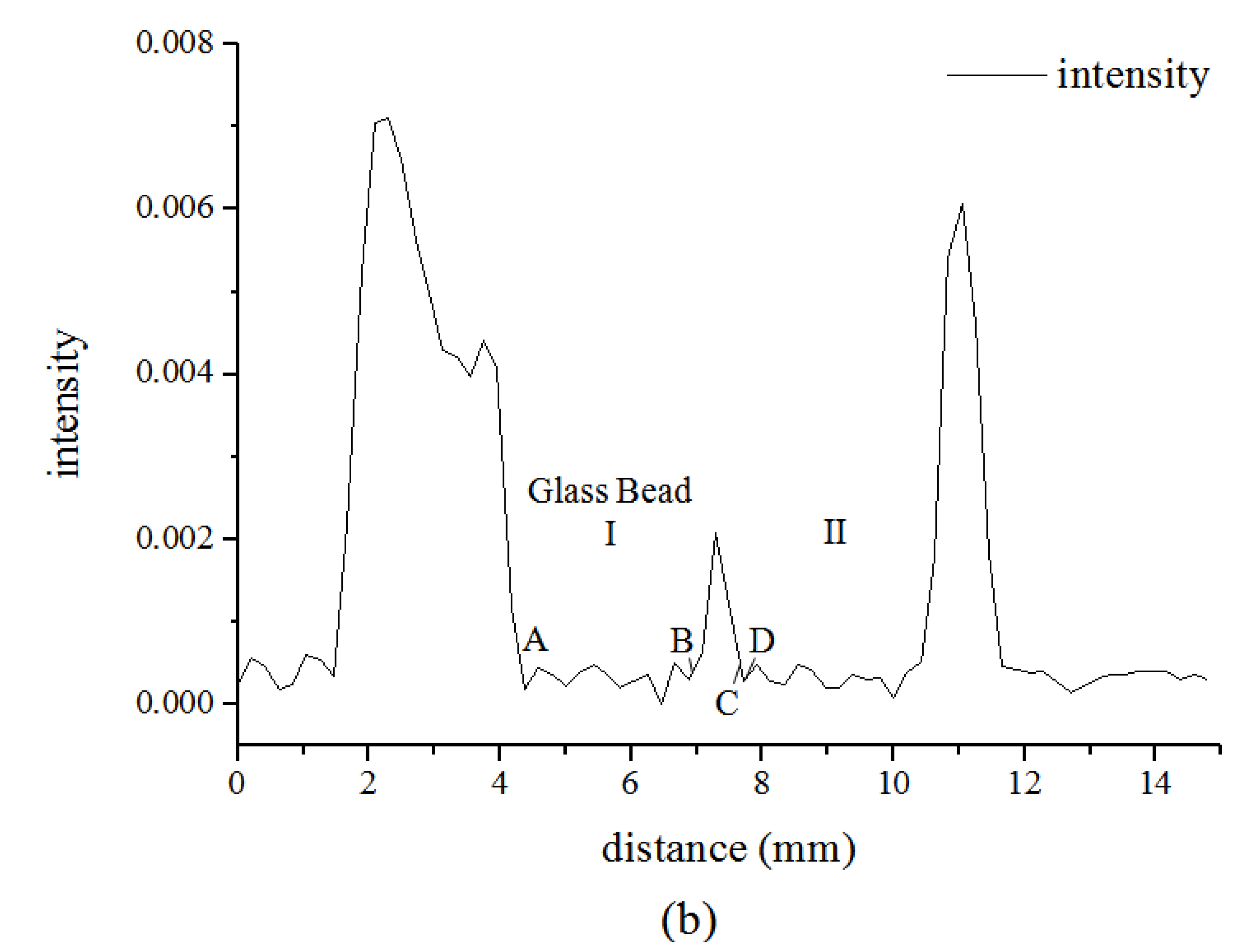
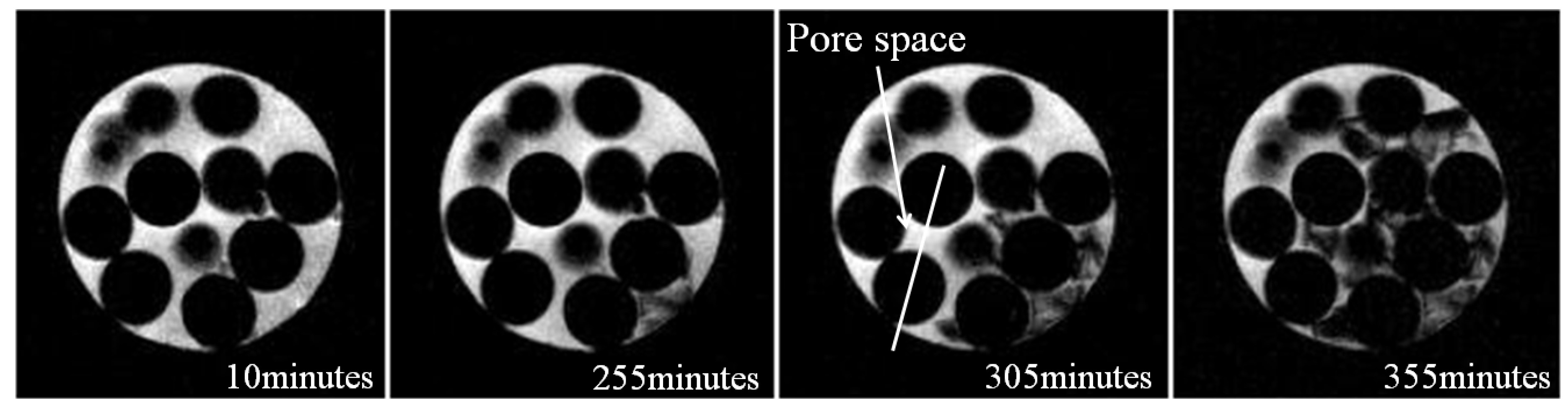
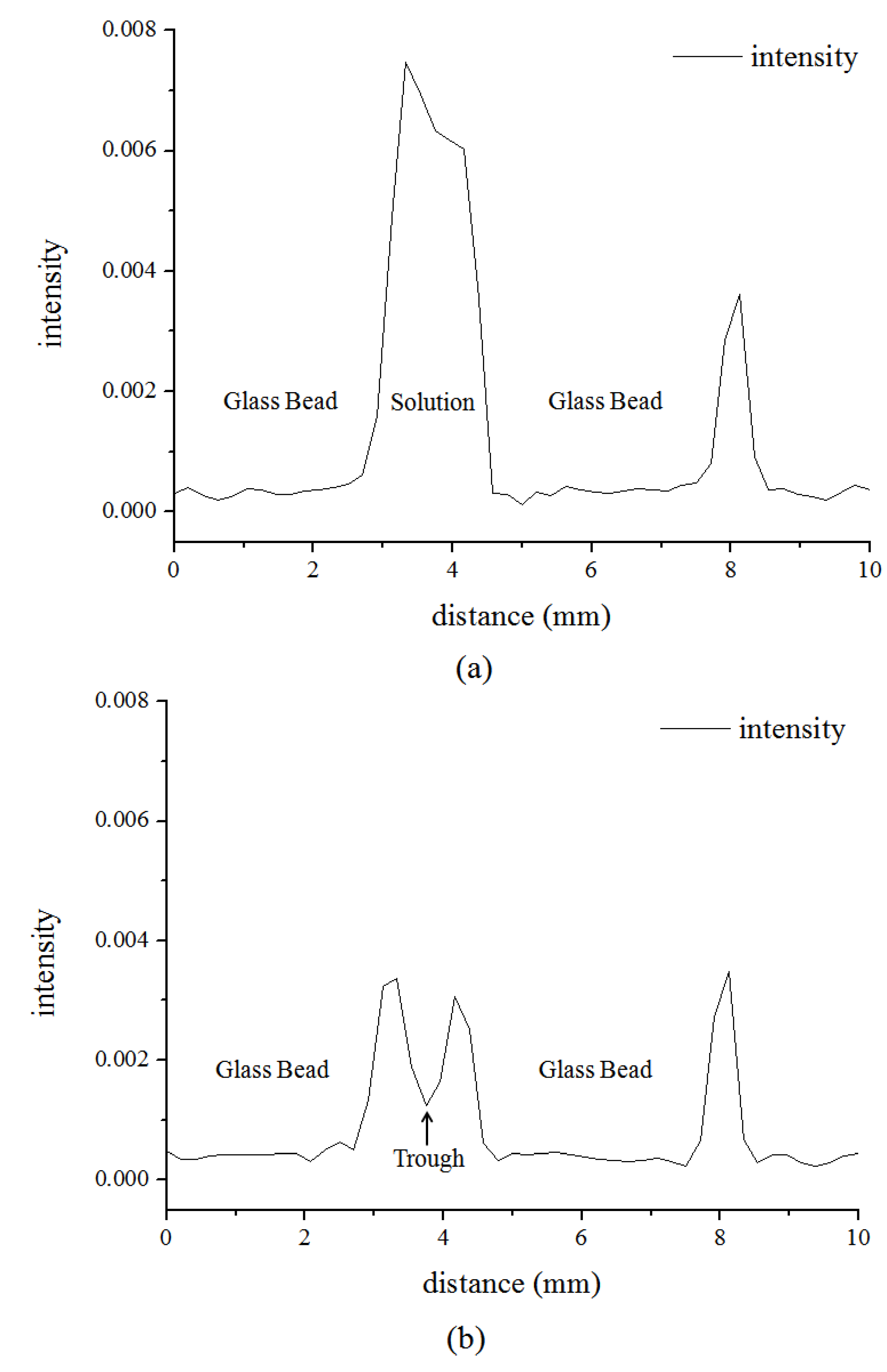
4. Conclusions
Acknowledgments
References
- Sloan, E.D. Clathrate Hydrates of Natural Gases; Marcel Dekker: New York, NJ, USA, 1990; p. 641. [Google Scholar]
- Makogon, Y.F. Hydrates of Hydrocarbons; Pennwell: Tulsa, OK, USA, 1997. [Google Scholar]
- Hacisalihoglu, B.; Demirbas, A.H.; Hacisalihoglu, S. Hydrogen from gas hydrate and hydrogen sulfide in the Black Sea. Energy Edu. Sci. Technol. 2008, 21, 109–115. [Google Scholar]
- Hovland, M.; Judd, A.G. Seabed Pockmarks and Seepages: Impact on Geology, Biology and the Marine Environment; Graham and Trotman: London, UK, 1998; p. 293. [Google Scholar]
- Dickens, G.R. Rethinking the global carbon cycle with a large, dynamic and microbially mediated gas hydrate capacitor. Earth Planet. Sci. Lett. 2003, 213, 169–183. [Google Scholar] [CrossRef]
- Kvenvolden, K.A. Gas hydrates—Global perspective and global change. Rev. Geophys. 1993, 31(2), 173–187. [Google Scholar] [CrossRef]
- Jones, K.W.; Kerkar, P.B.; Mahajan, D.; Lindquist, W.B.; Feng, H. Microstructure of natural hydrate host sediments. Nucl. Instrum. Meth. Phys. Res. 2007, 261, 504–507. [Google Scholar] [CrossRef]
- Kleinberg, R.L.; Flaum, C.; Griffin, D.D.; Brewer, P.G.; Malby, G.E.; Peltzer, E.T.; Yesinowski, J.P. Seafloor Nuclear Magnetic Resonance Assay of Methane Hydrate in Sediment and Rock. J. Geophys. Res. 2003, 108, 2508. [Google Scholar] [CrossRef]
- Huang, J.W.; Bellefleur, G.; Milkereit, B. Seismic modeling of multidimensional heterogeneity scales of Mallik gas hydrate reservoirs, Northwest Territories of Canada. J. Geophys. Res. 2009, 114, 1–22. [Google Scholar]
- Devarakonda, S.; Groysman, A.; Myerson, A.S. THF–water hydrate crystallization: an experimental investigation. J. Cryst. Growth 1999, 204, 525–538. [Google Scholar] [CrossRef]
- Kerkar, P.; Jones, K.W.; Kleinberg, R.; Lindquist, W.B.; Tomov, S.; Feng, H.; Mahajan, D. Direct observations of three dimensional growth of hydrates hosted in porous media. Appl. Phys. Lett. 2009, 95, 024102:1–024102:3. [Google Scholar] [CrossRef]
- Stern, L.A.; Lorenson, T.D.; Pinkston, J.C. Gas hydrate characterization and grain-scale imaging of recovered cores from the Mount Elbert Gas Hydrate Stratigraphic Test Well, Alaska North Slope. Mar. Petrol. Geol. 2009, 28, 394–403. [Google Scholar] [CrossRef]
- Guerin, G.; Goldberg, D.; Meltser, A. Characterization of in-situ elastic properties of gas-hydrate-bearing sediments on the Blake Ridge. J. Geophys. Res. B 1999, 104, 17781–17796. [Google Scholar] [CrossRef]
- Waite, W.F.; Winters, W.J.; Mason, D.H. Methane hydrate formation in partially water-saturated Ottawa sand. Am. Mineral. 2004, 89, 1202–1207. [Google Scholar]
- Kneafsey, T.J.; Tomutsa, L.; Moridis, G.J.; Seol, Y.; Freifeld, B.M.; Taylor, C.E.; Gupta, A. Methane hydrate formation and dissociation in partially saturated sand—measurements and observations. J. Pet. Sci. Eng. 2007, 56, 108–126. [Google Scholar] [CrossRef]
- Abegg, F.; Bohrmann, G.; Freitag, J.; Kuhs, W. Fabric of gas hydrate in sediments from Hydrate Ridge—Results from ODP Leg 204 samples. Geo-Mar. Lett. 2007, 27, 269–277. [Google Scholar] [CrossRef]
- Klapp, S.A.; Bohrmann, G.; Kuhs, W.F.; Murshed, M.M.; Pape, T.; Klein, H.; Techmer, K.S.; Heeschen, K.U.; Abegg, F. Microstructures of structure I and II gas hydrates from the Gulf of Mexico. Mar. Petrol. Geol. 2010, 27, 116–125. [Google Scholar] [CrossRef]
- Baldwin, B.A.; Moradi-Araghi, A.; Stevens, J.C. Monitoring hydrate formation and dissociation in sandstone and bulk with magnetic resonance imaging. Magn. Reson. Imag. 2003, 21, 1061–1069. [Google Scholar] [CrossRef]
- Mantle, M.D.; Sederman, A.J.; Gladden, L.F. Single- and two-phase flow in fixed-bed reactors: MRI flow visualization and lattice-Boltzmann simulations. Chem. Eng. Sci. 2001, 56 (2), 523–529. [Google Scholar] [CrossRef]
- Zhao, J.F.; Yao, L.; Song, Y.C.; Xue, K.H.; Cheng, C.X.; Liu, Y.; Zhang, Y. In situ observations by magnetic resonance imaging for formation and dissociation of tetrahydrofuran hydrate in porous media. Magn. Reson. Imag. 2011, 29, 281–288. [Google Scholar] [CrossRef]
- Leaist, D.G.; Murray, J.J.; Post, M.L.; Davidson, D.W. Enthalpies of decomposition and heat-capacities of ethylene-oxide and tetrahydrofuran hydrates. J. Chem. Phys. 1982, 86, 4175–4178. [Google Scholar] [CrossRef]
- Rueff, R.M.; Sloan, E.D. Effect of granular sediment on some thermal properties of tetrahydrofuran hydrate. Ind. Eng. Chem. 1985, 24, 882–885. [Google Scholar] [CrossRef]
- Lu, X.B.; Wang, L.; Wang, S.Y.; Li, Q.P. Study on the mechanical properties of the tetrahydrofuran hydrate deposit. In Proceedings of the 8th International Offshore and Polar Engineering Conference, Vancouver, Canada, 2008.
- Gough, S.R.; Davidson, D.W. Composition of tetrahydrofuran hydrate. Can. J. Chem. 1971, 49, 2691. [Google Scholar] [CrossRef]
- Kingston, E.; Clayton, C.; Priest, J. Gas hydrate growth morphologies and their effect on the stiffness and damping of a hydrate bearing sand. In Proceedings of the 6th International Conference on Gas Hydrates, Vancouver, Canada, 2008.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xue, K.; Zhao, J.; Song, Y.; Liu, W.; Lam, W.; Zhu, Y.; Liu, Y.; Cheng, C.; Liu, D. Direct Observation of THF Hydrate Formation in Porous Microstructure Using Magnetic Resonance Imaging. Energies 2012, 5, 898-910. https://doi.org/10.3390/en5040898
Xue K, Zhao J, Song Y, Liu W, Lam W, Zhu Y, Liu Y, Cheng C, Liu D. Direct Observation of THF Hydrate Formation in Porous Microstructure Using Magnetic Resonance Imaging. Energies. 2012; 5(4):898-910. https://doi.org/10.3390/en5040898
Chicago/Turabian StyleXue, Kaihua, Jiafei Zhao, Yongchen Song, Weiguo Liu, Weihaur Lam, Yiming Zhu, Yu Liu, Chuanxiao Cheng, and Di Liu. 2012. "Direct Observation of THF Hydrate Formation in Porous Microstructure Using Magnetic Resonance Imaging" Energies 5, no. 4: 898-910. https://doi.org/10.3390/en5040898



