Achieving a Green Solution: Limitations and Focus Points for Sustainable Algal Fuels
Abstract
:1. Introduction
2. Background
2.1. The Beginning for Algal Biofuels
2.2. Algae and Wastewater Treatment
| Wastewater | Algae | Growth Infrastructure | N Removal (%) | P Removal (%) | Productivity rate (mg/L/day) unless specified | Refs. |
|---|---|---|---|---|---|---|
| Synthetic | Scenedesmus obliquus | PBR | 70 | 94 | - | [35] |
| Synthetic | Chlorella vulgaris | PBR | 50 | 78 | - | [36] |
| Synthetic | Scenedesmus | PBR | 50–66 | >50 | 39.3 | [37] |
| Swine manure | Mixed species | Turf | 98 | 76 | - | [38] |
| Swine manure (pre-treated) | Chlorella sorokiniana | PBR | 65 (NH4) | - | - | [39] |
| Swine manure | Chlorella sorokiniana | PBR | 94–100 | 70–90 | - | [40] |
| Municipal wastewater | Scenedesmus, micratinium, chlorella, | Open pond | 96 | 99 | 24.4 (Lipid) | [27] |
| Municipal wastewater | Scenedesus | PBR | 99 | 99 | 250 | [41] |
| Municipal wastewater | Cyanobacteria | PBR | 88.3 | 64.8 | 10.9 g/m2/day | [42] |
| Municipal wastewater | Chlorella | PBR | 82.4 | 90.6 | 0.948/day | [43] |
| Dairy manure | Mixed culture | Turf scrubber | 51–83 | 62–91 | 8.3–25.1 | [44] |
2.3. The Possibility of Carbon Mitigation
| Algae Species | Gas | CO2 (%) | Productivity (g/m2/day) | Refs. |
|---|---|---|---|---|
| Chlorella sp. | Air | Air | 0.68 | [46] |
| Chlorella sp. | Synthetic | 2 | 1.45 | [46] |
| Chlorella sp. | Synthetic | 5 | 0.90 | [46] |
| Chlorella sp. | Synthetic | 10 | 0.11 | [46] |
| Chlorella vulgaris | Air | Air | 0.04 | [54] |
| Chlorella vulgaris | Flue gas (MSW incinerator) | 10–13 | 2.50 | [47] |
| Spirulina sp. | Synthetic | Air | 0.14 | [51] |
| Spirulina sp. | Synthetic | 6 | 0.22 | [51] |
| Spirulina sp. | Synthetic | 12 | 0.17 | [51] |
| S. Obliquus | Synthetic | Air | 0.04 | [51] |
| S. Obliquus | Synthetic | 6 | 0.10 | [51] |
| S. Obliquus | Synthetic | 12 | 0.14 | [51] |
2.4. Comparison of Open Ponds and Photo-Bioreactors
| Raceway Pond | Photobioreactor | Refs. | |
|---|---|---|---|
| Estimated productivity (g/m2/day) | 11 | 27 | [55] |
| Advantages | Low energy Simple technology Inexpensive Well researched | High productivity High controllability Small area required Concentrated biomass | [55] |
| Disadvantages | Low productivity Contamination Large area required High water use Dilute biomass | High energy Expensive Less researched | [55] |
2.5. Biomass Processing
2.5.1. Harvesting
| Flocculant | Algae | Removal (%) | Dosage (mg/L) | Media type | Refs. |
|---|---|---|---|---|---|
| FeCl3 | Chlorella | 98 | 250 | Piggery wastewater | [40] |
| FeCl3 | S. obliquus | 95 | 100 | ||
| Chlorococcum sp. | 90 | 150 | |||
| Fe2(SO4)3 | Chlorella | 90 | 250 | ||
| S. obliquus | 98 | 150 | |||
| C. sorokiniana | 98 | 250 | |||
| Chitosan | Spirulina, Oscillatoria, Chlorella | >90 | 15 | Nutrient media | [68] |
| Polyelectrolyte (Puriflocs 601 & 602) | Chlorella, Scenedesmus | 95 | 3 | Sewage | [59] |
2.5.2. Sedimentation
2.5.3. Flotation
2.5.4. Centrifugation
| Algae species | Harvesting Method | % TSS of Concentrate | Concentration Factor | Energy Requirement (kWh) | Reliability | Refs. |
|---|---|---|---|---|---|---|
| Coelastrum | Gravity filtration | 6 | 60 | 0.4 | Good | [62] |
| Coelastrum | Pressure filtration | 22–27 | 245 | 0.88 | Very high | [62] |
| Scenedesmus, Coelastrum proboscideum | Centrifuge (Westfalia self-cleaning) | 12 | 120 | 1 | Very good | [62] |
| Scenedesmus, Coelastrum proboscideum | Centrifuge (Westfalia screw) | 22 | 11 | 8 | Very good | [62] |
2.6. Fuels
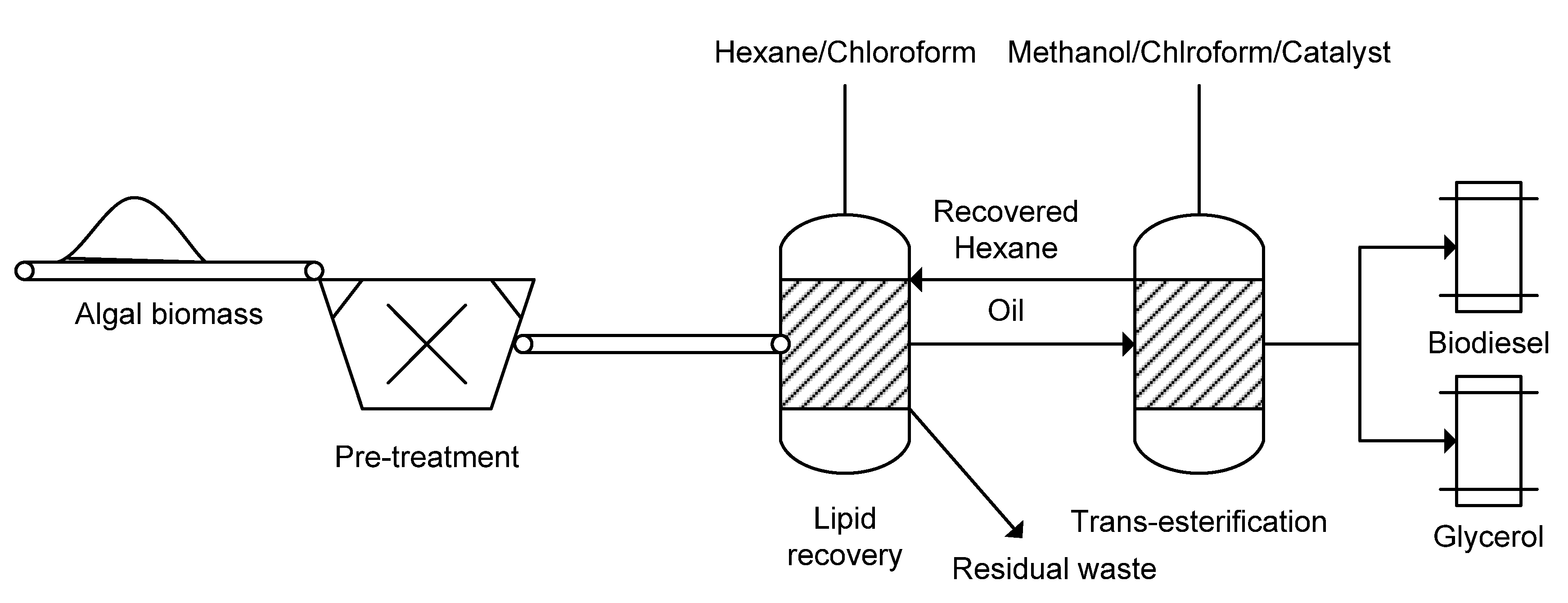
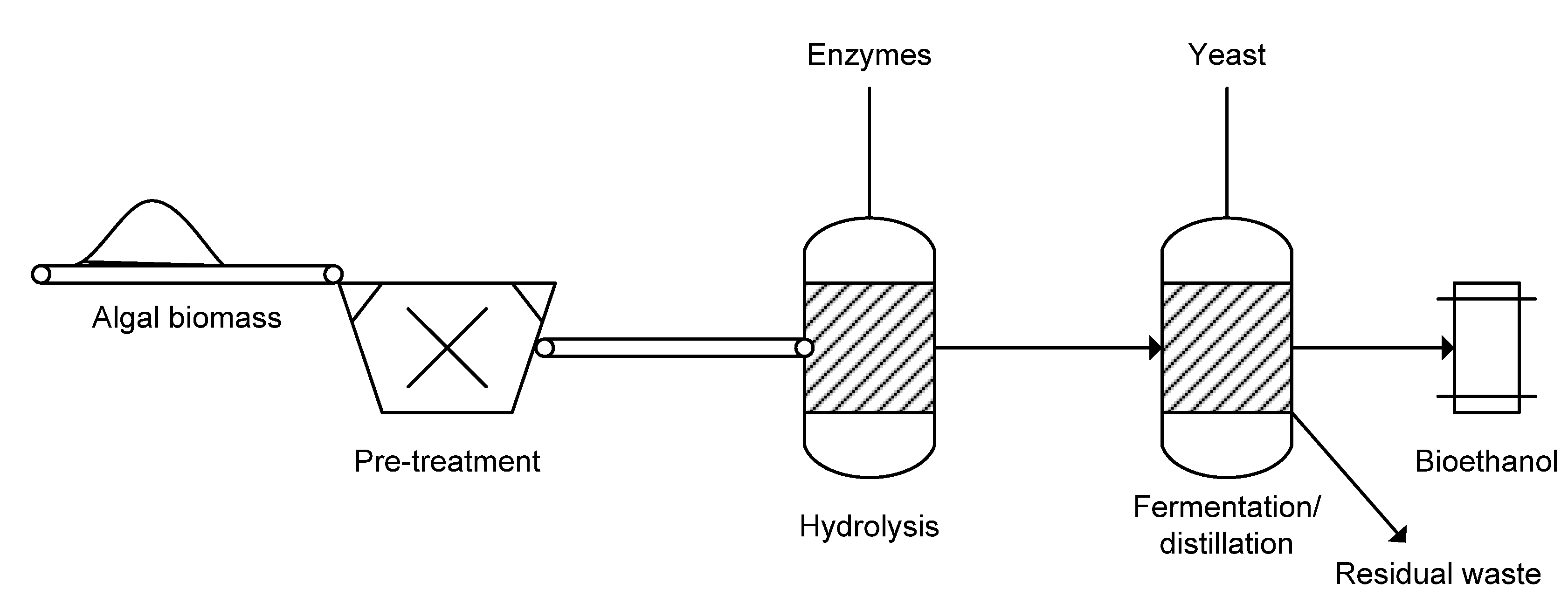
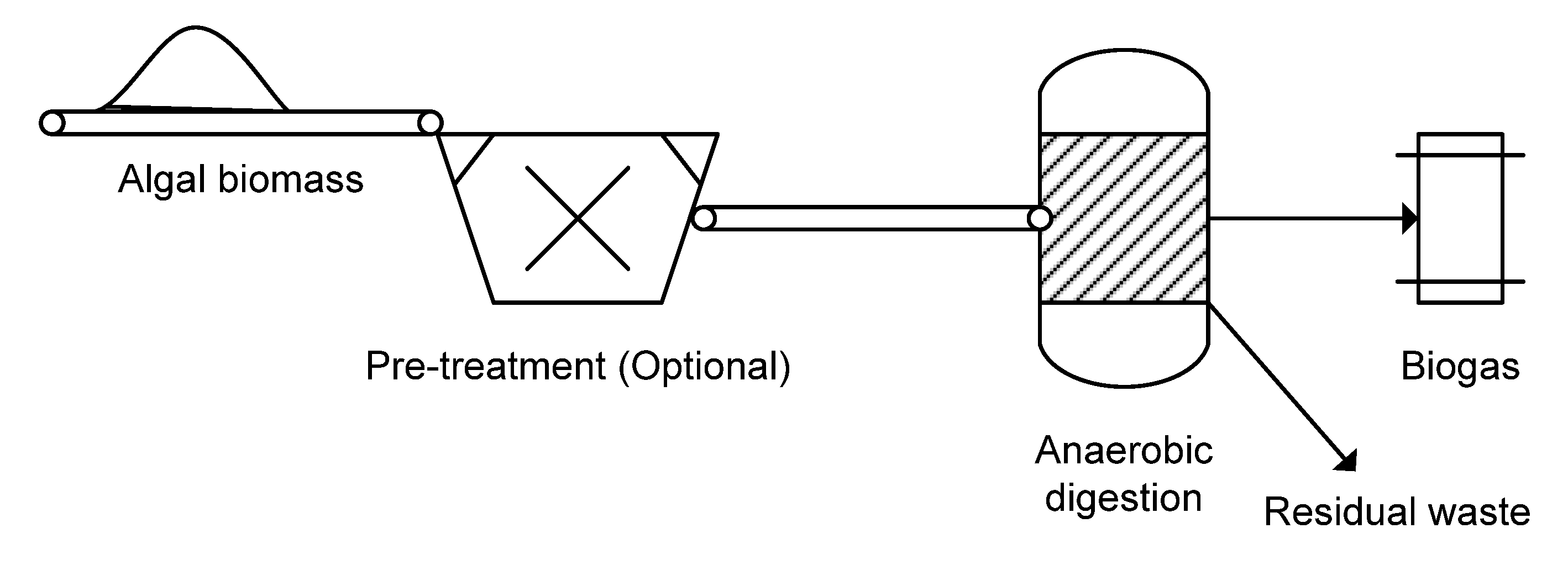
2.6.1. Biodiesel
2.6.2. Bioethanol
2.6.3. Biogas
| Substrate | L CH4/g VS |
|---|---|
| Proteins | 0.851 |
| Lipids | 1.014 |
| Carbohydrates | 0.415 |
| Algae Species | Proteins (%) | Lipids (%) | Carbohydrates (%) | CH4 (L/g) (Theoretical) [85] | CH4 (L/g) (Experimental) | Refs. |
|---|---|---|---|---|---|---|
| Euglena gracilis | 39–61 | 14–20 | 14–18 | 0.52–0.8 | - | [85] |
| Chlamydomonas reinhardtii | 48 | 21 | 17 | 0.69 | 0.59 | [88] |
| Chlorella pyrenoidosa | 57 | 2 | 26 | 0.8 | 0.17–0.32 (Chlorella-Scenedesmus) | [2] |
| Chlorella vulgaris | 51–58 | 14–22 | 12–17 | 0.63–0.79 | 0.24 | [8] |
| Dunaliella salina | 57 | 6 | 32 | 0.68–0.74 | 0.44–0.45 (Dunaliella) | [85] |
| Spirulina maxima | 60–71 | 6–7 | 13–16 | 0.63–0.74 | 0.32–0.31 (Spirulina) | [85] |
| Spirulina platensis | 46–63 | 4–9 | 8–14 | 0.47–0.69 | 0.32–0.31 (Spirulina) | [85] |
| Scenedesmus obliquus | 50–56 | 12–14 | 10–17 | 0.59–0.69 | 0.17–0.32 (Chlorella-Scenedesmus) | [2] |
3. Limitations
3.1. Open Pond Cultivation and Species Control
3.2. Water Resource Scarcity
3.3. Energy Consumption
| LCA Study | Energy Balance | LCA Method | Comments | Refs. |
|---|---|---|---|---|
| Algae-biodiesel | 0.95 | Well to fuel | Not taking into account wastewater treatment or CO2 from flue gas, both of these contributing the most energy use, cultivation in ponds | [96] |
| Algae-biodiesel | 6.7 | Well to pump | Co-product allocation provides greatest energy recovery, wastewater assumed to provide nutrients, harvesting greatest energy consumer, cultivation in ponds | [94] |
| Algae-biodiesel | 1.34 | Well to fuel | Wet biomass processing and low nitrogen addition for high lipid content, anaerobic digestion of oil cake essential for positive energy balance, cultivation in ponds | [76] |
| Algae-biodiesel | 3.05 | Cultivation | Considers just the cultivation stage and energy content of the oil in the biomass, cultivation in ponds | [55] |
| Algae-bioethanol | 5 | Well to wheel | 80% heat exchange efficiency | [97] |
| Algae-bioelectricity (combustion) | 4.10 | Well to wheel | Use of flue gas for CO2 | [24] |
3.4. Fertilisers
3.5. Carbon Dioxide
3.6. Environmental Impacts
| Feedstock | Biofuel | Cultivation | LCA Method | GHG Emissions (CO2e) kg CO2/MJ | Refs. |
|---|---|---|---|---|---|
| Algae | Biodiesel | PBR | Well to wheel | 0.32 | [95] |
| Algae | Biodiesel | Raceway pond | Well to fuel | 0.057 | [96] |
| Well to wheel | 0.18 | [95] | |||
| Well to pump | 0.2 | [76] | |||
| Well to fuel | −0.021 | [94] | |||
| Canola | Biodiesel | Agricultural | Well to fuel | −0.05 | [96] |
| Soy bean | Biodiesel | Agricultural | 0.030 | [102] | |
| Corn | Bioethanol | Agricultural | Well to fuel | −0.082 | [96] |
| Switchgrass | Bioethanol | Agricultural | Well to fuel | −0.076 | [96] |
| Well to fuel | −0.024 | [103] | |||
| Poplar | Bioethanol | Agricultural | Well to fuel | −0.024 | [103] |
4. A Sustainable Vision
4.1. Integrated and Localised Solutions
| Industry | Total N (mg/L) | Total P (mg/L) | Flue Gas Source | Advantages | Disadvantages |
|---|---|---|---|---|---|
| WWTP a | 15 b (NH4) | 11.5 b (PO4) | AD co-generator | Provides tertiary treatment Abatement of CO2 from co-digester AD of biomass available | Land requirement Contamination of wastewater could affect algae |
| Farm | 1210 c 5600 d | 303 c 1600 d | AD co-generator Composting facility | Treatment of excess nutrients Treated biomass for feed Available land | Potentially no CO2 source High nutrient loading may require dilution |
| Brewery/distillery | 56.5 e (NH4) 51 f 560–834 g (TKN) 3–106 h (NH3) | 177–215 e 57–325.8 h (PO4) | Fermentation process Boiler flue gas | Wastewater treatment Biomass for co-generator produced Sustainability targets | Land area requirement low pH wastewater |
| Oil refinery | 8 i (NH3) | 0.1 i | Flue gases | Abatement of GHGs Sustainability targets | Wastewater/flue gas may be too toxic Low nutrient loading |
4.2. Algal Species
4.3. Cultivation Methods
4.4. Low Energy Harvesting
4.5. Suggested Conversion Techniques
4.6. Resource Conservation and Recycling
4.7. Current State of Concept
4.8. Where to Go from Here
5. Conclusions
Acknowledgments
References
- Solazyme and algenol to make more algae. Chem. Eng. News 2011, 89, 20–21.
- Golueke, C.G.; Oswald, W.J.; Gotaas, H.B. Anaerobic digestion of algae. Appl. Microbiol. 1957, 5, 47–55. [Google Scholar] [PubMed]
- Golueke, C.G.; Oswald, W.J. Power from solar energy-via algae-produced methane. Sol. Energy 1963, 7, 86–92. [Google Scholar] [CrossRef]
- Oswald, W.J.; Gotaas, H.B.; Golueke, C.G.; Kellen, W.R. Algae in waste treatment. Sewage Ind. Wastes 1957, 29, 437–455. [Google Scholar]
- Oswald, W.J. My sixty years in applied algology. J. Appl. Phycol. 2003, 15, 99–106. [Google Scholar] [CrossRef]
- Saxena, V.K.; Tandon, S.M.; Singh, K.K. Anaerobic-digestions of green filamentous algae and waterhyacinth for methan production. Natl. Acad. Sci. Lett. 1984, 7, 283–284. [Google Scholar]
- Cecchi, F.; Vallini, G.; Pavan, P.; Bassetti, A.; Mataalvarez, J. Management of macroalgae from Venice lagoon through anaerobic co-digestion and co-composting with municipal solid-waste (msw). Water Sci. Technol. 1993, 27, 159–168. [Google Scholar]
- Ras, M.; Lardon, L.; Bruno, S.; Bernet, N.; Steyer, J.P. Experimental study on a coupled process of production and anaerobic digestion of Chlorella vulgaris. Bioresour. Technol. 2011, 102, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Hemanathan, K. Biodiesel production from freshwater algae. Energy Fuel 2009, 23, 5448–5453. [Google Scholar] [CrossRef]
- Weyer, K.M.; Bush, D.R.; Darzins, A.; Willson, B.D. Theoretical maximum algal oil production. Bioenergy Res. 2010, 3, 204–213. [Google Scholar] [CrossRef]
- Rodolfi, L.; Zittelli, G.C.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Yoo, C.; Jun, S.Y.; ahn, C.Y.; Oh, H.M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Production of biodiesel from algae oils. Energy Source A 2009, 31, 163–168. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P. A Look Back at the U.S. Department of Energy’s Aquatic Species Program—Biodiesel from Algae; Golden: New York, NY, USA, 1998. [Google Scholar]
- Chen, M.; Tang, H.Y.; Ma, H.Z.; Holland, T.C.; Ng, K.Y.S.; Salley, S.O. Effects of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour. Technol. 2011, 102, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Sturm, B.S.M.; Peltier, E.; Smith, V.; Denoyelles, F. Controls of microalgal biomass and lipid production in municipal wastewater-fed bioreactors. Environ. Prog. Sustain. Energy 2012, 31, 10–16. [Google Scholar] [CrossRef]
- Ruiz-Marin, A.; Mendoza-Espinosa, L.G.; Stephenson, T. Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour. Technol. 2010, 101, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.F.; Oswald, W.J.; Gotaas, H.B.; Lynch, V. Algae symbiosis in oxidation ponds. 1. Growth characteristics of Euglena gracilis cultured in sewage. Sewage Ind. Wastes 1951, 23, 1337–1355. [Google Scholar]
- Oswald, W.J.; Gotaas, H.B. Photoshynthesis in the algae-discussion. Ind. Eng. Chem. 1956, 48, 1457–1458. [Google Scholar]
- Oswald, W.J.; Gotaas, H.B.; Ludwig, H.F.; Lynch, V. Algae symbiosis in oxidation ponds. 2. Growth characteristics of Chlorella pyrenoidosa cultured in sewage. Sewage Ind. Wastes 1953, 25, 26–37. [Google Scholar]
- Oswald, W.J.; Gotaas, H.B.; Ludwig, H.F.; Lynch, V. Algae symbiosis in oxidation ponds. 3. Photosyntehtic oxygenation. Sewage Ind. Wastes 1953, 25, 692–705. [Google Scholar]
- Shelef, G. Combined Systems for Algal Wastewater Treatment and Reclamation and Protein Production; Technion: Haifa, Israel, 1981. [Google Scholar]
- Clarens, A.F.; Nassau, H.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental impacts of algae-derived biodiesel and bioelectricity for transportation. Environ. Sci. Technol. 2011, 45, 7554–7560. [Google Scholar] [CrossRef] [PubMed]
- Orpez, R.; Martinex, M.E.; Hodaifa, G.; El Yousfi, F.; Jbari, N.; Sanchez, S. Growth of the microalga Botryococcus braunii in secondarily treated sewage. Desalination 2009, 246, 625–630. [Google Scholar] [CrossRef]
- Delanoue, J.; Laliberte, G.; Proulx, D. Algae and waste-water. J. Appl. Phycol. 1992, 4, 247–254. [Google Scholar] [CrossRef]
- Woertz, I.; Feffer, A.; Lundquist, T.; Nelson, Y. Algae grown on dairy and municipal wastewater for simultaneous nutrient removal and lipid production for biofuel feedstock. J. Environ. Eng. 2009, 135, 1115–1122. [Google Scholar] [CrossRef]
- Patel, A.; Zhu, J.; Nakhla, G. Simultaneous carbon, nitrogen and phosphorous removal from municipal wastewater in a circulating fluidized bed bioreactor. Chemosphere 2006, 65, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, A.C.; Mulbry, W.W. Recovery of dairy manure nutrients by benthic freshwater algae. Bioresour. Technol. 2002, 84, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Shalaru, V.M.; Shalaru, V.V.; Dudnicenco, T.I.; Ichim, M.D. The use of algae in wastewater treatment from animal farms. Phycologia 2005, 44, 93–93. [Google Scholar]
- Pizarro, C.; Mulbry, W.; Blersch, D.; Kangas, P. An economic assessment of algal turf scrubber technology for treatment of dairy manure effluent. Ecol. Eng. 2006, 26, 321–327. [Google Scholar] [CrossRef]
- Park, K.Y.; Lim, B.R.; Lee, K. Growth of microalgae in diulted process water of the animal wastewater treatment plant. Water Sci. Technol. 2009, 59, 2111–2116. [Google Scholar] [CrossRef] [PubMed]
- Chinnasamy, S.; Bhatnagar, A.; Claxton, R.; Das, K.C. Biomass and bioenergy production potential of microalgae consortium in open and closed bioreactors using untreated carpet industry effluent as growth medium. Bioresour. Technol. 2010, 101, 6751–6760. [Google Scholar] [CrossRef] [PubMed]
- Travieso, L.; Benitez, F.; Sanchez, E.; Borja, R.; Leon, M.; Raposo, F.; Rincon, B. Performance of a laboratory-scale microalgae pond for secondary treatment of distillery wastewaters. Chem. Biochem. Eng. Q. 2008, 22, 467–473. [Google Scholar]
- Fierro, S.; del Pilar Sanchez-Saavedra, M.; Copalcua, C. Nitrate and phosphate removal by chitosan immobilized Scenedesmus. Bioresour. Technol. 2008, 99, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Kapdan, I.K. Batch kinetics of nitrogen and phosporus removal from synthetic wastewater by algae. Ecol. Eng. 2006, 28, 67–70. [Google Scholar] [CrossRef]
- Voltolina, D.; Gomez-Villa, H.; Correa, G. Nitrogen removal and recycling by Scenedesmus obliquus in semicontinuous cultures using artificial wastewater and a simulated light and temperature cycle. Bioresour. Technol. 2005, 96, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Kebede-Westhead, E.; Pizarro, C.; Mulbry, W.W. Treatment of swine manure effluent using freshwater algae: Production, nutrient recovery, and elemental composition of algal biomass at four effluent loading rates. J. Appl. Phycol. 2006, 18, 41–46. [Google Scholar] [CrossRef]
- Gonzalez, C.; Marciniak, J.; Villaverde, S.; Garcia-Encina, P.A.; Munoz, R. Microalgae-based processes for the biodegradation of pretreated piggery wastewaters. Appl. Microbiol. Biotechnol. 2008, 80, 891–898. [Google Scholar] [CrossRef] [PubMed]
- De Godos, I.; Gonzalez, C.; Becares, E.; Garcia-Encina, P.A.; Munoz, R. Simultaneous nutrients and carbon removal during pretreated swine slurry degradation in a tubular biofilm photobioreactor. Appl. Microbiol. Biotechnol. 2009, 82, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Di Termini, I.; Prassone, A.; Cattaneo, C.; Rovatti, M. On the nitrogen and phosphorus removal in algal photobioreactors. Ecol. Eng. 2011, 37, 976–980. [Google Scholar] [CrossRef]
- Su, Y.; Mennerich, A.; Urban, B. Municipal wastewater treatment and biomass accumulation with a wastewater-born and settleable algal-bacterial culture. Water Res. 2011, 45, 3351–3358. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kondrad, S.; Pizarro, C.; Kebede-Westhead, E. Cultivation of green algae Chlorella sp. in different wastwaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol. 2008, 162, 1174–1186. [Google Scholar] [CrossRef]
- Mulbry, W.; Kondrad, S.; Buyer, J. Treatment of dairy and swine manure effluents using freshwater algae: Fatty acid content and composition of algal biomass at different manure loading rates. J. Appl. Phycol. 2008, 20, 1079–1085. [Google Scholar] [CrossRef]
- Guine, J.B.; Heijungs, R.; Van Der Voet, E. A greenhouse gas indicator for bioenergy: Some theoretical issues with practical implications. Int. J. Life Cycle Ass. 2009, 14, 328–339. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Kao, C.Y.; Chen, C.H.; Kuan, T.C.; Ong, S.C.; Lin, C.S. Reduction of CO2 by a high density culture of chlorella sp in a siemicontinuous photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Douskova, I.; CDoucha, J.; Livansky, K.; Machat, J.; Novak, P.; Umysova, D.; Zachleder, V.; Vitova, M. Simulataneous flue gas bioremediation and reduction of microalgal biomass production costs. Appl. Microbiol. Biotechnol. 2009, 82, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Brune, D.E.; Lundquist, T.; Benemann, J. Microalgal biomass for greenhouse gas reductions: Potential for replacement of fossil fuels and animal feeds. J. Environ. Eng. 2009, 135, 1136–1144. [Google Scholar] [CrossRef]
- Maeda, K.; Owada, M.; Kimura, N.; Omata, K.; Karube, I. CO2 fixation from the flue-gas on cola-fired thermal power-plant by microalgae. Energy Convers. Manag. 1995, 36, 717–720. [Google Scholar] [CrossRef]
- Stepan, D.J.; Shockey, R.E.; Moe, T.A.; Dorn, R. Subtask 2.3—Carbon Dioxide Sequestering Using Microalgal Systems; U.S. Department of Energy: Pittsburgh, PA, USA, 2002.
- de Morais, M.G.; Costa, J.A.V. Isolation and selection of microalgae from coal fired thermoelectric power plant for biofixation of carbon dioxide. Energy Convers. Manag. 2007, 48, 2169–2173. [Google Scholar] [CrossRef]
- De Morais, M.G.; Costa, J.A.V. Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J. Biotechnol. 2007, 129, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Doucha, J.; Straka, F.; Livansky, K. Utilization of flue gas for cultivation of microalgae (chlorella sp) in an outdoor open thin-layer photobioreactor. J. Appl. Phycol. 2005, 17, 403–412. [Google Scholar] [CrossRef]
- Scragg, A.H.; Illman, A.M.; Carden, A.; Shales, S.W. Growth of microalgae with increased calorific values in a tubular bioreactor. Biomass Bioenergy 2022, 23, 67–73. [Google Scholar] [CrossRef]
- Jorquera, O.; Kiperstok, A.; Sales, E.A.; Embirucu, M.; Ghirardi, M.L. Comparative energy lifecycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresour. Technol. 2010, 101, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Soratana, K.; Landis, A.E. Evaluating industrial symbiosis and algae cultivation from a life cycle perspective. Bioresour. Technol. 2011, 102, 6892–6901. [Google Scholar] [CrossRef] [PubMed]
- Grima, E.M.; Belarbi, E.H.; Fernandez, F.G.A.; Medina, A.R.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef] [PubMed]
- Benemann, J.; Koopman, B.; Wieissman, J.; Eisenberg, D.; Goebel, R. Development of Microalgae Harvesting and High-Rate Pond Technologies in California; Elsevier/North-Holland Biomedical Press: Amsterdam, The Netherlands, 1980. [Google Scholar]
- Golueke, C.G.; Oswald, W.J. Harvesting and processing sewage-grown planktonic algae. Water Pollut. Control Fed. 1965, 37, 471–498. [Google Scholar]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Uduman, N.; Qi, Y.; Danquah, M.K.; Forde, G.M.; Hoadley, A. Dewatering of microalgal cultures: A major bottleneck to algae-based fuels. J. Renew. Sustain. Energy 2010, 2, 12701–12716. [Google Scholar] [CrossRef]
- Mohn, F.M. Experiences and strategies in the recovery of biomass from mass cultures of microalgae. In Algal Biomass; Shelef, G., Soeder, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1980. [Google Scholar]
- Spilling, K.; Seppala, J.; Tamminen, T. A potential low-cost method for harvesting microalgae using high pH and regulation of particle encournter rate. Water Sci. Technol. 2009, 52, 9–18. [Google Scholar]
- Gutzeit, G.; Lorch, D.; Weber, A.; Engels, M.; Neis, U. bioflocculent algal-bacterial biomass improves low-cost wastewater treatment. Water Sci. Technol. 2005, 52, 9–18. [Google Scholar] [PubMed]
- Salim, S.; Bosma, R.; Vermue, M.H.; VWijffels, R.H. Harvesting of microalgae by bioflocculations. J. Appl. Phycol. 2011, 23, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Sukenik, A.; Bilanovic, D.; Shelef, G. Flocculation of microalgae in brackish and sea waters. Biomass 1988, 15, 187–199. [Google Scholar] [CrossRef]
- de Godos, I.; Gonzalez, C.; Becares, E.; Garcia-Encina, P.A.; Munoz, R. Simultaneous nutrients and carbon removal during pretreated swine slurry degradation in a tubular biofilm photobioreactor. Appl. Microbiol. Biotechnol. 2009, 82, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Divakaran, R.; Pillai, V.N.S. Flocculation of algae usin chitosan. J. Appl. Phycol. 2002, 14, 419–422. [Google Scholar] [CrossRef]
- Shelef, G.; Sukenick, A.; Green, M. Microalgae Harvesting and Processing: A Literature Review; Report Number: SERI/STR-231-2396; Solar Energy Research Institute, US Department of Energy: Oak Ridge, TN, USA, 1984.
- Antizar-Ladislao, B.; Turrion-Gomez, J.L. Decentralized energy from waste systems. Energies 2010, 3, 194–205. [Google Scholar] [CrossRef]
- Demirbas, A. Microalgae as a feedstck for biodiesel. Energy Educ. Sci. Tech. A 2010, 25, 31–43. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Patil, P.D.; Gude, V.G.; Mannarswamy, A.; Deng, S.G.; Cooke, P.; Munson-McGee, S.; Rhodes, I.; Lammers, P.; Nirmalakhandan, N. Optimization of direct conversion of wet algae to biodiesel under supercritical methanol conditions. Bioresour. Technol. 2011, 102, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.B.; Wen, Z.Y. Development of an attached microalgal growth system for biofuel production. Appl. Microbiol. Biotechnol. 2010, 85, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Wahlen, B.D.; Willis, R.M.; Seefeldt, L.C. Biodiesel production by simultaneous extraction and conversion of total lipids from microalgae, cyanobacteria, and wild mixed cultures. Bioresour. Technol. 2011, 102, 2724–2730. [Google Scholar] [CrossRef] [PubMed]
- Lardon, L.; Helias, A.; Sialve, B.; Stayer, F.P.; Bernard, O. Life-cycle assessment of biodiesesl production from microalgae. Environ. Sci. Technol. 2009, 43, 6475–6481. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, S.; Ueda, R.; Ogushi, Y.; Hirano, A.; Samejima, Y.; Hon-Nami, K.; Kunito, S. Ethanol production from carbon dioxide by fermentative microalgae. Adv. Chem. Convers. Mitig. Carbon Dioxide 1998, 114, 657–660. [Google Scholar]
- Choi, S.P.; Nguyen, M.T.; Sim, S.J. Enzymatic pretreatment of Chloamydomonas reinhardtii biomass for ethanol production. Bioresour. Technol. 2010, 101, 5330–5336. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Ueda, R.; Hirayama, S.; Ogushi, Y. CO2 fixation and ethanol production with microalgal photosynthesis and intracellular anaerobic fermentation. Energy 1997, 22, 137–142. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K.; Forde, G.M. Microalgal biomass as a fermentation feedstock for bioethanol production. J. Chem. Technol. Biotechnol. 2010, 85, 199–203. [Google Scholar]
- Hromadko, J.; Hromadko, J.; Miler, P.; Honig, V.; Sterba, P. Use of bioethanol in combustion engines. Chem. Listy 2011, 105, 122–128. [Google Scholar]
- Nguyen, M.T.; Choi, S.P.; Lee, J.; Lee, J.H. Hydrothermal acid pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. J. Microbil. Biotechnol. 2009, 19, 161–166. [Google Scholar] [CrossRef]
- Luo, L.; van Der Voet, E.; Huppes, G. An energy analysis of ethanol from cellulosic feedstock corn stover. Renew. Sustain. Energy Rev. 2009, 27, 409–416. [Google Scholar]
- Luo, L.; van der Voet, E.; Huppes, G. An energy analysis of ethanol from cellulosic feedstock—Corn stover. Renew. Sustain. Energy Rev. 2009, 13, 2003–2011. [Google Scholar] [CrossRef]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Collet, P.; Helias, A.; Lardon, L.; Ras, M.; Goy, R.A.; Steyer, J.P. Life-cycle assessment of mciroalgae culture coupled to biogas production. Bioresour. Technol. 2011, 102, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Clarens, A.F.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef]
- Mussnug, J.H.; Klassen, V.; Schluter, A.; Kruse, O. Microgalgae as a substrate for fermentative biogas production in a combined biorefinery concept. J. Biotechnol. 2010, 150, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, H.; Baky, A.; Bernesson, S.; Nordberg, A.; Noren, O.; Hansson, P.A. Use of on-farm produced biofuels on organic farms—Evaluation of energy balances and environmental loads for three possible fuels. Agric. Syst. 2006, 89, 184–203. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J.; Shilton, A.N. Recycling algae to improve species control and harvest efficiency from a high rate algal pond. Water Res. 2011, 45, 6637–6649. [Google Scholar] [CrossRef] [PubMed]
- Tseng, K.F.; Huang, J.S.; Liao, I.C. Species control of microalgae in an aquaculture pond. Water Res. 1991, 25, 1431–1437. [Google Scholar]
- Williams, P.J.L.; Laurens, L.M.L. Microalgae as biodiesel and biomass feedstocks: Review and analysis of the biochemistry, energetics and economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar] [CrossRef]
- Murphy, C.F.; Allen, D.T. Energy-water nexus for mass cultivation of algae. Environ. Sci. Technol. 2011, 45, 5861–5868. [Google Scholar] [CrossRef] [PubMed]
- Sander, K.; Murthy, G.S. Life cycle analysis of algae biodiesel. Int. J. Life Cycle Ass. 2010, 15, 704–714. [Google Scholar] [CrossRef]
- Stephenson, A.L.; Kazamia, E.; Dennis, J.S.; Howe, C.J.; Scott, S.A.; Smith, A.G. Life-cycle assessment of potential algal biodiesel production in the United Kingdom: A comparison of raceways and air-lift tubular bioreactors. Energy Fuel 2010, 24, 4062–4077. [Google Scholar] [CrossRef]
- Clarens, A.F.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.X.; Hu, Z.S.; choi, D.G.; Thomas, V.M.; Realff, M.J.; Chance, R.R. Life cycle energy and greenhouse gas emissions for an ethanol production process based on blue-green algae. Environ. Sci. Technol. 2010, 44, 8670–8677. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.L.; Kazamia, E.; Dennis, J.S.; Howe, C.J.; Scott, S.A.; Smith, A.G. Life-cycle assessment of potential algal biodiesel production in the United Kingdom: A comparison of raceways and air-lift tubular bioreactors. Energy Fuel 2010, 24, 4062–4077. [Google Scholar] [CrossRef]
- Shirvani, T.; Yan, X.Y.; inderwildi, O.R.; Edwards, P.P.; King, D.A. Life cycle energy and greenhouse analysis for algae-derived biodiesel. Energy Environ. Sci. 2011, 4, 3773–3778. [Google Scholar] [CrossRef]
- Packer, M. Algal capture of carbon dioxide, biomass generation as a tool for greenhouse gas mitigation with reference to New Zealand energy strategy and policy. Energy Policy 2009, 37, 3428–3437. [Google Scholar] [CrossRef]
- Antizar-Ladislao, B.; Turrion-Gomez, J.L. Second-generation biofuels and local bioenergy systems. Biofuel Bioprod. Biorefin. 2008, 2, 455–469. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Tana, P.Q.; Yan, X.Y.; Low, D.M. Life cycle energy, environment and economic assessment of soybean-based biodiesel as an alternative automotive fuel in China. Energy 2008, 33, 1654–1658. [Google Scholar] [CrossRef]
- Adler, P.R.; Del Grosso, S.J.; Parton, W.J. Life cycle assessment of net greenhosue gas flux for bioenergy cropping systems. Ecol. Appl. 2007, 17, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Reiinders, L.; Huijbregts, M.A.J. Life cycle greenhouse gas emissions, fossil fuel demand and solar energy conversion efficiency in European bioethanol production for automotive purposes. J. Clean. Prod. 2007, 15, 1806–1812. [Google Scholar] [CrossRef]
- Karakashev, D.; Schmidt, J.E.; Angelidaki, I. Innovative process scheme for removal of organic matter, phosphorus and nitrogen from pig manure. Water Res. 2008, 42, 4083–4090. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.S.; Jia, X.Q.; Wen, J.P. Purification of high strength wastewater originating from bioethnaol production with simultaneous biogas production. World J. Microbiol. Biotechnol. 2011, 27, 2711–2722. [Google Scholar] [CrossRef]
- Douskova, I.; Kastanek, F.; Maleterova, Y.; Kastanek, P.; Doucha, J.; Zachleder, V. Utilization of distillery stillage for energy generation and concurrent production of valuable microalgal biomass in the sequence: Biogas-cogeneration-microalgae-products. Energy Convers. Manag. 2010, 51, 606–611. [Google Scholar] [CrossRef]
- Musee, N.; Trerise, M.A.; Lorenzen, L. Post-treatment of distillery wastewater after UASB using aerobic techniques. South Afr. J. Enol. Vitic. 2007, 28, 50–55. [Google Scholar]
- Raposo, M.F.D.; Oliveira, S.E.; Castro, P.M.; Bandarra, N.M.; Morais, R.M. On the utilization of microalgae for brewery effluent treatment and possible application of the produced biomass. J. Inst. Brew. 2010, 116, 285–292. [Google Scholar] [CrossRef]
- Moreno, C.; Farhbakhshazad, N.; Morrison, G.M. Ammonia removal from oil refinery effluent in vertical upflow macrophyte column systems. Water Air Soil Pollut. 2002, 135, 237–247. [Google Scholar] [CrossRef]
- Kim, C.J.; Jung, Y.H.; Ko, S.R.; Kim, H.I.; Park, Y.H.; Oh, H.M. Raceway cultivation of Spirulina platensis using underground water. J. Microbiol. Biotechnol. 2007, 17, 853–857. [Google Scholar] [PubMed]
- Celeckli, A.; Yavuzatmac, M.; Bozkurt, H. Modeling of biomass production by Spirulina platensis as function of phosphate concentrations and pH regimes. Bioresour. Technol. 2009, 100, 6325–6329. [Google Scholar]
- Olguin, E.J.; Galicia, S.; Mercado, G.; Perez, T. Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J. Appl. Phycol. 2003, 15, 249–257. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aitken, D.; Antizar-Ladislao, B. Achieving a Green Solution: Limitations and Focus Points for Sustainable Algal Fuels. Energies 2012, 5, 1613-1647. https://doi.org/10.3390/en5051613
Aitken D, Antizar-Ladislao B. Achieving a Green Solution: Limitations and Focus Points for Sustainable Algal Fuels. Energies. 2012; 5(5):1613-1647. https://doi.org/10.3390/en5051613
Chicago/Turabian StyleAitken, Douglas, and Blanca Antizar-Ladislao. 2012. "Achieving a Green Solution: Limitations and Focus Points for Sustainable Algal Fuels" Energies 5, no. 5: 1613-1647. https://doi.org/10.3390/en5051613





