Esterification and Deacidification of a Waste Cooking Oil (TAN 68.81 mg KOH/g) for Biodiesel Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Methanol/WCO Ratios on the Esterification
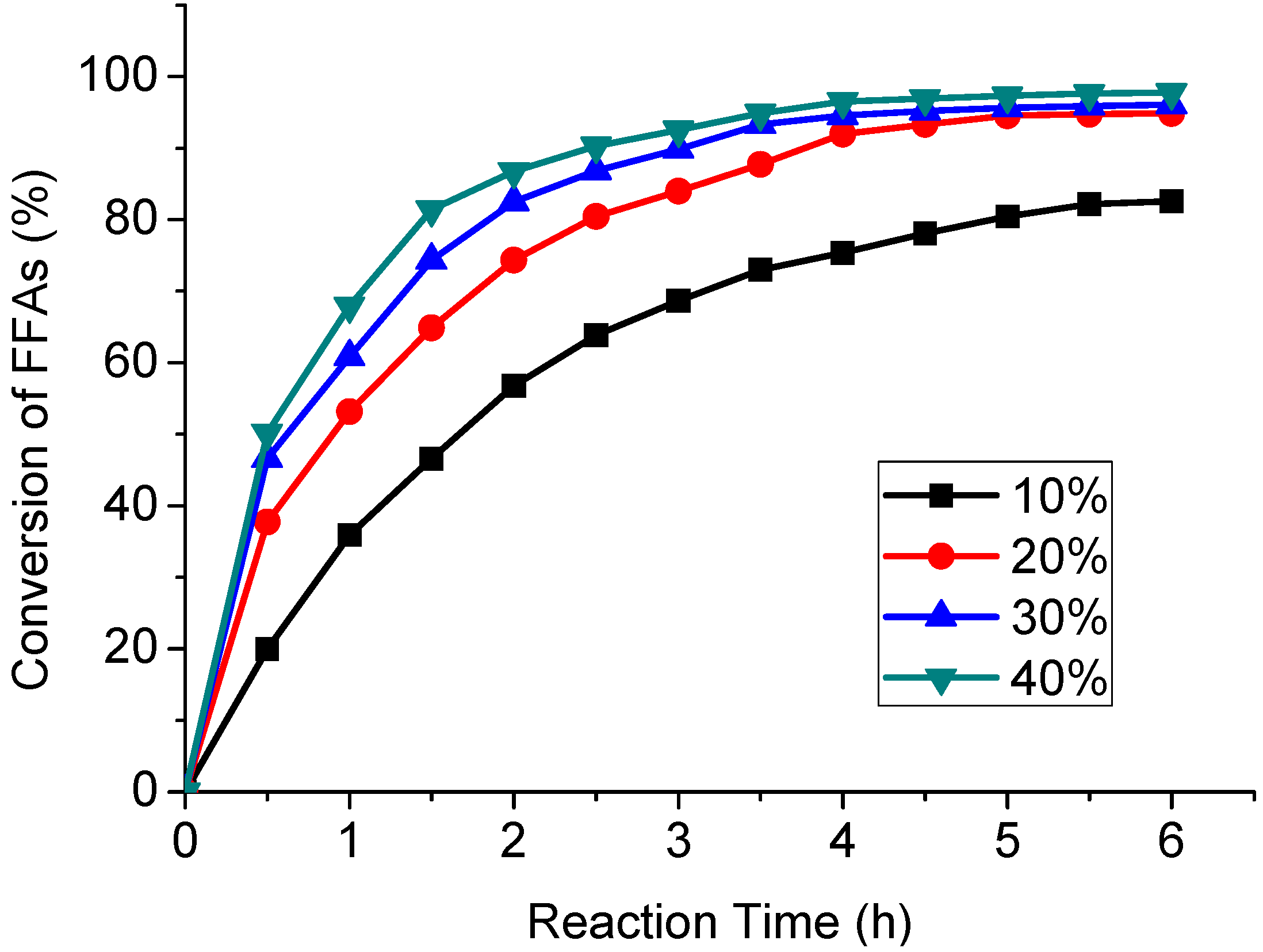
2.2. Effect of the Amount of Sulphuric Acid on the Esterification
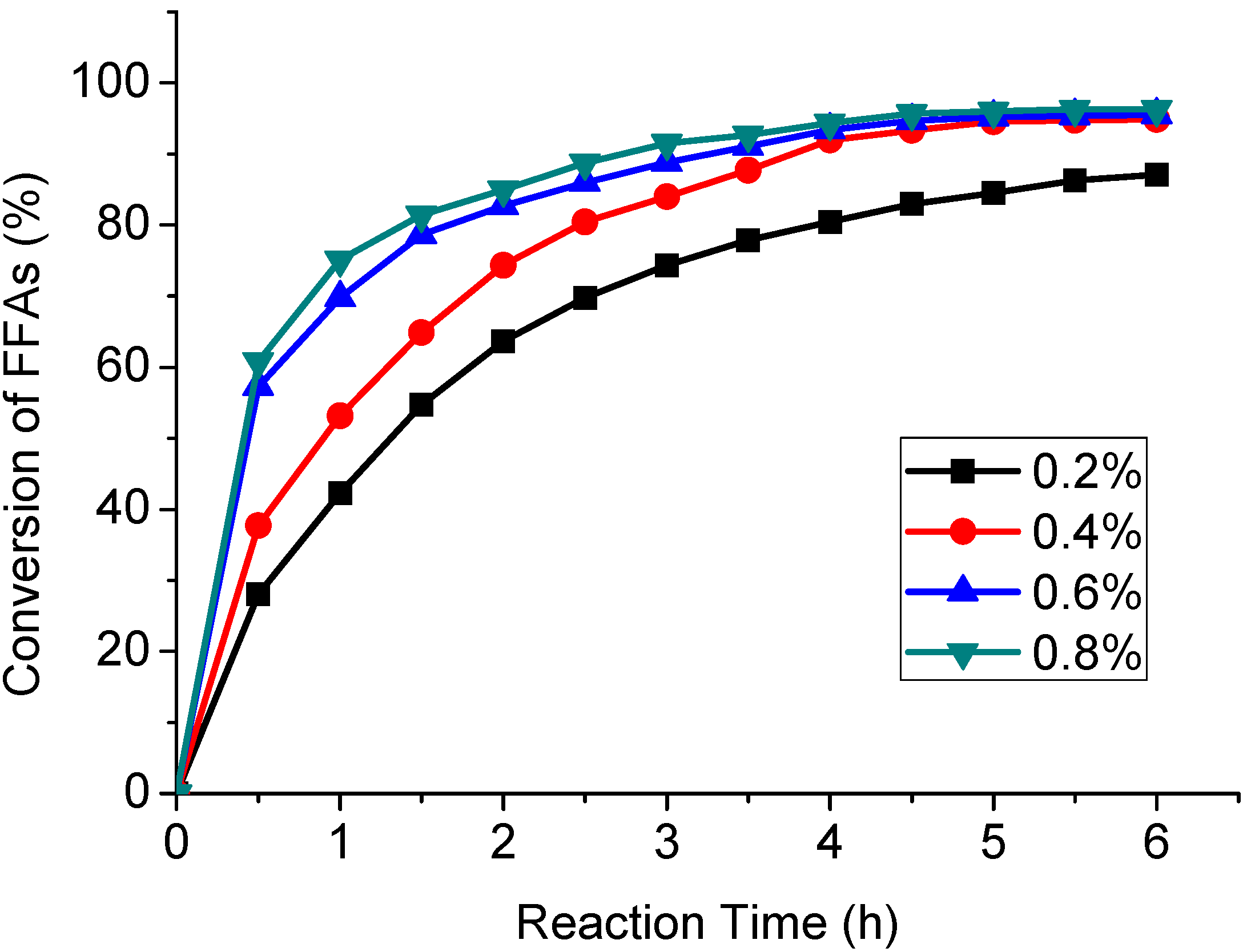
2.3. Effect of Reaction Temperature on the Esterification

2.4. Effect of the Alkali Concentration on the Deacidification
| Alkali concentration (N) | Conversion (δ, %) | Excess Alkali (%) | Conversion (δ, %) | Temperature (°C) | Conversion (δ, %) | Time (min) | Conversion (δ, %) |
|---|---|---|---|---|---|---|---|
| 0.1 | 56.61 | 5 | 64.89 | 30 | 33.35 | 10 | 59.81 |
| 0.3 | 67.87 | 10 | 71.46 | 40 | 48.12 | 20 | 67.65 |
| 0.5 | 77.11 | 15 | 77.11 | 50 | 64.76 | 30 | 72.33 |
| 0.8 | 78.36 | 20 | 78.35 | 60 | 77.11 | 40 | 77.11 |
| 1 | 78.52 | 25 | 78.79 | 70 | 76.79 | 50 | 74.54 |
2.5. Effect of Excess Alkali on the Deacidification
2.6. Effect of Temperature on the Deacidification
2.7. Effect of Time on the Deacidification
3. Experimental Section
3.1. Materials
3.2. Esterification Procedure
3.3. Determination of the Acid Value and FFA Conversion
3.4. Deacidification
4. Conclusions
Acknowledgments
References
- Martyanov, I.N.; Sayari, A. Comparative study of triglyceride transesterification in the presence of catalytic amounts of sodium, magnesium, and calcium methoxides. Appl. Catal. A Gen. 2008, 339, 45–52. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, D.K.; Lee, J.P.; Park, S.C.; Kim, Y.J.; Lee, J.S. Blending effects of biodiesels on oxidation stability and low temperature flow properties. Bioresour. Technol. 2008, 99, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Biodiesel production from high FFA rubber seed oil. Fuel 2005, 84, 335–340. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, S.; Liu, P.; Xue, F.; Tang, S. Comparison of two different processes to synthesize biodiesel by waste cooking oil. J. Mol. Catal. A Chem. 2006, 252, 107–112. [Google Scholar] [CrossRef]
- Barnwal, B.K.; Sharma, M.P. Prospects of biodiesel production from vegetable oils in India. Renew. Sustain. Energy Rev. 2005, 9, 363–378. [Google Scholar] [CrossRef]
- Coronado, C.R.; de Carvalho, J.A., Jr.; Silveira, J.L. Biodiesel CO2 emissions: A comparison with the main fuels in the Brazilian market. Fuel Process. Technol. 2009, 90, 204–211. [Google Scholar] [CrossRef]
- Haas, M.J.; McAloon, A.J.; Yee, W.C.; Foglia, T.A. A process model to estimate biodiesel production costs. Bioresour. Technol. 2006, 97, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; He, B.; Li, J. Biodiesel production from acidified oils via supercritical methanol. Energies 2011, 4, 2212–2223. [Google Scholar] [CrossRef]
- Sree, R.; Babu, N.S.; Prasad, P.S.S.; Lingaiah, N. Transesterification of edible and non-edible oils over basic solid Mg/Zr catalysts. Fuel Process. Technol. 2009, 90, 152–157. [Google Scholar] [CrossRef]
- Wichmann, H.; Sahlabji, T.; Ohnesorge, M.; Vogt, R.; Bahadir, M. Feasibility study on membrane-aided clean up and fractionation of fatty acid esters produced from waste fats. Clean. Soil Air Water 2008, 36, 840–844. [Google Scholar] [CrossRef]
- Ding, J.; He, B.; Li, J. Cation ion-exchange resin/polyethersulfone hybrid catalytic membrane for biodiesel production. J. Biobased Mater. Bioenergy 2011, 5, 82–91. [Google Scholar] [CrossRef]
- Gan, S.; Ng, H.K.; Ooi, C.W.; Motala, N.O.; Ismail, M.A.F. Ferric sulphate catalysed esterification of free fatty acids in waste cooking oil. Bioresour. Technol. 2010, 101, 7338–7343. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Deng, S.; Rhodes, J.I.; Lammers, P.J. Conversion of waste cooking oil to biodiesel using ferric sulfate and supercritical methanol processes. Fuel 2010, 89, 360–364. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T.; Mohamed, A.R. Potential of waste palm cooking oil for catalyst-free biodiesel production. Energy 2011, 36, 2085–2088. [Google Scholar] [CrossRef]
- Berrios, M.; Siles, J.; Martin, M.A.; Martin, A. A kinetic study of the esterification of free fatty acids (FFA) in sunflower oil. Fuel 2007, 86, 2383–2388. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef] [PubMed]
- De la Iglesia, Ó.; Mallada, R.; Menendez, M.; Coronas, J. Continuous zeolite membrane reactor for esterification of ethanol and acetic acid. Chem. Eng. J. 2007, 131, 35–39. [Google Scholar]
- Liu, Y.; Lotero, E.; Goodwin, J.G., Jr. Effect of carbon chain length on esterification of carboxylic acids with methanol using acid catalysis. J. Catal. 2006, 243, 221–228. [Google Scholar] [CrossRef]
- Veljković, V.B.; Lakićević, S.H.; Stamenković, O.S.; Todorović, Z.B.; Lazić, M.L. Biodiesel production from tobacco (Nicotiana tabacum L.) seed oil with a high content of free fatty acids. Fuel 2006, 85, 2671–2675. [Google Scholar] [CrossRef]
- Ghadge, S.V.; Raheman, H. Biodiesel production from mahua (Madhuca indica) oil having high free fatty acids. Biomass Bioenergy 2005, 28, 601–605. [Google Scholar] [CrossRef]
- Park, J.Y.; Wang, Z.M.; Kim, D.K.; Lee, J.S. Effects of water on the esterification of free fatty acids by acid catalysts. Renew. Energy 2010, 35, 614–618. [Google Scholar] [CrossRef]
- Essid, K.; Trabelsi, M.; Frikha, M.H. Effects of neutralization with lime on the quality of acid olive oil. J. Am. Oil Chem. Soc. 2006, 83, 879–884. [Google Scholar] [CrossRef]
- Bhattacharyya, A.C.; Bhattacharyya, D.K. Deacidification of high FFA rice bran oil by reesterification and alkali neutralization. J. Am. Oil Chem. Soc. 1987, 64, 128–131. [Google Scholar] [CrossRef]
- Berchmans, H.J.; Hirata, S. Biodiesel production from crude Jatropha curcas L. seed oil with a high content of free fatty acids. Bioresour. Technol. 2008, 99, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Bhosle, B.M.; Subramanian, R. New approaches in deacidification of edible oils—A review. J. Food Eng. 2005, 69, 481–494. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ding, J.; Xia, Z.; Lu, J. Esterification and Deacidification of a Waste Cooking Oil (TAN 68.81 mg KOH/g) for Biodiesel Production. Energies 2012, 5, 2683-2691. https://doi.org/10.3390/en5082683
Ding J, Xia Z, Lu J. Esterification and Deacidification of a Waste Cooking Oil (TAN 68.81 mg KOH/g) for Biodiesel Production. Energies. 2012; 5(8):2683-2691. https://doi.org/10.3390/en5082683
Chicago/Turabian StyleDing, Jincheng, Zheng Xia, and Jie Lu. 2012. "Esterification and Deacidification of a Waste Cooking Oil (TAN 68.81 mg KOH/g) for Biodiesel Production" Energies 5, no. 8: 2683-2691. https://doi.org/10.3390/en5082683




