Gamma Irradiation Induced Degradation of Orange Peels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Proximate Analysis
2.2. Comparison of Radiation Doses and Absorbance for Total Sugars
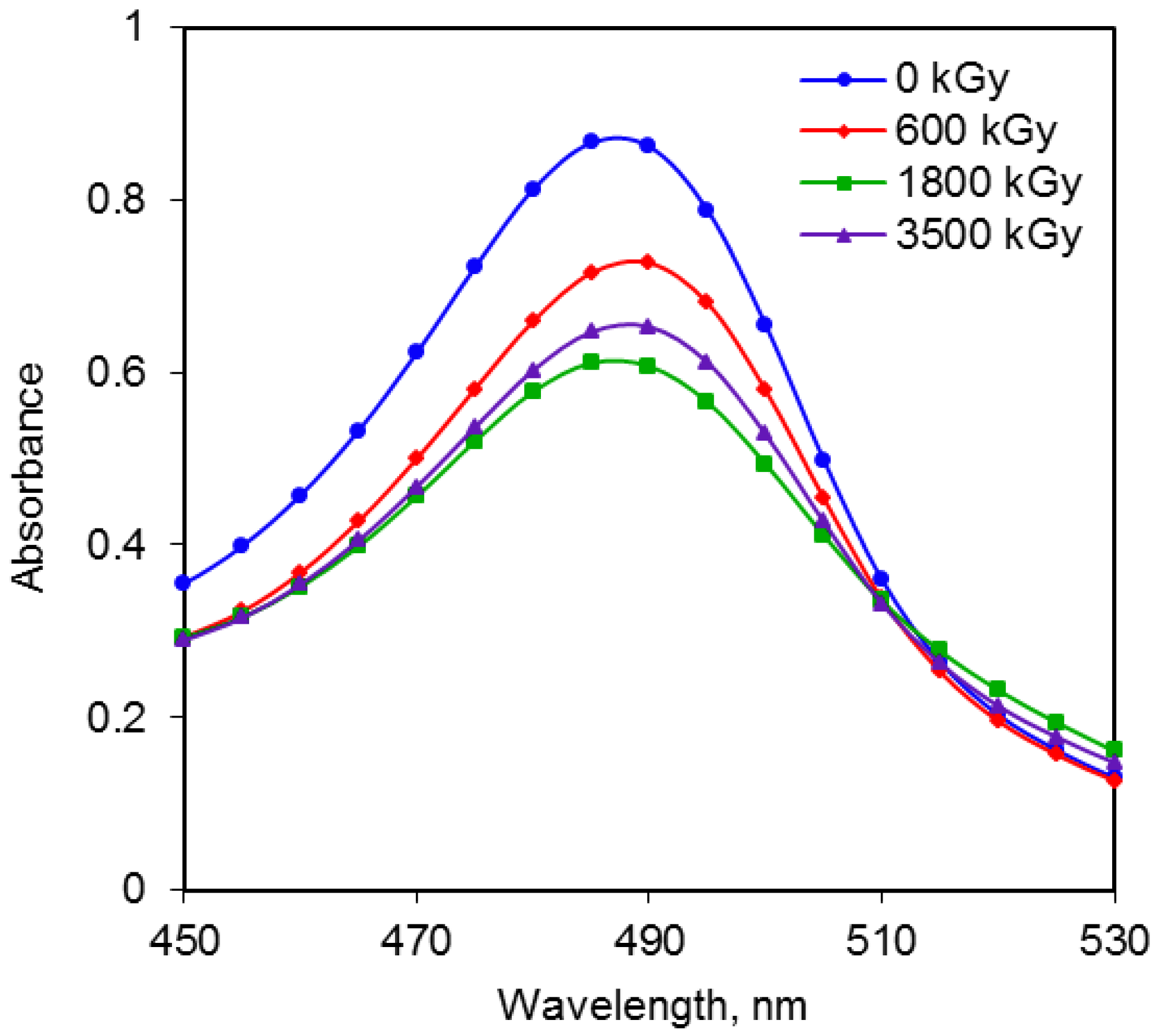
2.3. Comparison of Radiation Doses and Absorbance for Reducing Sugars
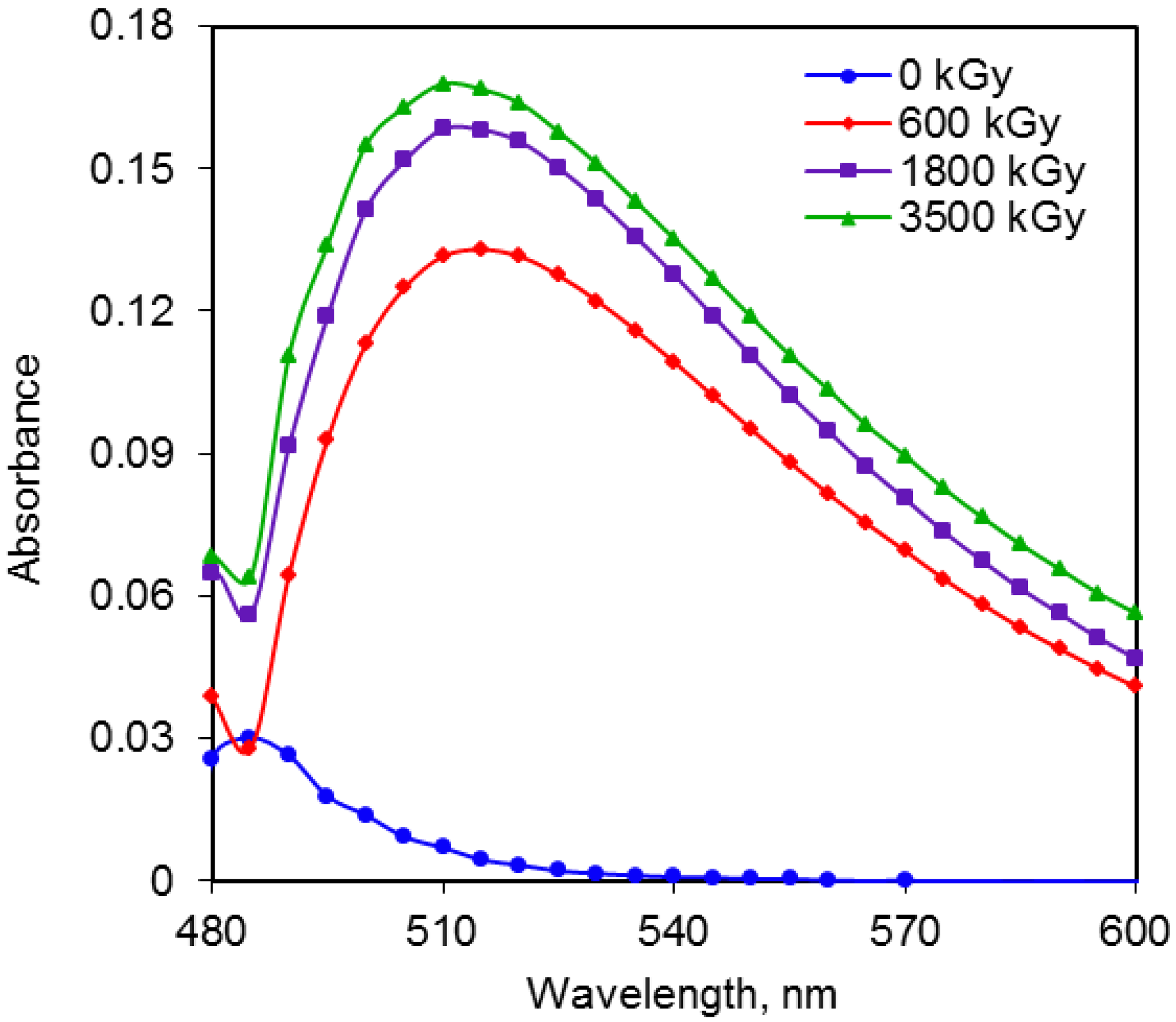
2.4. Liquid Chromatographic Analysis of Sugars
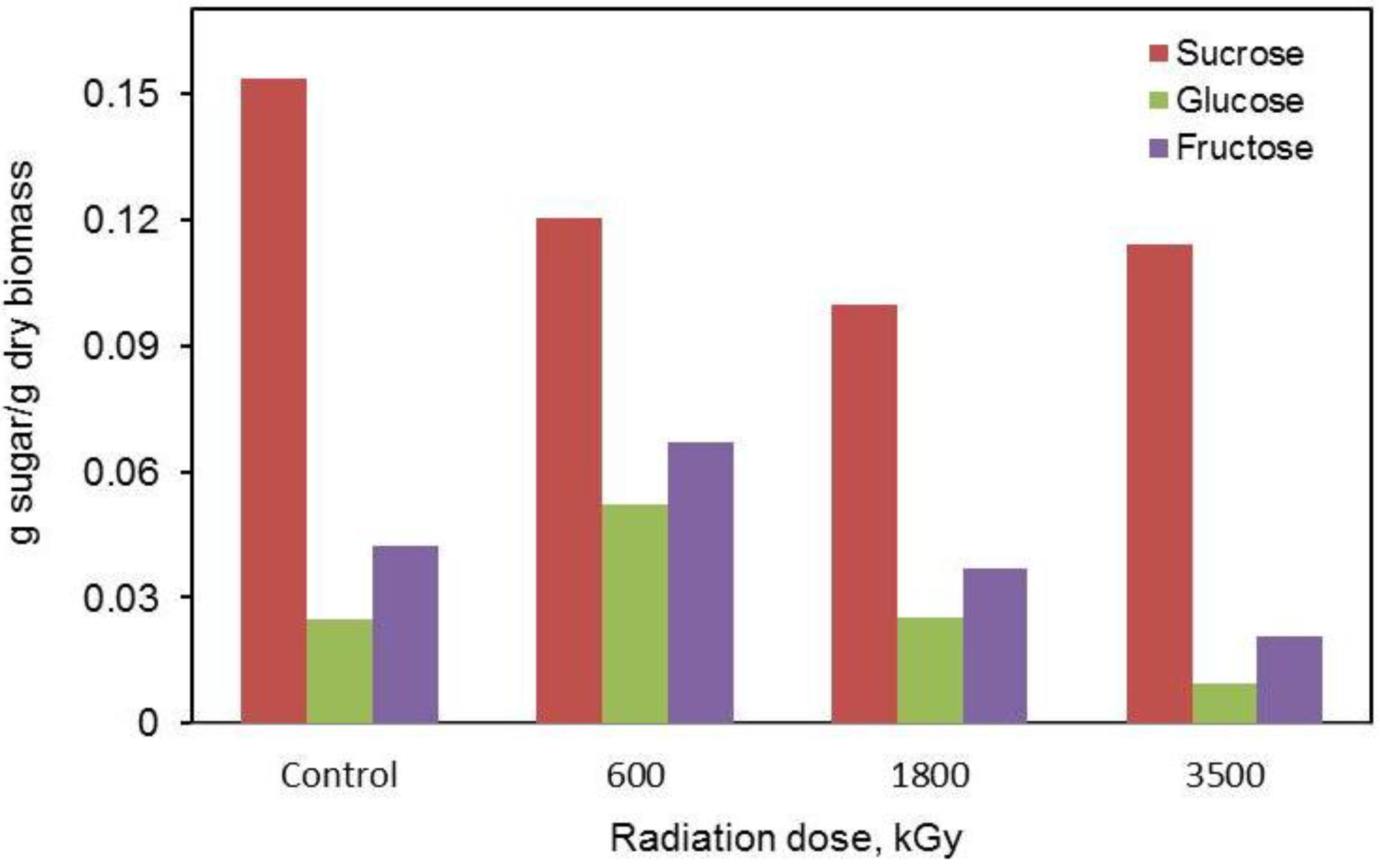
2.5. FTIR Spectra of the Irradiated Substrates at Different Doses

2.6. Thermal Behavior
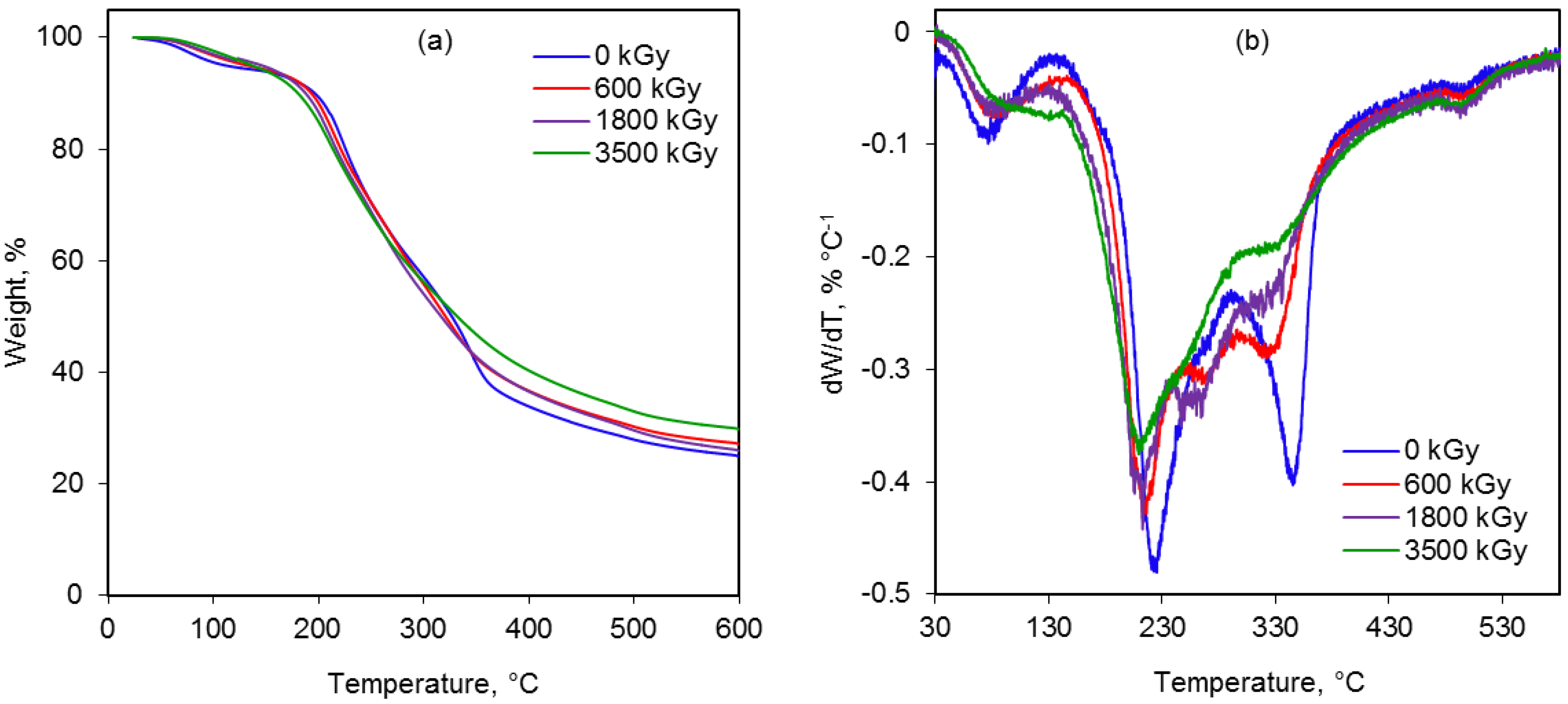
| Radiation dose, kGy | Peak temperature (Tmax), °C | Weight loss, % | Residue, % | ||||
|---|---|---|---|---|---|---|---|
| Stage 2 | Stage 3 | Stage 4 | Stage 2 | Stage 3 | Stage 4 | ||
| 0 | 346 | 223 | 496 | 29 | 27 | 9 | 23 |
| 600 | 324 | 218 | 498 | 28 | 26 | 9 | 25 |
| 1800 | 322 | 212 | 495 | 27 | 24 | 7 | 26 |
| 3500 | 321 | 210 | 495 | 26 | 24 | 7 | 28 |
2.7. Cleavage of the Glycosidic Bonds
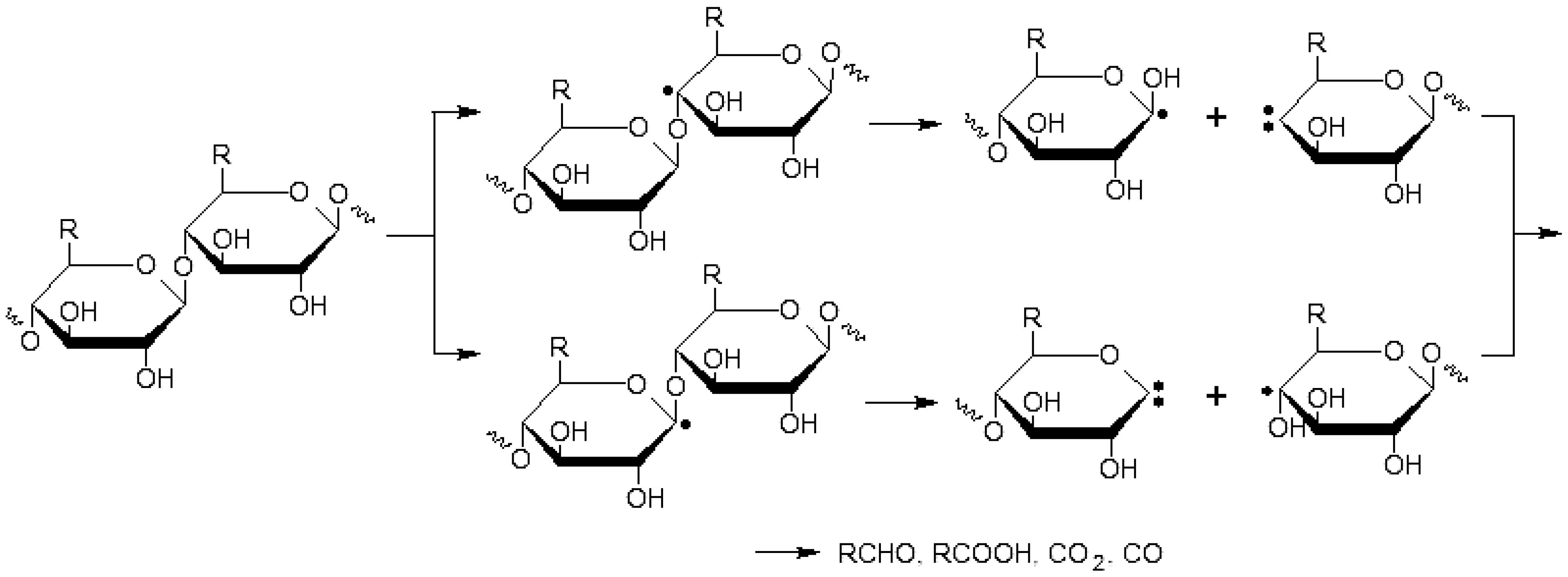
3. Experimental Section
3.1. Materials
3.2. Proximate Analysis
3.3. Gamma Irradiation
3.4. Total Sugars
3.5. Reducing Sugars
3.6. Analysis of Sugars by HPLC
3.7. Instrumentation and Characterization Methods
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Haberl, H.; Erb, K.H.; Krausmann, F.; Bondeau, A.; Lauk, C.; Muller, C.; Plutzar, C.; Steinberger, J.K. Global bioenergy potentials from agricultural land in 2050: Sensitivity to climate change diets and yields. Biomass Bioenergy 2011, 35, 4753–4769. [Google Scholar] [CrossRef] [PubMed]
- Champagne, P.; Li, C. Enzymatic hydrolysis of cellulosic municipal wastewater treatment process residuals as feedstocks for the recovery of simple sugars. Bioresour. Technol. 2009, 100, 5700–5706. [Google Scholar] [CrossRef] [PubMed]
- Matías, J.; González, J.; Royano, L.; Barrera, R.A. Analysis of sugars by liquid chromatography-mass spectrometry in Jerusalem artichoke tubers for bioethanol production optimization. Biomass Bioenergy 2011, 35, 2006–2012. [Google Scholar] [CrossRef]
- Peng, L.; Chen, Y. Conversion of paper sludge to ethanol by separate hydrolysis and fermentation (SHF) using Saccharomyces cerevisiae. Biomass Bioenergy 2011, 35, 1600–1606. [Google Scholar]
- Mamma, D.; Kourtoglau, E.; Christakopoulos, P. Fungal multienzyme production on industrial by-products of the citrus-processing industry. Bioresour. Technol. 2008, 99, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- Ververis, C.; Georghiou, K.; Danielidis, D.; Hatzinikolaou, D.G.; Santas, P.; Santas, R.; Corleti, V. Cellulose, hemicelluloses, lignin and ash content of some organic materials and their suitability for use as paper pulp supplements. Bioresour. Technol. 2007, 98, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Boluda-Aguilar, M.; García-Vidal, L.; González-Castañeda, F.P.; López-Gómez, A. Mandarin peel wastes pretreatment with steam explosion for bioethanol production. Bioresour. Technol. 2010, 101, 3506–3513. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Widmer, W.W.; Grohmann, K. Simultaneous saccharification and fermentation of citrus peel waste by Saccharomyces cerevisiae to produce ethanol. Process Biochem. 2007, 42, 1614–1619. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Vadlani, P.V.; Madl, R.L.; Saida, L.; Abeykon, J.P. Ethanol production from orange peels: Two-stage hydrolysis and fermentation studies using optimized parameters though experimental design. J. Agric. Food Chem. 2010, 58, 3422–3429. [Google Scholar] [CrossRef] [PubMed]
- Cardona, C.A.; Sánchez, O.J. Fuel ethanol production: Process design trends and integration opportunities. Bioresour. Technol. 2007, 98, 2415–2457. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, O.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef] [PubMed]
- Hamelinck, C.N.; Hooijdonk, G.V.; Faaij, A.P. Ethanol from lignocellulosic biomass: Techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 2005, 28, 384–410. [Google Scholar] [CrossRef]
- Cunha, J.A.; Pereira, M.M.; Valente, L.M.M.; de la Piscina, P.R.; Homs, N.; Santos, M.R. Waste biomass to liquids: Low temperature conversion of sugarcane bagasse to bio-oil. The effect of combined hydrolysis treatments. Biomass Bioenergy 2011, 35, 2106–2116. [Google Scholar] [CrossRef]
- Moxley, G.; Zhu, Z.; Percival, Y.H. Efficient sugar release by the cellulose solvent-based lignocellulose fractionation technology and enzymatic cellulose hydrolysis. J. Agric. Food Chem. 2008, 56, 7885–7890. [Google Scholar] [CrossRef] [PubMed]
- Widmer, W.; Zhou, W.; Grohmann, K. Pretreatment effects on orange processing waste for making ethanol by simultaneous saccharification and fermentation. Bioresour. Technol. 2010, 101, 5242–5249. [Google Scholar] [CrossRef] [PubMed]
- Talebnia, F.; Pourbafrani, M.; Lundin, M.; Taherzadeh, M.J. Optimization study of citrus wastes saccharification by dilute-acid hydrolysis. Bioresources 2008, 3, 108–122. [Google Scholar]
- Severiano, L.C.; Lahr, F.A.R.; Bardi, M.A.G.; Santos, A.C.; Machado, L.D.B. Influence of gamma radiation on properties of common Brazilian wood species used in artwork. Prog. Nucl. Energy 2010, 52, 730–734. [Google Scholar] [CrossRef]
- Chunping, Y.; Zhiqiang, S.; Guoce, Y.; Jianlong, W. Effect and aftereffect of γ radiation pretreatment on enzymatic hydrolysis of wheat straw. Bioresour. Technol. 2008, 99, 6240–6245. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Ahmad, S.R.; Kronfli, E. γ-Radiation induced changes in the physical and chemical properties of lignocellulose. Biomacromolecules 2006, 7, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, J.; Xia, J.; Kennedy, J.F.; Yie, X.; Liu, T.G. Effect of gamma irradiation on the condensed state structure and mechanical properties of konjac glucomannan/chitosan blend films. Carbohydr. Polym. 2011, 83, 44–51. [Google Scholar] [CrossRef]
- Byun, E.H.; Kim, J.H.; Sung, N.Y.; Choi, J.I.; Lim, S.T.; Kim, K.H.; Yook, H.S.; Byun, M.W.; Lee, J.W. Effects of gamma irradiation on the physical and structural properties of β-glucan. Radiat. Phys. Chem. 2008, 77, 781–786. [Google Scholar] [CrossRef]
- Ajila, C.M.; Bhat, S.G.; Prasada, U.J.S. Valuable components of raw and ripe peels from two Indian mango varieties. Food Chem. 2007, 102, 1006–1011. [Google Scholar] [CrossRef]
- Emaga, T.H.; Robert, C.; Ronkart, S.N.; Wathelet, B.; Paquot, M. Dietary fiber components and pectin chemical features of peels during ripening in banana and plantain varieties. Bioresour. Technol. 2008, 10, 4346–4354. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.; Reberts, P.; Smithd, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasakib, N.; Majima, T.; Nishimura, S.I.; Lee, Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Ezekiel, R.; Rana, G.; Singh, N.; Singh, S. Physicochemical, thermal and pasting properties of starch separated from γ-irradiated and stored potatoes. Food Chem. 2007, 105, 1420–1429. [Google Scholar] [CrossRef]
- Wu, D.; Shu, Q.; Wang, Z.; Xia, Y. Effect of gamma irradiation on starch viscosity and physicochemical properties of different rice. Radiat. Phys. Chem. 2002, 65, 79–86. [Google Scholar] [CrossRef]
- Choi, J.; Kim, H.J.; Kim, J.H.; Byun, M.W.; Chun, B.S.; Ahn, D.H.; Hwang, Y.J.; Kim, D.J.; Kim, G.H.; Lee, J.W. Application of gamma irradiation for the enhanced physiological properties of polysaccharides from seaweeds. Appl. Radiat. Isot. 2009, 67, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.K.; Srinivasan, P.; Kim, J.H.; Park, H.J.; Byun, M.W.; Lee, J.W. Comparison of gamma ray and electron beam irradiation on extraction yield, morphological and antioxidant properties of polysaccharides from tamarind seed. Radiat. Phys. Chem. 2009, 78, 605–609. [Google Scholar] [CrossRef]
- Zapata, B.; Balmaseda, J.; Fregoso-Israel, E.; Torres-García, E. Thermo-kinetics study of orange peel in air. J. Therm. Anal. Calorimetry. 2009, 98, 309–315. [Google Scholar] [CrossRef]
- Hernández-Montoya, V.; Montes-Mora, M.A.; Elizalde-González, M.P. Study of the thermal degradation of citrus seeds. Biomass Bioenergy 2009, 33, 1295–1299. [Google Scholar] [CrossRef]
- Aguiar, L.; Márquez-Montesinos, F.; Gonzalo, A.; Sánchez, J.L.; Arauzo, J.L. Influence of temperature and particle size on the fixed bed pyrolysis of orange peel residues. J. Anal. Appl. Pyrolysis 2008, 83, 124–130. [Google Scholar] [CrossRef]
- Zeng, J.; Singh, D.; Chen, S. Thermal decomposition kinetics of wheat straw treated by Phanerochaete chrysosporium. Int. Biodeterior. Biodegrad. 2011, 65, 410–414. [Google Scholar] [CrossRef]
- Ershov, B.G. Radiation-chemical degradation of cellulose and other polysaccharides. Russ. Chem. Rev. 1998, 67, 314–334. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 16th ed.; AOAC: Washington, DC, USA, 1998. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sánchez Orozco, R.; Balderas Hernández, P.; Flores Ramírez, N.; Roa Morales, G.; Saucedo Luna, J.; Castro Montoya, A.J. Gamma Irradiation Induced Degradation of Orange Peels. Energies 2012, 5, 3051-3063. https://doi.org/10.3390/en5083051
Sánchez Orozco R, Balderas Hernández P, Flores Ramírez N, Roa Morales G, Saucedo Luna J, Castro Montoya AJ. Gamma Irradiation Induced Degradation of Orange Peels. Energies. 2012; 5(8):3051-3063. https://doi.org/10.3390/en5083051
Chicago/Turabian StyleSánchez Orozco, Raymundo, Patricia Balderas Hernández, Nelly Flores Ramírez, Gabriela Roa Morales, Jaime Saucedo Luna, and Agustín Jaime Castro Montoya. 2012. "Gamma Irradiation Induced Degradation of Orange Peels" Energies 5, no. 8: 3051-3063. https://doi.org/10.3390/en5083051




