1. Introduction
For decades, concerns about limited fossil fuel reservoirs have increased alarmingly. In addition, increases in fossil-fuel consumption are causing global environmental issues due to CO
2 emissions. As the most promising alternative fuel, hydrogen is attractive as a clean energy source. Unlike petroleum, hydrogen can be produced from renewable sources such as water, sunlight, and biomass, thereby reducing the growing dependence on fossil fuels [
1,
2]. Furthermore, it can generate electricity by reacting with oxygen in PEM fuel cells with only water as the by-product without greenhouse gas emissions for hydrogen-fueled vehicles. Storage of hydrogen for on-board applications, however, is difficult because of its physical characteristics such as its very light weight. Despite the availability of a few hydrogen storage methods, solid-state hydrogen storage has been considered as a promising method to employ for on-board hydrogen storage [
3,
4].
Li-based complex hydrides such as lithium aluminum hydride (LiAlH
4) and lithium borohydride (LiBH
4) have been considered as promising hydrogen storage materials due to their high theoretical hydrogen capacities of about 10.5 and 18.5 wt %, respectively [
5,
6,
7]. For LiAlH
4, the balling time for sample preparation and transition metals were reported to affect its decomposition behaviors [
6,
8,
9]. Even though LiBH
4 has a high hydrogen desorption capacity, it is a very stable and needs a very high temperature, up to 600 °C, for any significant amount of desorbed hydrogen to be observed [
10]. Jin
et al. [
5] reported that a LiBH
4 and LiAlH
4 mixture added with 3 mol % TiF
3 stored hydrogen up to 7.2 wt % with a decomposition temperature between 177 and 247 °C. In addition, LiAlH
4+2LiBH
4 doped with 5 mol % TiF
3 showed the best hydrogen storage properties, with the decrease in the onset temperature of the first and second dehydrogenation steps [
11]. Moreover, it could absorb 3.76 and 4.78 wt % in 1 and 14 h, respectively, at 600 °C and 4 MPa hydrogen.
In this work, LiBH4 and LiAlH4 were selected as the base materials for hydrogen storage. Mixtures of these hydrides were prepared by mechanical ball-milling. A small amount of Ti-based catalysts (TiCl3 and TiO2), VCl3, and ZrCl4 were added during the milling process to study their effects on the hydrogen storage capacity, decomposition temperature, kinetics, and reversibility of hydrogen desorption/absorption. Phase transformations during the hydrogen desorption/absorption were also investigated.
2. Results and Discussion
Various molar ratios of LiAlH
4 and LiBH
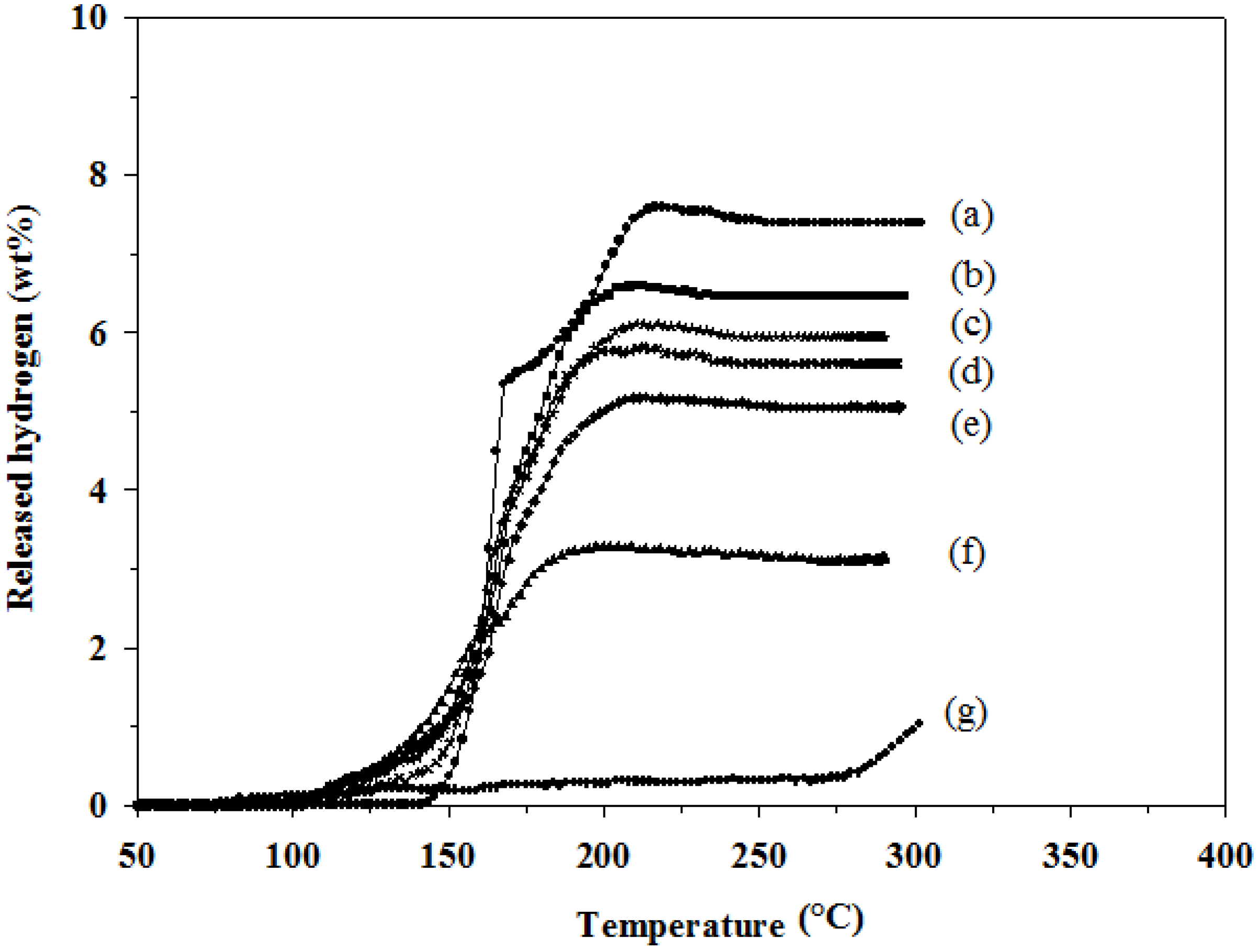
4 in the mixtures were studied in order to understand effects of the hydride combination on the amount of desorbed hydrogen and desorption temperature. As shown in
Figure 1, LiAlH
4 (
Figure 1a) decomposes in two steps starting at 145 °C and continues to 220 °C with a total hydrogen amount of 7.6 wt %. LiBH
4 (
Figure 1g) starts to decompose a small amount of hydrogen at a low temperature of 95 °C, which is believed to occur due to the reduction in the crystallite size and hydrogen desorption distance, and the distortion of hydride structure during the high energy milling process [
12]. LiBH
4 starts to release 1.0 wt % hydrogen at a temperature higher than 285 °C, which is the melting point of LiBH
4. In the case of the LiAlH
4 and LiBH
4 mixtures (
Figure 1b–f), all mixtures release lower amounts of hydrogen than LiAlH
4. A 2:1 molar ratio of LiAlH
4:LiBH
4 (2LiAlH
4+LiBH
4) (
Figure 1b) provides the highest amount of hydrogen at 6.6 wt %, and it desorbs hydrogen in the temperature range of 100–220 °C. The hydrogen desorption temperatures of the LiAlH
4/LiBH
4 mixture correspond with the data is previous works [
11]. The amounts of desorbed hydrogen and temperature of each mixture are also shown in
Table 1. However, the desorbed samples cannot re-absorb hydrogen under the tested conditions, as we will further discuss in the phase transformation section.
Figure 1.
Hydrogen desorption profiles of: (a) as-milled LiAlH4; (b) 2LiAlH4+LiBH4; (c) 3LiAlH4+LiBH4; (d) 4LiAlH4+LiBH4; (e) LiAlH4+LiBH4; (f) LiAlH4+2LiBH4; and (g) as-milled LiBH4.
Figure 1.
Hydrogen desorption profiles of: (a) as-milled LiAlH4; (b) 2LiAlH4+LiBH4; (c) 3LiAlH4+LiBH4; (d) 4LiAlH4+LiBH4; (e) LiAlH4+LiBH4; (f) LiAlH4+2LiBH4; and (g) as-milled LiBH4.
Table 1.
Hydrogen desorption temperature and amounts of desorbed hydrogen of LiAlH4–LiBH4 mixtures.
Table 1.
Hydrogen desorption temperature and amounts of desorbed hydrogen of LiAlH4–LiBH4 mixtures.
| Sample | Desorption temperature (°C) | Desorption amount (wt %) |
|---|
| Initial | Final |
|---|
| LiAlH4 | 145 | 220 | 7.6 |
| 1:1 | 105 | 220 | 5.2 |
| 2:1 | 100 | 220 | 6.6 |
| 3:1 | 95 | 220 | 6.1 |
| 4:1 | 105 | 220 | 5.8 |
| 1:2 | 95 | 210 | 3.3 |
| LiBH4 | 75 | 370 * | 1.0 * |
One mol % of TiO
2, TiCl
3, VCl
3, or ZrCl
4 were also added to the 2LiAlH
4+LiBH
4 mixture in order to investigate effects of the transition metals on the hydrogen desorption/absorption behaviors.
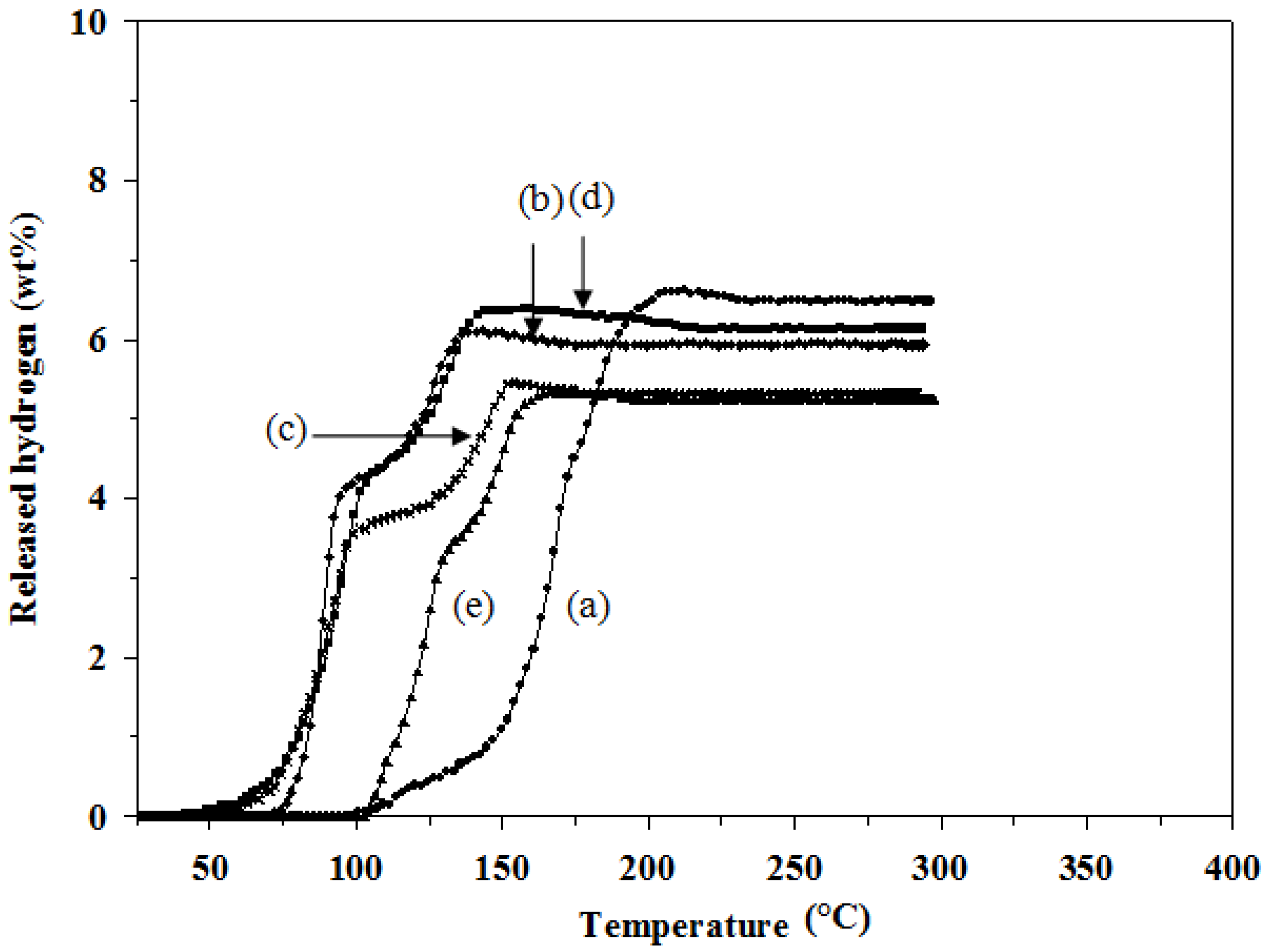
Figure 2 shows the two-step decomposition reactions and the decrease in the desorption temperature for all doped mixtures. The mixture in the presence of TiCl
3 (
Figure 2d) starts to decompose and release hydrogen at the lowest temperature (40 °C), which is lower than the undoped one by 60 °C. In addition, 1 mol % TiCl
3–2LiAlH
4+LiBH
4 provides the highest amount of hydrogen desorption among the doped samples, 6.4 wt %. A possible reason is the fact that TiCl
3 could facilitate the crystallite size reduction of the hydride during the milling process resulting in the hydrogen storage improvement [
13]. However, the hydrogen desorption capacities of all the doped mixtures are significantly lower than that of the undoped one. The results are summarized in
Table 2.
Figure 2.
Hydrogen desorption profiles of: (a) 2LiAlH4+LiBH4; (b) 1 mol % ZrCl4–2LiAlH4+LiBH4; (c) 1 mol % VCl3–2LiAlH4+LiBH4; (d) 1 mol % TiCl3–2LiAlH4+LiBH4; and (e) 1 mol % TiO2–2LiAlH4+LiBH4.
Figure 2.
Hydrogen desorption profiles of: (a) 2LiAlH4+LiBH4; (b) 1 mol % ZrCl4–2LiAlH4+LiBH4; (c) 1 mol % VCl3–2LiAlH4+LiBH4; (d) 1 mol % TiCl3–2LiAlH4+LiBH4; and (e) 1 mol % TiO2–2LiAlH4+LiBH4.
Table 2.
Hydrogen desorption temperature and amounts of desorbed hydrogen of 2LiAlH4+LiBH4 mixed with 1 mol % TiO2, TiCl3, VCl3, and ZrCl4 in the first (R1) and second (R2) steps.
Table 2.
Hydrogen desorption temperature and amounts of desorbed hydrogen of 2LiAlH4+LiBH4 mixed with 1 mol % TiO2, TiCl3, VCl3, and ZrCl4 in the first (R1) and second (R2) steps.
| Sample | Desorption temperature (°C) | Desorption amount (wt %) | Total (wt %) |
|---|
| R1 | R2 | Final | R1 | R2 |
|---|
| 2LiAlH4+LiBH4 | 100 | - | 220 | - | - | 6.6 |
| TiO2–2LiAlH4+LiBH4 | 105 | 138 | 190 | 3.5 | 1.8 | 5.3 |
| TiCl3–2LiAlH4+LiBH4 | 40 | 110 | 170 | 4.4 | 2.0 | 6.4 |
| VCl3–2LiAlH4+LiBH4 | 53 | 110 | 155 | 3.7 | 1.8 | 5.5 |
| ZrCl4–2LiAlH4+LiBH4 | 72 | 105 | 150 | 4.3 | 1.8 | 6.1 |
To investigate effects of an amount of catalyst on the hydrogen desorption properties of the 2LiAlH
4+LiBH
4 mixture, 3 and 5 mol % TiCl
3 were further added. The results, as shown in
Table 3 and
Figure 3, show that the hydrogen desorption capacities of all TiCl
3-doped mixtures are significantly lower than that of the undoped one. The hydrogen desorption capacity decreases with the increase in the doping amount, 6.4, 5.6, and 2.7 wt % for 1, 3, and 5 mol % doping, respectively. The desorption temperature of all TiCl
3-doped samples are lower than the undoped one by 40–70 °C; however, there is no significant difference in the desorption temperature among the TiCl
3-doped samples. Jin
et al. [
5] reported that the mixture of LiAlH
4+2.2LiBH
4 together with 3 mol % TiF
3 started to decompose between 177 °C and 247 °C and the weight loss reached up to 7.2 wt % at 387 °C. Mao
et al. [
11] also reported that LiAlH
4+2LiBH
4 doped with 5 mol % TiF
3 could decrease the onset temperature of the first and second decomposition steps by 64 and 150 °C, respectively. They further studied that doping with 5 mol % TiO
2 decreased the temperature of the first and second decomposition steps by 27 and 50 °C, respectively. In this work, no hydrogen absorption was observed for any of the doped samples at 300 °C and 8.5 MPa hydrogen for 6 h. Jin
et al. [
5] confirmed that the dehydrogenated product of 3 mol% TiF
3–LiAlH
4+2.2LiBH
4 could be hydrogenated to about 5.1 wt % H
2 at 350 °C and 70 bar hydrogen for 6 h. But the reaction kinetics was rather slow. Mao
et al. [
11] reported that 5 mol % TiF
3–LiAlH
4+2LiBH
4 absorbed 3.76 wt % and 4.78 wt % in 1 h and 14 h, respectively, at 600 °C and 4 MPa hydrogen.
Table 3.
Hydrogen desorption temperature and amounts of desorbed hydrogen of TiCl3-doped 2LiAlH4+LiBH4 in the first (R1) and second (R2) steps.
Table 3.
Hydrogen desorption temperature and amounts of desorbed hydrogen of TiCl3-doped 2LiAlH4+LiBH4 in the first (R1) and second (R2) steps.
| Sample | Desorption temperature (°C) | Desorption amount (wt %) | Total (wt %) |
|---|
| R1 | R2 | Final | R1 | R2 |
|---|
| 2LiAlH4+LiBH4 | 100 | - | 220 | - | - | 6.6 |
| 1 mol % TiCl3–2LiAlH4+LiBH4 | 40 | 110 | 170 | 4.4 | 2.0 | 6.4 |
| 3 mol % TiCl3–2LiAlH4+LiBH4 | 60 | 95 | 170 | 3.1 | 2.5 | 5.6 |
| 5 mol % TiCl3–2LiAlH4+LiBH4 | 45 | 100 | 150 | 1.4 | 1.3 | 2.7 |
Figure 3.
Hydrogen desorption profiles of: (a) 2LiAlH4+LiBH4; (b) 1 mol % TiCl3–2LiAlH4+LiBH4; (c) 3 mol % TiCl3–2LiAlH4+LiBH4; and (d) 5 mol % TiCl3–2LiAlH4+LiBH4.
Figure 3.
Hydrogen desorption profiles of: (a) 2LiAlH4+LiBH4; (b) 1 mol % TiCl3–2LiAlH4+LiBH4; (c) 3 mol % TiCl3–2LiAlH4+LiBH4; and (d) 5 mol % TiCl3–2LiAlH4+LiBH4.
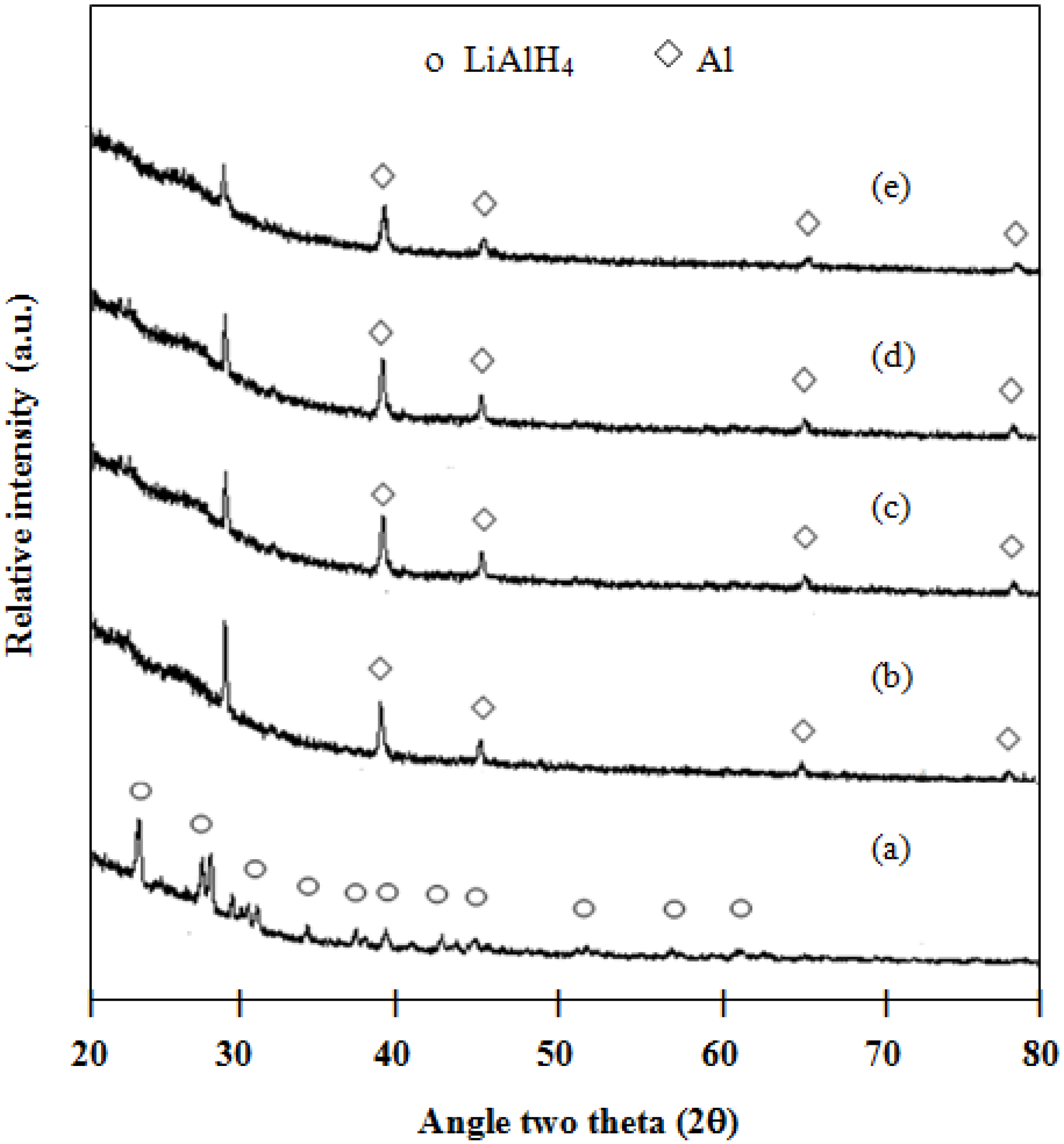
Phase transformation of the 2:1 molar ratio of LiAlH
4:LiBH
4 mixture after the milling process is similar to LiAlH
4, without any peak of LiBH
4. In the case of the undoped mixture, as shown in
Figure 4a, only LiAlH
4 is left after the 120 min milling process. In the case of the doped mixture, as shown in
Figure 4b–e, Al is observed after the milling process. It is possible that the milling process and a dopant might destabilize the structure of LiAlH
4 [
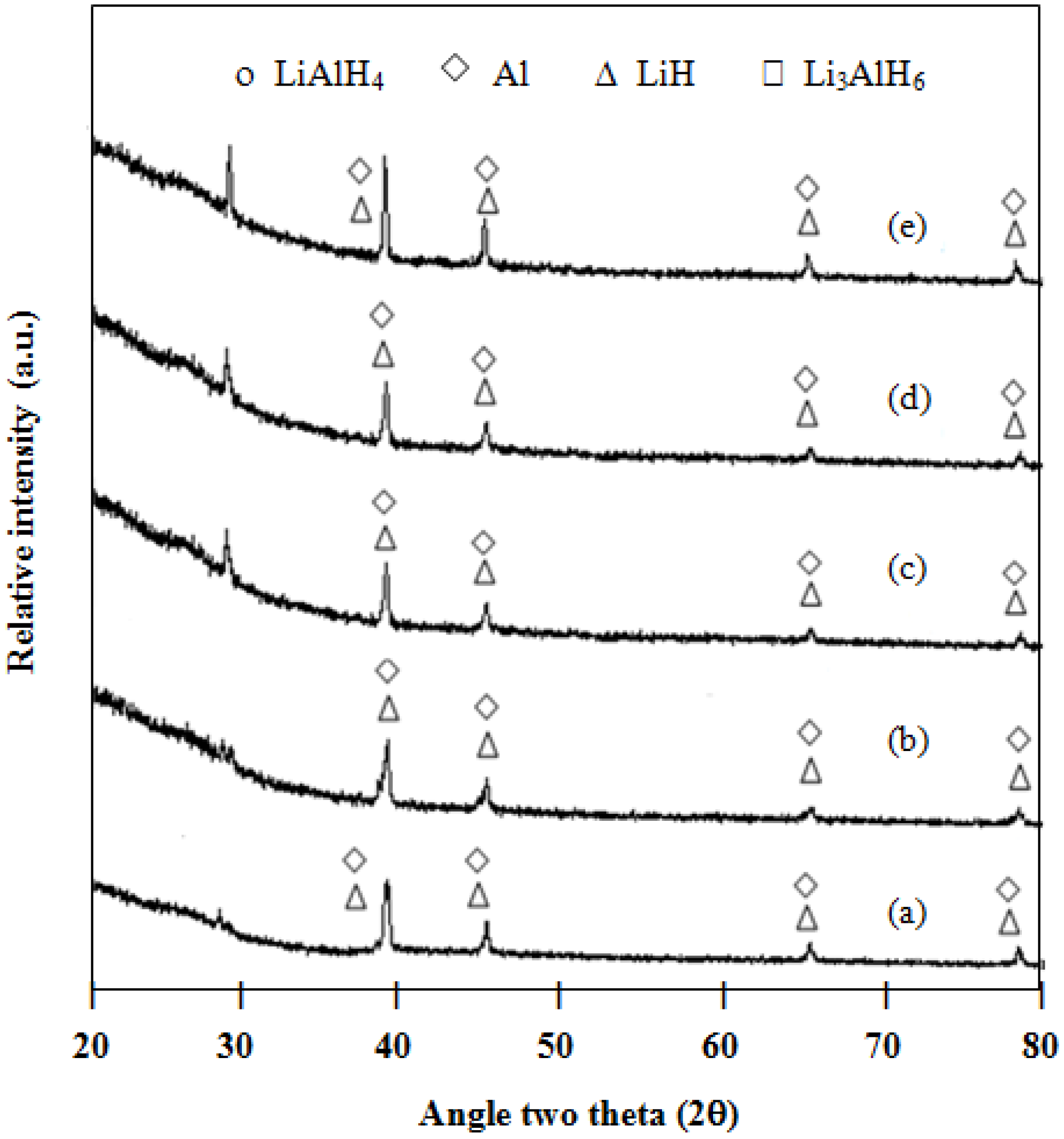
9]. After the hydrogen desorption at 300 °C (
Figure 5), Al and LiH are present in both undoped and doped mixtures, without the formation of AlB
2. It is the AlB
2 phase that is claimed to facilitate the reversibility of LiAlH
4 and LiBH
4 [
11,
14,
15,
16]. This could be a possible reason why the LiAlH
4/LiBH
4 mixture is not reversible. In addition, as shown in
Figure 6, the XRD patterns of the desorbed samples in the presence of a higher amount of the catalyst (3 or 5 mol % TiCl
3) exhibit new peaks, which are those of LiCl [
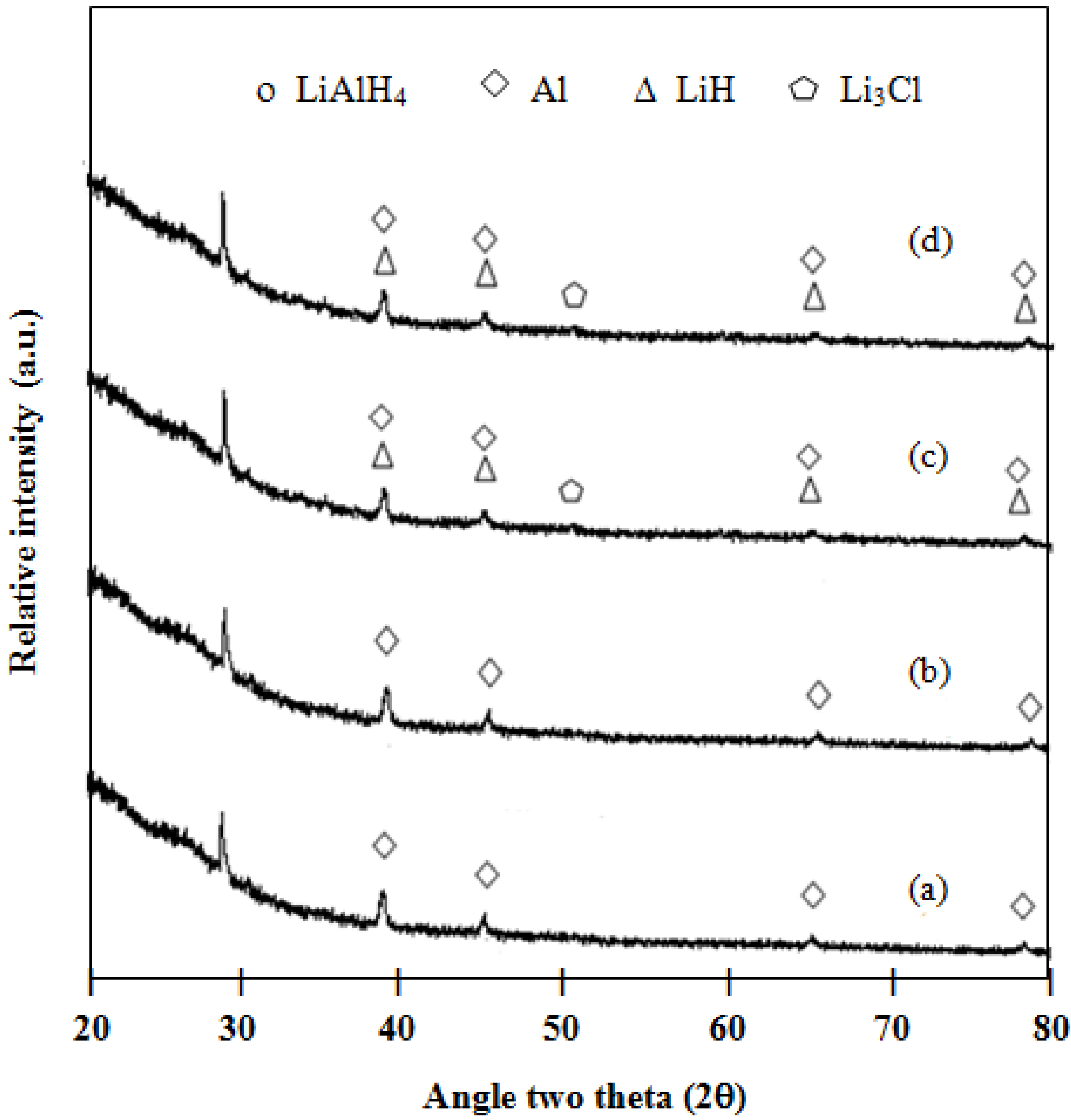
17]. The formation of LiCl might deteriorate the hydrogen desorption ability of the samples, consequently decreasing the hydrogen desorption capacity.
Figure 4.
XRD patterns of milled samples for 120 min: (a) 2LiAlH4+LiBH4; (b) 1 mol % ZrCl4–2LiAlH4+LiBH4; (c) 1 mol % VCl3–2LiAlH4+LiBH4; (d) 1 mol % TiCl3–2LiAlH4+LiBH4; and (e) 1 mol % TiO2–2LiAlH4+LiBH4.
Figure 4.
XRD patterns of milled samples for 120 min: (a) 2LiAlH4+LiBH4; (b) 1 mol % ZrCl4–2LiAlH4+LiBH4; (c) 1 mol % VCl3–2LiAlH4+LiBH4; (d) 1 mol % TiCl3–2LiAlH4+LiBH4; and (e) 1 mol % TiO2–2LiAlH4+LiBH4.
Figure 5.
XRD patterns of desorbed samples at 300 °C: (a) 2LiAlH4+LiBH4; (b) 1 mol % ZrCl4–2LiAlH4+LiBH4; (c) 1 mol % VCl3–2LiAlH4+LiBH4; (d) 1 mol % TiCl3–2LiAlH4+LiBH4; and (e) 1 mol % TiO2–2LiAlH4+LiBH4.
Figure 5.
XRD patterns of desorbed samples at 300 °C: (a) 2LiAlH4+LiBH4; (b) 1 mol % ZrCl4–2LiAlH4+LiBH4; (c) 1 mol % VCl3–2LiAlH4+LiBH4; (d) 1 mol % TiCl3–2LiAlH4+LiBH4; and (e) 1 mol % TiO2–2LiAlH4+LiBH4.
Figure 6.
XRD patterns of: (a) milled 3 mol % TiCl3–2LiAlH4+LiBH4; (b) milled 5 mol % TiCl3–2LiAlH4+LiBH4; (c) desorbed 3 mol % TiCl3–2LiAlH4+LiBH4; (d) desorbed 5 mol % TiCl3–2LiAlH4+LiBH4.
Figure 6.
XRD patterns of: (a) milled 3 mol % TiCl3–2LiAlH4+LiBH4; (b) milled 5 mol % TiCl3–2LiAlH4+LiBH4; (c) desorbed 3 mol % TiCl3–2LiAlH4+LiBH4; (d) desorbed 5 mol % TiCl3–2LiAlH4+LiBH4.
The LiAlH
4 and LiBH
4 mixtures doped with TiO
2, TiCl
3, VCl
3, or ZrCl
4 partially decompose during the milling process because the structure of LiAlH
4 is unstable and the additives might destabilize its structure [
9]. These metal catalysts in the hydride systems are reduced by the metal hydrides and transformed to TM-neutral [
6,
18], as shown in Reactions (1) and (2), where TM is the transition metal, M is the metal, and X can be either V or Ti:
It may be this reduction that increases the hydrogen desorption capacity of LiAlH
4 as compared to the undoped one. Moreover, after the decomposition, these catalysts can form M
2O and MCl, which are left in the sample as stable materials and cannot further decompose [
7]. In this work, MCl formation in Reaction (2) is LiCl, the presence of which might cause the decrease in the hydrogen desorption capacity with the increase in the TiCl
3 amount.