Optimal Conditions for Fast Charging and Long Cycling Stability of Silicon Microwire Anodes for Lithium Ion Batteries, and Comparison with the Performance of Other Si Anode Concepts
Abstract
:1. Introduction
2. Results and Discussion

2.1. Variation of Charging Conditions
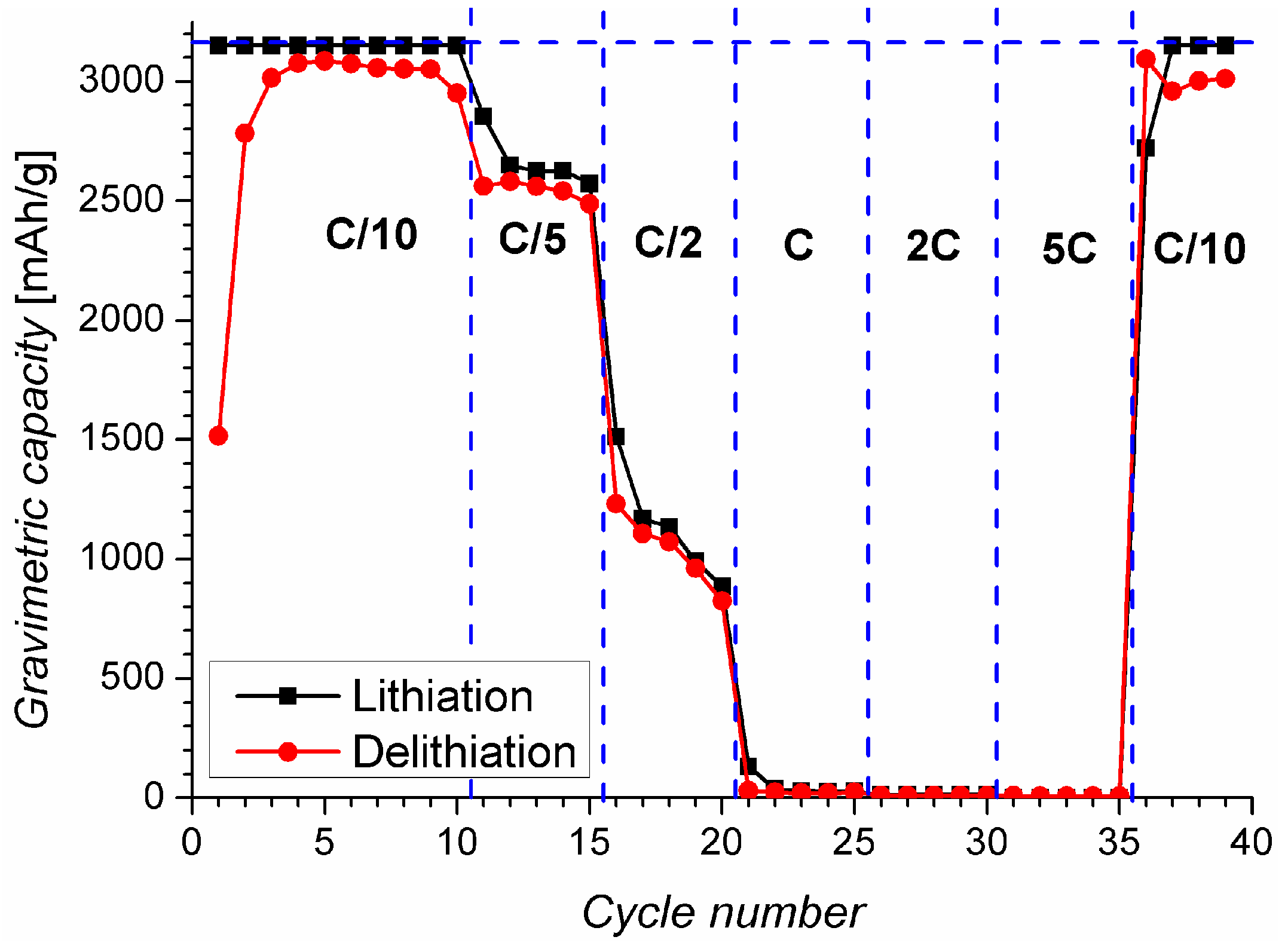
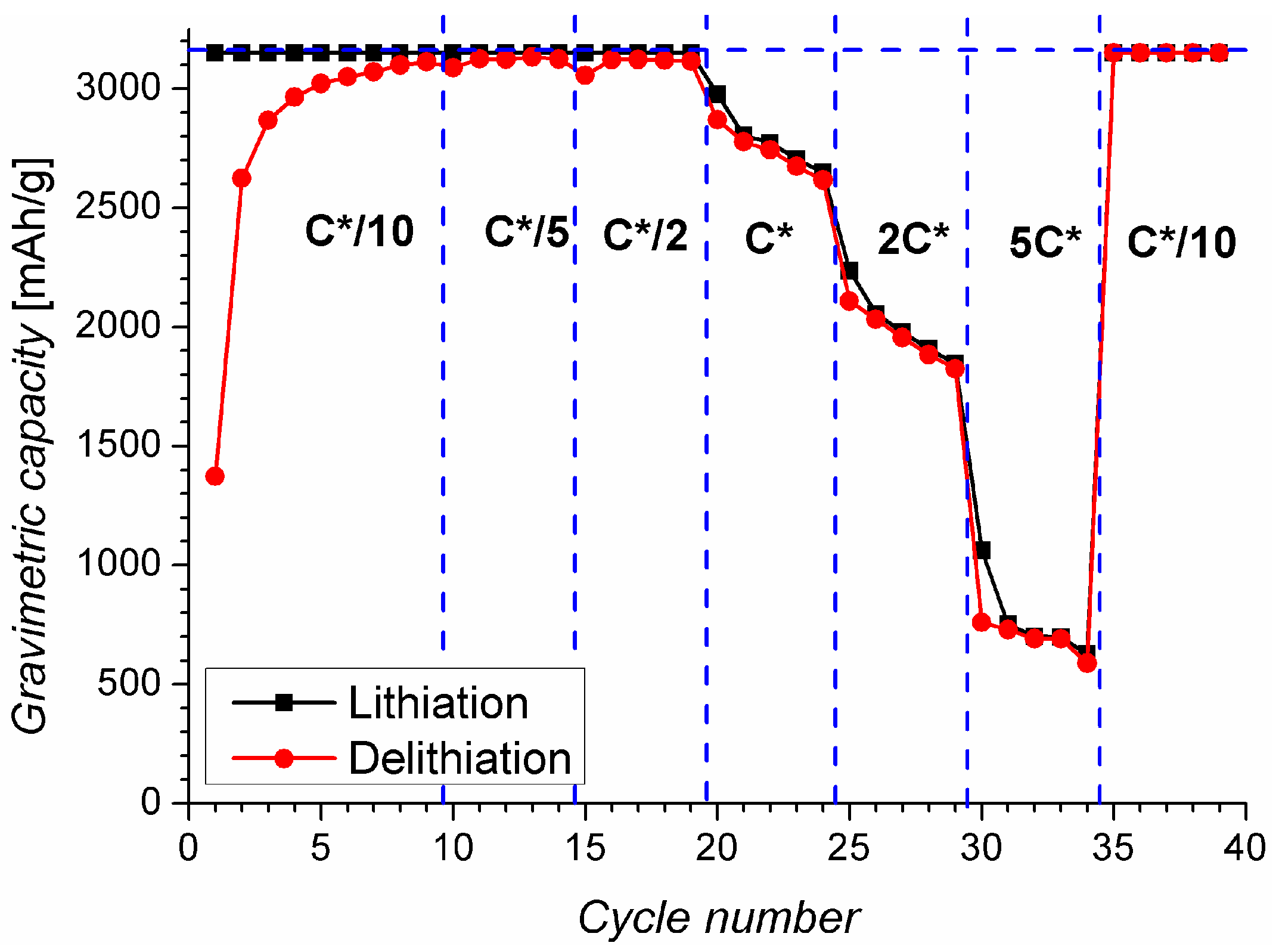
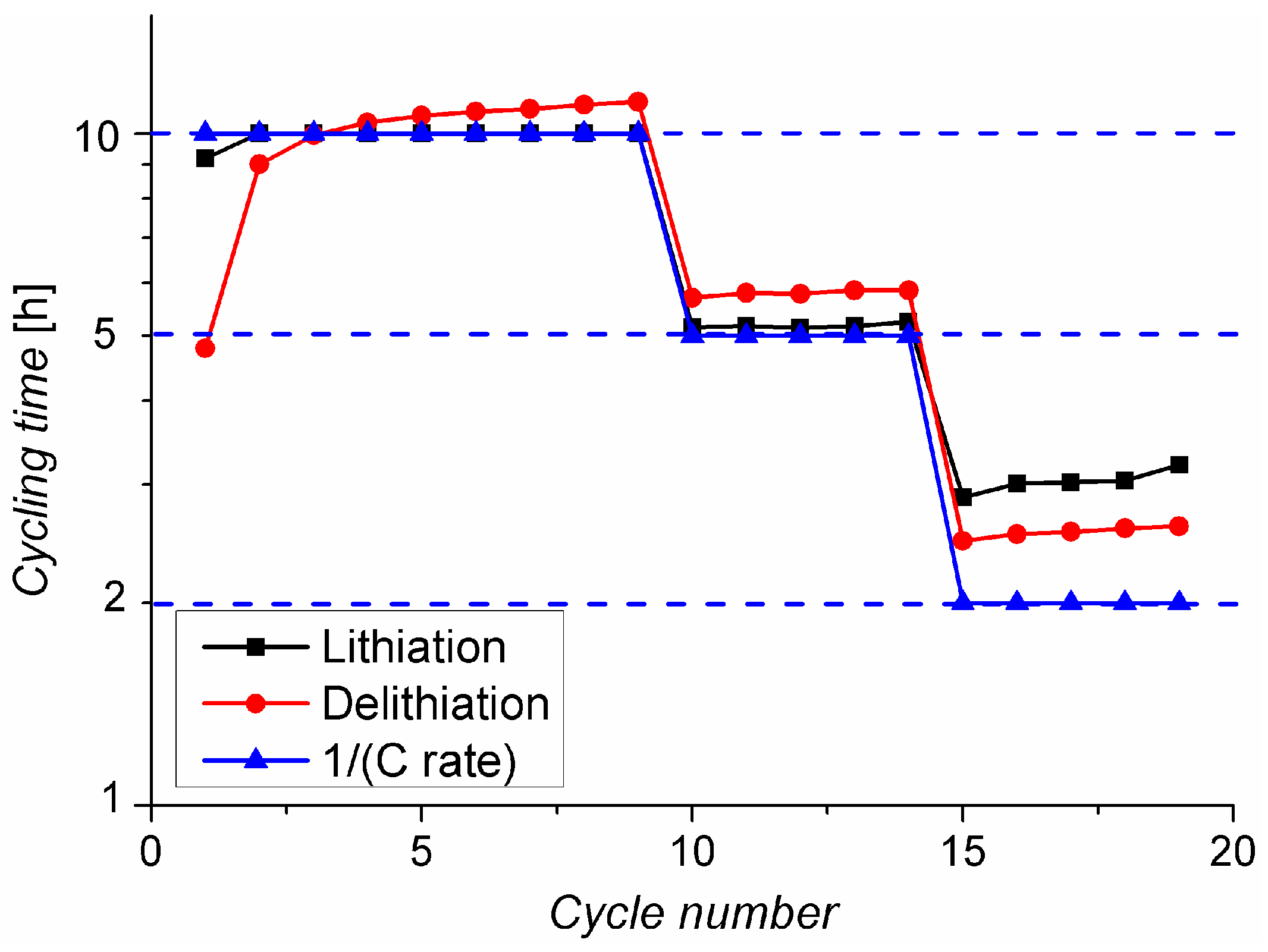
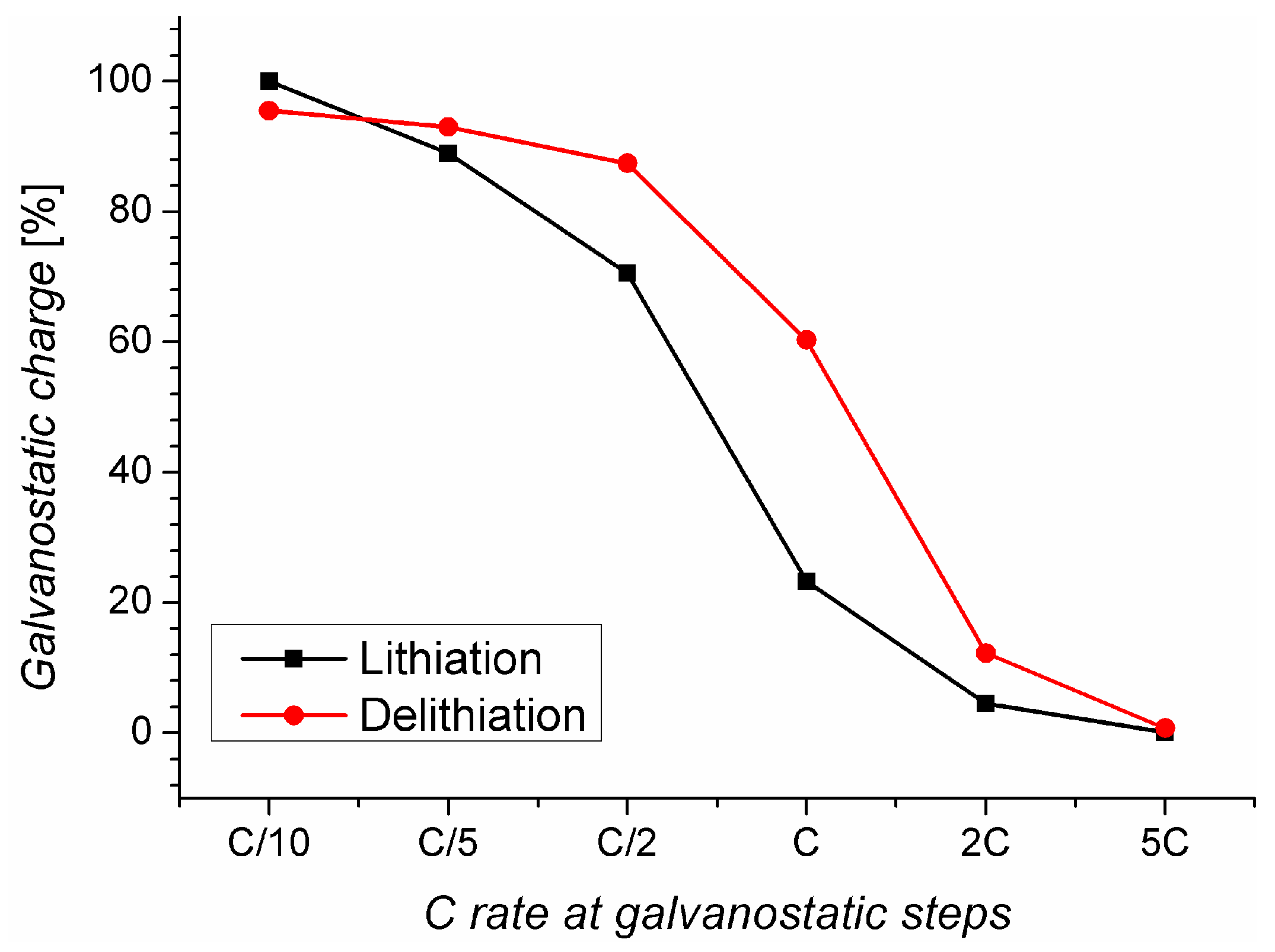
2.2. Charging under Optimal Conditions
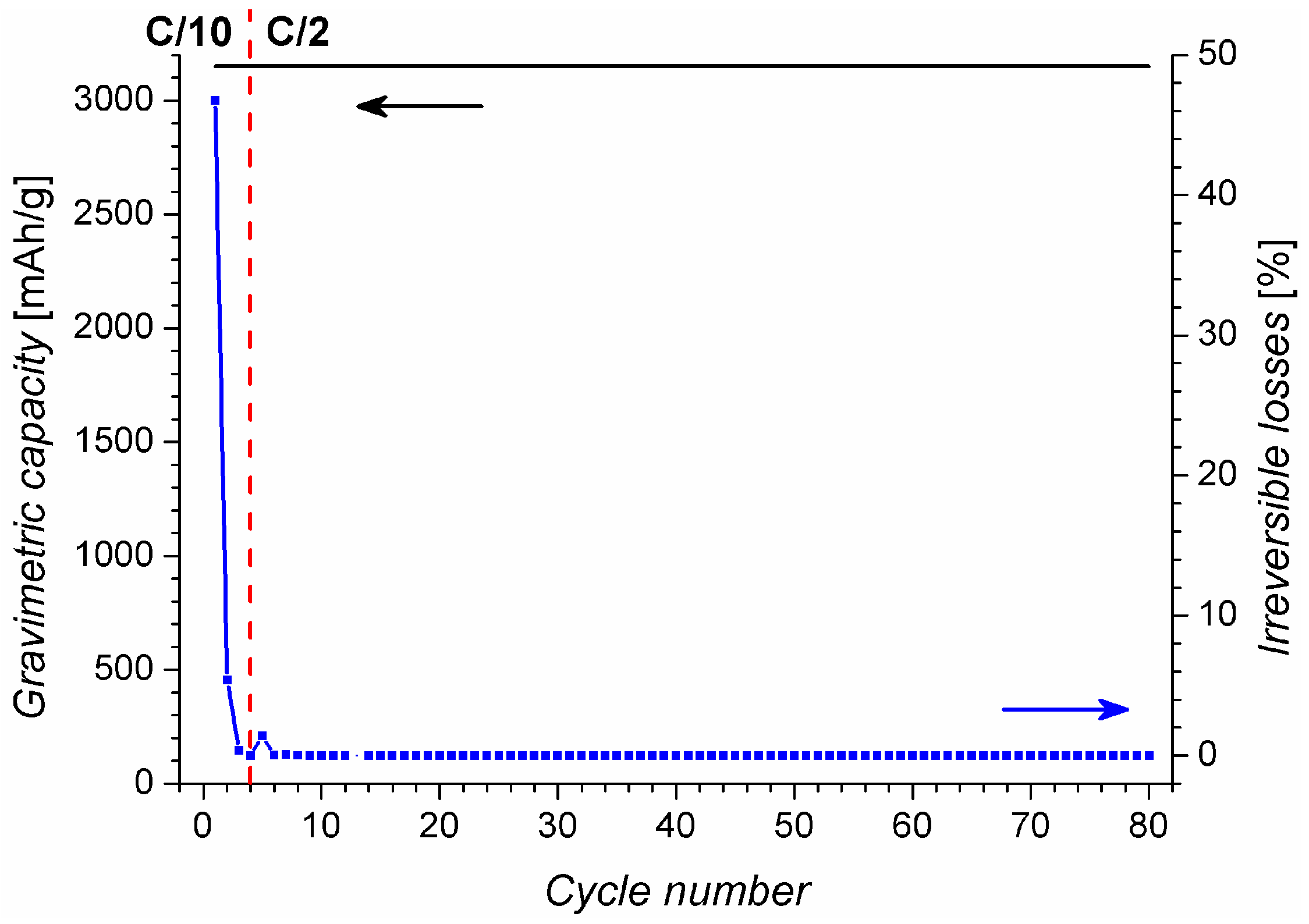

2.3. Comparative Measures
| Form of Si | Φ Si (nm) | Weight active (mg/cm2) | C rate (galv. steps) | Capacity start (mAh/g) | Capacity end (mAh/g) | Cycles | V min (V) | V max (V) | Areal capacity (mAh/cm2) | Ch. density rate (mAh/cm2h) | Fading/cycle (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Si microwires (charged to 75%) | 1000 | 1.35 | 0.500 | 3150 | 3150 | 80 | 0.110 | 0.7 | 4.25 | 2.84 | 0.00 | - |
| Si microwires (charged to 100%) | 1000 | 1.35 | 0.500 | 4200 | 4200 | 50 | 0.110 | 0.7 | 5.67 | 2.84 | 0.00 | [2] |
| Free standing Si wires/tubes | ||||||||||||
| Si nanowires | 100 | 0.20 | 0.200 | 2600 | 1900 | 80 | 0.007 | 0.7 | 0.52 | 0.17 | 0.34 | [4] |
| C-Si core shell nanowires | 50 | 2.00 | 0.119 | 2000 | 1600 | 55 | 0.010 | 1.0 | 4.00 | 1.00 | 0.36 | [16] |
| Si nanowires with Si nanoparticles | 50 | 1.50 | 0.190 | 1730 | 1200 | 40 | 0.050 | 1.0 | 2.60 | 1.20 | 0.77 | [17] |
| Si nanopillars | 580 | 0.06 | 3.788 | 1718 | 859 | 50 | 0.025 | 2.0 | 0.10 | 0.95 | 1.00 | [18] |
| Cu-coated Si nanowires | 100 | 0.60 | 0.050 | 2680 | 2500 | 15 | 0.020 | 2.0 | 1.61 | 0.13 | 0.45 | [19] |
| Si nanotube with SiOx | 350 | 0.06 | 5.710 | 675 | 550 | 5000 | 0.010 | 1.0 | 0.04 | 1.44 | 0.00 | [20] |
| p-doped Si nanowires | 30 | 0.14 | 2.000 | 2150 | 1900 | 20 | 0.000 | 2.0 | 0.30 | 1.18 | 0.58 | [21] |
| Si particles in slurry | ||||||||||||
| C-coated porous particles | 4000 | 3.50 | 0.024 | 2100 | 1600 | 30 | 0.020 | 1.5 | 7.35 | 0.35 | 0.79 | [13] |
| C-coated Si nanowires with CNTs | 24.6 | 0.20 | 0.200 | 1600 | 1050 | 70 | 0.010 | 1.0 | 0.32 | 0.17 | 0.49 | [22] |
| Si nano-particles | 78 | 2.00 | 0.015 | 2100 | 1260 | 20 | 0.000 | 0.8 | 4.20 | 0.125 | 2.00 | [23] |
| Si nanotubes nominal | 225 | 10.00 | 1.000 | 3250 | 3150 | 80 | 0.000 | 1.5 | 32.50 | 42.00 | 0.04 | [14] |
| Si nanotubes calculated | 225 | 1.40 | 1.000 | 3250 | 3150 | 80 | 0.000 | 1.5 | 4.55 | 5.88 | 0.04 | - |
| Si thin films | ||||||||||||
| Si film on CNF | 200 * | 0.05 | 0.100 | 1600 | 1200 | 102 | 0.010 | 3.0 | 0.08 | 0.02 | 0.25 | [24] |
| Si film on rough Cu substrate | 350 * | 0.07 | 0.200 | 2300 | 2100 | 100 | 0.010 | 1.2 | 0.16 | 0.06 | 0.09 | [25] |
| Film of Si nanoparticles | 35 * | 0.10 | 0.024 | 2200 | 650 | 100 | 0.020 | 1.5 | 0.21 | 0.01 | 0.70 | [26] |
| Porous Si | ||||||||||||
| C-coated macroporous Si | 1500 | 5.82 | 0.008 | 1250 | 950 | 20 | 0.070 | 1.5 | 7.28 | 0.20 | 1.20 | [15] |
| Au-coated macroporous Si | 1000 | 1.00 | 0.024 | 1619 | 2452 | 10 | 0.100 | 2.0 | 1.62 | 0.10 | −5.15 | [27] |
3. Experimental Section
4. Conclusions
Acknowledgements
Conflicts of Interest
References
- Okamoto, H. The Li-Si (lithium-silicon) system. J. Phase Equilib. 1990, 11, 306–312. [Google Scholar]
- Quiroga-González, E.; Carstensen, J.; Föll, H. Good cycling performance of high-density arrays of Si microwires as anodes for Li ion batteries. Electrochim. Acta 2013, 101, 93–98. [Google Scholar] [CrossRef]
- Graetz, J.; Ahn, C.C.; Yazami, R.; Fultz, B. Highly reversible lithium storage in nanostructured silicon. Electrochem. Solid-State Lett. 2003, 6, A194–A197. [Google Scholar] [CrossRef]
- Ruffo, R.; Hong, S.S.; Chan, C.K.; Huggins, R.A.; Cui, Y. Impedance analysis of silicon nanowire lithium ion battery anodes. J. Phys. Chem. C 2009, 113, 11390–11398. [Google Scholar] [CrossRef]
- Huang, R.; Fan, X.; Shen, W.; Zhu, J. Carbon-coated silicon nanowire array films for high-performance lithium-ion battery anodes. Appl. Phys. Lett. 2009, 95. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Lee, H.S.; Han, D.W.; Kim, G.S.; Lee, D.; Lee, G.; Kang, Y.M.; Jo, M.H. Maximum Li storage in Si nanowires for the high capacity three dimensional Li-ion battery. Appl. Phys. Lett. 2010, 96. [Google Scholar] [CrossRef]
- Yang, Y.; McDowell, M.T.; Jackson, A.; Cha, J.J.; Hong, S.S.; Cui, Y. New nanostructured Li2S/Silicon rechargeable battery with high specific energy. Nano Lett. 2010, 10, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, H.; Au, M.; Chen, N.; Heiden, P.A.; Yassar, R.S. In situ electrochemical lithiation/delithiation observation of individual amorphous si nanorods silicon nanotube battery anodes. ACS Nano 2011, 5, 7805–7811. [Google Scholar] [CrossRef] [PubMed]
- Quiroga-González, E.; Carstensen, J.; Föll, H. Structural and electrochemical investigation during the first charging cycles of silicon microwire array anodes for high capacity lithium ion batteries. Materials 2013, 6, 626–636. [Google Scholar] [CrossRef]
- Föll, H.; Carstensen, J.; Ossei-Wusu, E.; Cojocaru, A.; Quiroga-González, E.; Neumann, G. Optimized Cu contacted Si nanowire anodes for Li ion batteries made in a production near process. J. Electrochem. Soc. 2011, 158, A580–A584. [Google Scholar] [CrossRef]
- Quiroga-González, E.; Ossei-Wusu, E.; Carstensen, J.; Föll, H. How to make optimized arrays of Si nanowires suitable as superior anode for Li-ion batteries. J. Electrochem. Soc. 2011, 158, E119–E123. [Google Scholar] [CrossRef]
- Xiao, J.; Xu, W.; Wang, D.; Choi, D.; Wang, W.; Li, X.; Graff, G.L.; Liu, J.; Zhang, J.G. Stabilization of silicon anode for Li-ion batteries. J. Electrochem. Soc. 2010, 157, A1047–A1051. [Google Scholar] [CrossRef]
- Park, M.-H.; Kim, M.G.; Joo, J.; Kim, K.; Kim, J.; Ahn, S.; Cui, Y.; Cho, J. Silicon nanotube battery anodes. Nano Lett. 2009, 9, 3844–3847. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.T.; Pernites, R.B.; Nitta, N.; Isaacson, M.; Sinsabaugh, S.L. Freestanding macroporous Silicon and pyrolyzed polyacrylonitrile as a composite anode for lithium ion batteries. Chem. Mater. 2012, 24, 2998–3003. [Google Scholar] [CrossRef]
- Cui, L.-F.; Yang, Y.; Hsu, C.-M.; Cui, Y. Carbon-silicon core-shell nanowires as high capacity electrode for Lithium ion batteries. Nano Lett. 2009, 9, 3370–3374. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wu, H.; Hong, S.S.; Cui, L.; McDonough, J.R.; Bohy, S.; Cui, Y. Si nanoparticle-decorated Si nanowire networks for Li-ion battery anodes. Chem. Commun. 2011, 47, 367–369. [Google Scholar] [CrossRef]
- Green, M.; Fielder, E.; Scrosati, B.; Wachtler, M.; Moreno, J.S. Structured silicon anodes for lithium battery applications. Electrochem. Solid-State Lett. 2003, 6, A75–A79. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, Y.; Wang, L.; Yang, Y. Silicon nanowires coated with copper layer as anode materials for lithium-ion batteries. J. Power Sources 2011, 196, 6657–6662. [Google Scholar] [CrossRef]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; McDowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L.; Cui, Y. Stable cycling of double-walled silicon nanotube battery anodes through solid-electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Choi, W.; Byun, D.; Lee, J.K. Electrochemical characteristics of phosphorus doped silicon for the anode material of lithium secondary batteries. Solid State Ionics 2012, 212, 43–46. [Google Scholar] [CrossRef]
- Chan, C.K.; Patel, R.N.; O’Connell, M.J.; Korgel, B.A.; Cui, Y. Solution-grown silicon nanowires for lithium-ion battery anodes. ACS Nano 2010, 4, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, X.; Chen, L.; Wu, Z.; Liang, Y. A high capacity nano Si composite anode material for lithium rechargeable batteries. Electrochem. Solid-State Lett. 1999, 2, 547–549. [Google Scholar] [CrossRef]
- Chen, P.-C.; Xu, J.; Chen, H.; Zhou, C. Hybrid silicon-carbon nanostructured composites as superior anodes for lithium ion batteries. Nano Res. 2011, 4, 290–296. [Google Scholar] [CrossRef]
- Cho, G.; Noh, J.; Sung, H.; Lee, S.; Ahn, H.; Nam, T.; Kim, K. Improved electrochemical properties of patterned Si film electrodes. Microelectron. Eng. 2012, 89, 104–108. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, N.; Fan, Z.; Dhanabalan, A.; Chen, C.; Li, Y.; Yang, M.; Wang, C. Facile synthesis of silicon films by photosintering as anode materials for lithium-ion batteries. J. Power Sources 2012, 214, 21–27. [Google Scholar] [CrossRef]
- Thakur, M.; Isaacson, M.; Sinsabaugh, S.L.; Wong, M.S.; Biswal, S.S. Gold-coated porous silicon films as anodes for lithium ion batteries. J. Power Sources 2012, 205, 426–432. [Google Scholar] [CrossRef]
- Shu, Z.X.; McMillan, R.S.; Murray, J.J. Electrochemical intercalation of lithium into graphite. J. Electrochem. Soc. 1993, 140, 922–927. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Quiroga-González, E.; Carstensen, J.; Föll, H. Optimal Conditions for Fast Charging and Long Cycling Stability of Silicon Microwire Anodes for Lithium Ion Batteries, and Comparison with the Performance of Other Si Anode Concepts. Energies 2013, 6, 5145-5156. https://doi.org/10.3390/en6105145
Quiroga-González E, Carstensen J, Föll H. Optimal Conditions for Fast Charging and Long Cycling Stability of Silicon Microwire Anodes for Lithium Ion Batteries, and Comparison with the Performance of Other Si Anode Concepts. Energies. 2013; 6(10):5145-5156. https://doi.org/10.3390/en6105145
Chicago/Turabian StyleQuiroga-González, Enrique, Jürgen Carstensen, and Helmut Föll. 2013. "Optimal Conditions for Fast Charging and Long Cycling Stability of Silicon Microwire Anodes for Lithium Ion Batteries, and Comparison with the Performance of Other Si Anode Concepts" Energies 6, no. 10: 5145-5156. https://doi.org/10.3390/en6105145






