The Effect of Effluent Recirculation in a Semi-Continuous Two-Stage Anaerobic Digestion System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Inoculums
2.2. Experimental Set-Up
2.2.1. Reactors
2.2.2. Reactors Seeding and Start Up
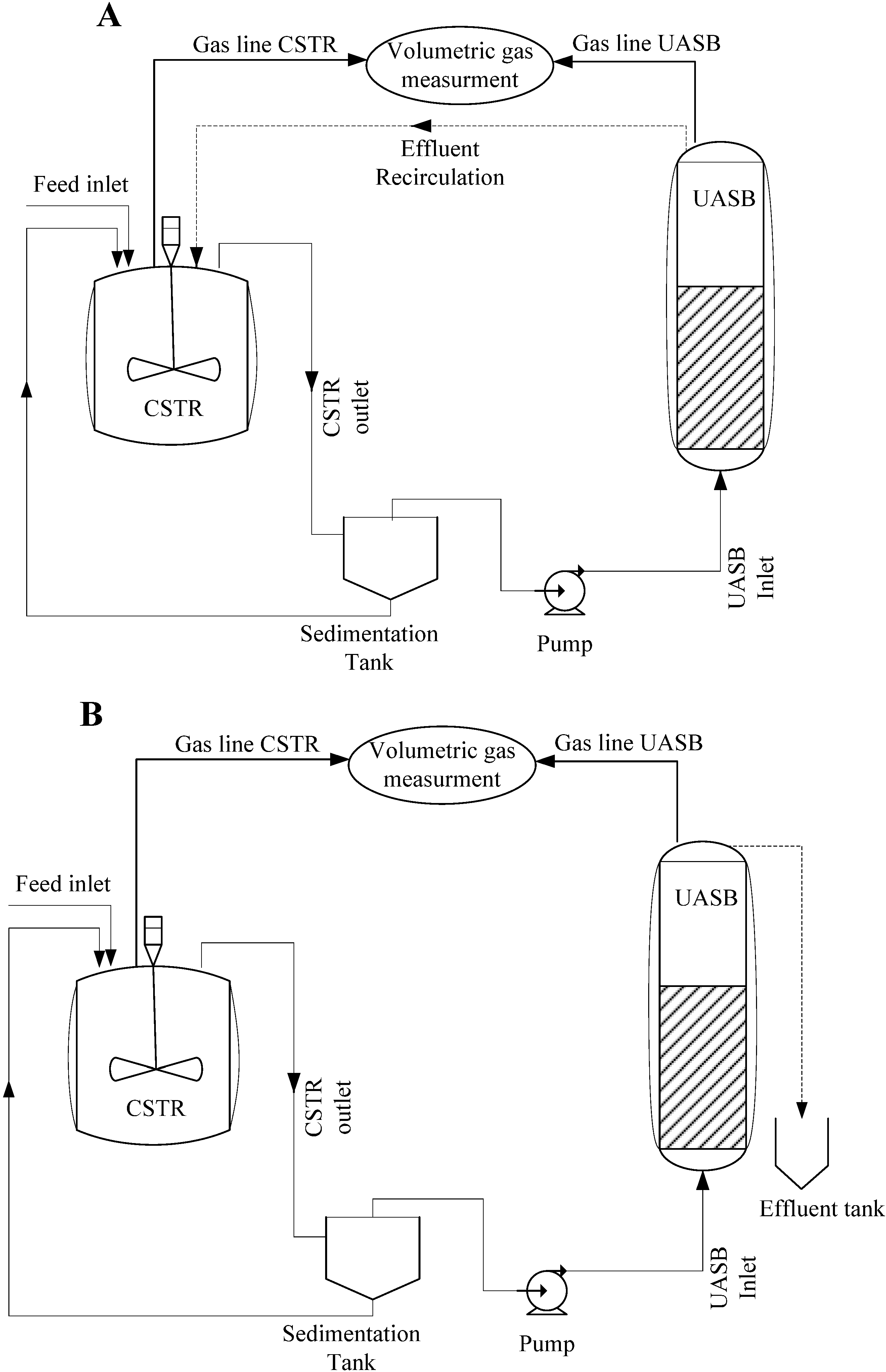
2.2.3. Reactors Configuration
2.2.4. Experimental Procedure
| Stage | OLR (gVS/L/day) | HRT in CSTR (day) | HRT in UASB (day) | Duration (day) |
|---|---|---|---|---|
| 1 | 2.0 | 10.0 | 7.50 | 30.0 |
| 2 | 2.7 | 7.5 | 5.62 | 30.0 |
| 3 | 4.0 | 5.0 | 3.75 | 15.0 |
| 4 | 8.0 | 2.5 | 1.88 | 8.0 |
| 5 | 10 | 2.0 | 1.50 | 6.0 |
| 6 | 20 | 1.0 | 0.75 | 6.0 |
2.2.5. Analytical Methods
3. Results

3.1. Gas Production
3.1.1. Biogas Production in Open System (without Effluent Recirculation)

3.1.2. Biogas Production in Closed System (with Effluent Recirculation)

3.2. COD and Its Removal
3.2.1. Open System
| Substate | OLR (gVS/L/day) | COD (mg/L) | COD removal efficiency (%) | COD (mg/L) | COD removal efficiency (%) | ||
|---|---|---|---|---|---|---|---|
| Open system | Closed system | ||||||
| CSTR | UASB | CSTR | UASB | ||||
| Cotton | 2 | 3,259 ± 638 | 1,194 ± 95 | 61.9 ± 16.3 | 4,204 ± 742 | 1,958 ± 791 | 49.2 ± 21.6 |
| 2.7 | 3,699 ± 844 | 229 ± 82 | 93.3 ± 3.5 | 2,651 ± 572 | 1,525 ± 172 | 39.4 ± 16.5 | |
| 4 | 3,241 ± 545 | 196 ± 33 | 93.9 ± 1.8 | 2,571 ± 204 | 1,476 ± 357 | 45.6 ± 15.9 | |
| Starch | 2 | 4,324 ± 1,345 | 1,308 ± 335 | 69.7 ± 14.2 | 5,041 ± 430 | 3,273 ± 674 | 35.0 ± 12.8 |
| 2.7 | 9,034 ± 1,127 | 317 ± 143 | 96.49 ± 1.2 | 4,463 ± 626 | 3,353 ± 374 | 24.8 ± 16.2 | |
| 4 | 19,500 ± 2,493 | 350 ± 165 | 98.2 ± 2.1 | 4,396 ± 565 | 3,051 ± 494 | 30.5 ± 7.8 | |
| 8 | 21,450 ± 2,185 | 708 ± 186 | 96.6 ± 2.7 | 17,865 ± 2,767 | 3,091 ± 423 | 82.69 ± 3.8 | |
| 10 | 27,716 ± 1,606 | 876 ± 270 | 96.8 ± 1.3 | 25,833 ± 3,333 | 3,995 ± 873 | 84.5 ± 4.2 | |
| 20 | 23,800 ± 2,347 | 898 ± 98 | 96.2 ± 2.5 | 12,516 ± 2,171 | 3,256 ± 658 | 73.9 ± 9.5 | |
3.2.2. Closed System
3.3. Effect of Nutrients
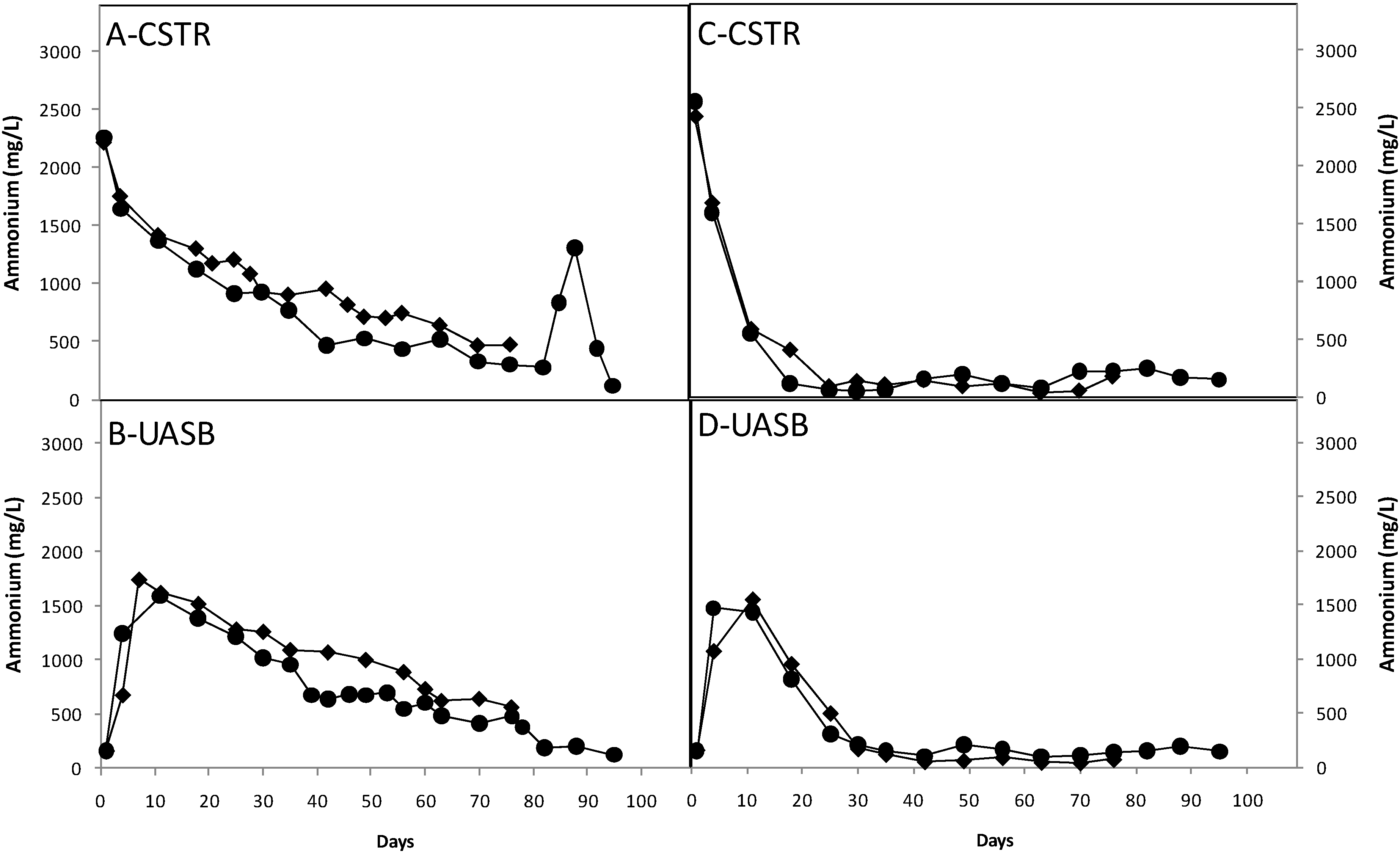
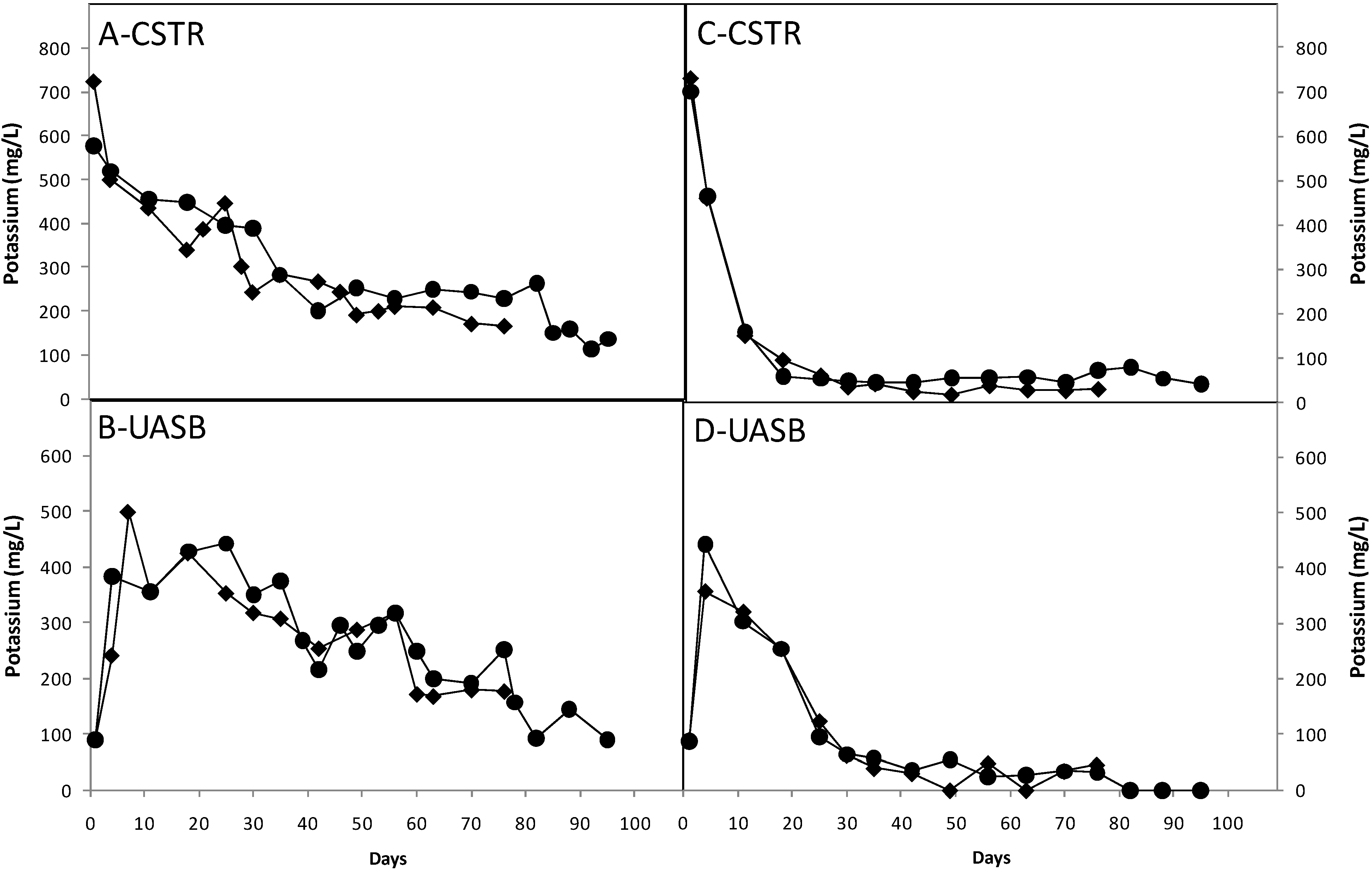
3.4. Ratio of Methane to Carbon Dioxide
| Substrate | OLR (gVS/L/day) | Ratio of methane to carbon dioxide | |||
|---|---|---|---|---|---|
| Open system | Closed system | ||||
| CSTR | UASB | CSTR | UASB | ||
| Cotton | 2 | 1.8 ± 0.2 | 4.4 ± 0.8 | 1.9 ± 0.2 | 4.4 ± 0.4 |
| 2.7 | 1.7 ± 0.1 | 4.9 ± 0.6 | 2.0 ± 0.1 | 4.2 ± 0.2 | |
| 4 | 2.0 ± 0.0 | 4.9 ± 0.2 | 2.1 ± 0.0 | 4.1 ± 0.1 | |
| Starch | 2 | 2.0 ± 1.0 | 4.0 ± 0.5 | 2.1 ± 0.2 | 4.1 ± 0.6 |
| 2.7 | 1.3 ± 0.3 | 4.5 ± 0.6 | 2.2 ± 0.0 | 3.4 ± 0.2 | |
| 4 | 1.0 ± 0.1 | 4.9 ± 0.2 | 1.8 ± 0.5 | 3.4 ± 0.1 | |
| 8 | 0.3 ± 0.2 | 4.4 ± 0.1 | 0.9 ± 0.1 | 4.2 ± 0.3 | |
| 10 | 0.1 ± 0.0 | 4.2 ± 0.2 | 0.8 ± 0.1 | 4.7 ± 0.4 | |
| 20 | 0.07 ± 0.04 | 3.5 ± 0.8 | 0.7 ± 0.1 | 4.3 ± 0.8 | |
4. Discussion
5. Conclusions
Acknowledgements
Conflicts of Interest
References
- Asam, Z.-U.-Z.; Poulsen, T.G.; Nizami, A.-S.; Rafique, R.; Kiely, G.; Murphy, J.D. How can we improve biomethane production per unit of feedstock in biogas plants? Appl. Energy 2011, 88, 2013–2018. [Google Scholar] [CrossRef]
- Chynoweth, D.P.; Owens, J.M.; Legrand, R. Renewable methane from anaerobic digestion of biomass. Renew. Energy 2001, 22, 1–8. [Google Scholar] [CrossRef]
- Yadvika; Santosh; Sreekrishnan, T.R.; Kohli, S.; Rana, V. Enhancement of biogas production from solid substrates using different techniques––A review. Bioresour. Technol. 2004, 95, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.; Zeeman, G.; Gijzen, H.; Lettinga, G. Solids removal in upflow anaerobic reactors, a review. Bioresour. Technol. 2003, 90, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vartak, D.R.; Engler, C.R.; McFarland, M.J.; Ricke, S.C. Attached-film media performance in psychrophilic anaerobic treatment of dairy cattle wastewater. Bioresour. Technol. 1997, 62, 79–84. [Google Scholar] [CrossRef]
- Tay, J.-H.; Zhang, X. Stability of high-rate anaerobic systems. I: Performance under shocks. J. Environ. Eng. 2000, 126, 713–725. [Google Scholar] [CrossRef]
- Lettinga, G. Anaerobic digestion and wastewater treatment systems. Antonie Van Leeuwenhoek 1995, 67, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.E.; Ahring, B.K. Granular sludge formation in upflow anaerobic sludge blanket (UASB) reactors. Biotechnol. Bioeng. 1996, 49, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Lyberatos, G.; Skiadas, I.V. Modelling of anaerobic digestion—A review. Glob. Nest. Int. J. 1999, 1, 63–76. [Google Scholar]
- Lettinga, G.; van Velsen, A.F.M.; Hobma, S.W.; de Zeeuw, W.; Klapwijk, A. Use of the upflow sludge blanket (USB) reactor concept for biological wastewater treatment, especially for anaerobic treatment. Biotechnol. Bioeng. 1980, 22, 699–734. [Google Scholar] [CrossRef]
- Bal, A.S.; Dhagat, N.N. Upflow anaerobic sludge blanket reactor—A review. Indian J. Environ. Health 2001, 43, 1–83. [Google Scholar] [PubMed]
- Shin, H.S.; Han, S.K.; Song, Y.C.; Lee, C.Y. Performance of UASB reactor treating leachate from acidogenic fermenter in the two-phase anaerobic digestion of food waste. Water Res. 2001, 35, 3441–3447. [Google Scholar] [CrossRef] [PubMed]
- Ince, O. Performance of a two-phase anaerobic digestion system when treating dairy wastewater. Water Res. 1998, 32, 2707–2713. [Google Scholar] [CrossRef]
- Demirel, B.; Yenigün, O. Two-phase anaerobic digestion processes: A review. J. Chem. Technol. Biotechnol. 2002, 77, 743–755. [Google Scholar] [CrossRef]
- Verrier, D.; Roy, F.; Albagnac, G. Two-phase methanization of solid vegetable wastes. Biol. Wastes 1987, 22, 163–177. [Google Scholar] [CrossRef]
- Bouallagui, H.; Torrijos, M.; Godon, J.J.; Moletta, R.; Ben Cheikh, R.; Touhami, Y.; Delgenes, J.P.; Hamdi, M. Two-phases anaerobic digestion of fruit and vegetable wastes: Bioreactors performance. Biochem. Eng. J. 2004, 21, 193–197. [Google Scholar] [CrossRef]
- Chanakya, H.N.; Borgaonkar, S.; Rajan, M.G.C.; Wahi, M. Two-phase anaerobic digestion of water hyacinth or urban garbage. Bioresour. Technol. 1992, 42, 123–131. [Google Scholar] [CrossRef]
- Ghosh, S.; Ombregt, J.P.; Pipyn, P. Methane production from industrial wastes by two-phase anaerobic digestion. Water Res. 1985, 19, 1083–1088. [Google Scholar] [CrossRef]
- Yu, H.W.; Samani, Z.; Hanson, A.; Smith, G. Energy recovery from grass using two-phase anaerobic digestion. Waste Manag. 2002, 22, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Calzada, J.F.; de Porres, E.; Yurrita, A.; de Arriola, M.C.; de Micheo, F.; Rolz, C.; Menchú, J.F.; Cabello, A. Biogas production from coffee pulp juice: One- and two-phase systems. Agric. Wastes 1984, 9, 217–230. [Google Scholar] [CrossRef]
- Koster, I.W. Liquefaction and acidogenesis of tomatoes in an anaerobic two-phase solid-waste treatment system. Agric. Wastes 1984, 11, 241–252. [Google Scholar] [CrossRef]
- Yeoh, B.G. Two-phase anaerobic treatment of cane-molasses alcohol stillage. Water Sci. Technol. 1997, 36, 441–448. [Google Scholar] [CrossRef]
- Goel, B.; Pant, D.C.; Kishore, V.V.N. Two-phase anaerobic digestion of spent tea leaves for biogas and manure generation. Bioresour. Technol. 2001, 80, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.V.; Liao, P.H. Two-phase anaerobic digestion of screened dairy manure. Biomass 1985, 8, 81–90. [Google Scholar] [CrossRef]
- Liao, P.H.; Lo, K.V. Two-phase thermophilic anaerobic digestion of screened dairy manure. Biomass 1985, 8, 185–194. [Google Scholar] [CrossRef]
- Lo, K.V.; Chen, W.Y.; Liao, P.H. Mesophilic digestion of screened dairy manure using anaerobic rotating biological contact reactor. Biomass 1986, 9, 81–92. [Google Scholar] [CrossRef]
- Demirer, G.N.; Chen, S. Two-phase anaerobic digestion of unscreened dairy manure. Process Biochem. 2005, 40, 3542–3549. [Google Scholar] [CrossRef]
- Fezzani, B.; Ben Cheikh, R. Two-phase anaerobic co-digestion of olive mill wastes in semi-continuous digesters at mesophilic temperature. Bioresour. Technol. 2010, 101, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Banks, C.J. Evaluation of a two stage anaerobic digester for the treatment of mixed abattoir wastes. Process Biochem. 2003, 38, 1267–1273. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Madura, R.L.; Walling, D.A.; Farrell, J.B. Volatile solids reduction in two-phase and conventional anaerobic sludge digestion. Water Res. 1996, 30, 1041–1048. [Google Scholar] [CrossRef]
- Diamantis, V.; Aivasidis, A. Two-stage uasb design enables activated-sludge free treatment of easily biodegradable wastewater. Bioproc. Biosyst. Eng. 2010, 33, 287–292. [Google Scholar] [CrossRef]
- Diamantis, V.I.; Aivasidis, A. Comparison of single- and two-stage UASB reactors used for anaerobic treatment of synthetic fruit wastewater. Enzym. Microb. Technol. 2007, 42, 6–10. [Google Scholar] [CrossRef]
- Feng, C.; Shimada, S.; Zhang, Z.; Maekawa, T. A pilot plant two-phase anaerobic digestion system for bioenergy recovery from swine wastes and garbage. Waste Manag. 2008, 28, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Sabry, T. Application of the UASB inoculated with flocculent and granular sludge in treating sewage at different hydraulic shock loads. Bioresour. Technol. 2008, 99, 4073–4077. [Google Scholar] [CrossRef] [PubMed]
- Joubert, W.A.; Britz, T.J.; Lategan, P.M. The effect of effluent recirculation on the performance of a two stage anaerobic process. Biotechnol. Lett. 1985, 7, 853–858. [Google Scholar] [CrossRef]
- Suss, H.U.; Kronis, J.D. The Correlation of Cod and Yield in Chemical Pulp Bleaching. In Presented at Tappi Breaking the Pulp Yield Barrier Symposium, Atlanta, GA, USA, 8–12 March 1998.
- Osuna, M.B.; Zandvoort, M.H.; Iza, J.M.; Lettinga, G.; Lens, P.N.L. Effects of trace element addition on volatile fatty acid conversions in anaerobic granular sludge reactors. Environ. Technol. 2003, 24, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Jeihanipour, A.; Aslanzadeh, S.; Rajendran, K.; Balasubramanian, G.; Taherzadeh, M. High-rate biogas production from waste textiles using a two-stage process. Renew. Energy 2013, 52, 128–135. [Google Scholar] [CrossRef]
- Alimahmoodi, M.; Mulligan, C.N. Anaerobic bioconversion of carbon dioxide to biogas in an upflow anaerobic sludge blanket reactor. J. Air Waste Manag. Assoc. 2008, 58, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Deublein, D.; Steinhauser, A. Biology. In Biogas from Waste and Renewable Resources; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 93–128. [Google Scholar]
- Kosaric, N.; Blaszczyk, R.; Orphan, L. Factors influencing formation and maintenance of granules in anaerobic sludge blanket reactors (UASBR). Water Sci. Technol. 1990, 22, 275–282. [Google Scholar]
- Blaszczyk, R.; Gardner, D.; Kosaric, N. Response and recovery of anaerobic granules from shock loading. Water Res. 1994, 28, 675–680. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aslanzadeh, S.; Rajendran, K.; Jeihanipour, A.; Taherzadeh, M.J. The Effect of Effluent Recirculation in a Semi-Continuous Two-Stage Anaerobic Digestion System. Energies 2013, 6, 2966-2981. https://doi.org/10.3390/en6062966
Aslanzadeh S, Rajendran K, Jeihanipour A, Taherzadeh MJ. The Effect of Effluent Recirculation in a Semi-Continuous Two-Stage Anaerobic Digestion System. Energies. 2013; 6(6):2966-2981. https://doi.org/10.3390/en6062966
Chicago/Turabian StyleAslanzadeh, Solmaz, Karthik Rajendran, Azam Jeihanipour, and Mohammad J. Taherzadeh. 2013. "The Effect of Effluent Recirculation in a Semi-Continuous Two-Stage Anaerobic Digestion System" Energies 6, no. 6: 2966-2981. https://doi.org/10.3390/en6062966






