Effects of Environmental Factors and Nutrient Availability on the Biochemical Composition of Algae for Biofuels Production: A Review
Abstract
:1. Introduction
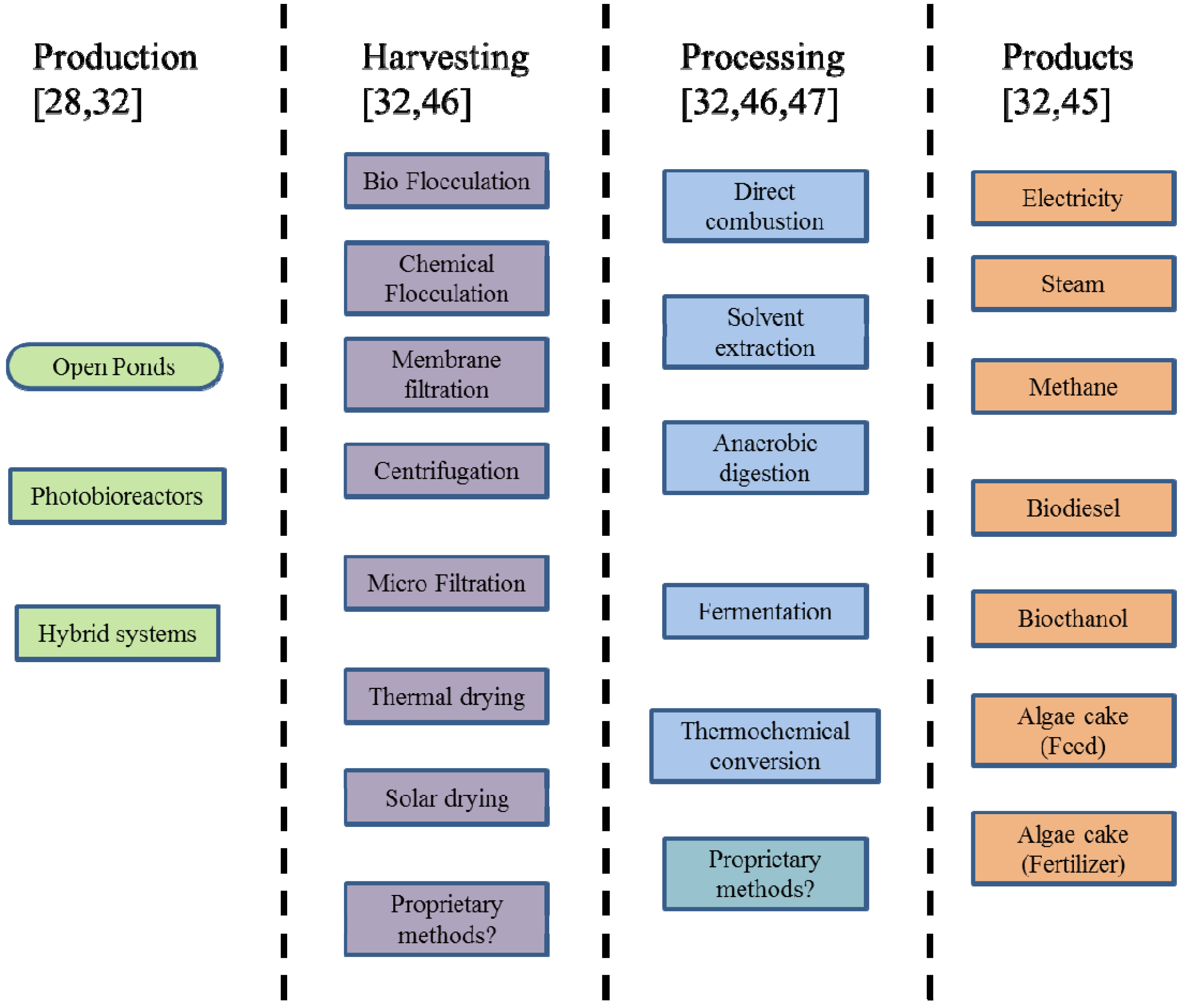
1.1. Algae Production, Processing and Use
| Process | Advantages | Limitations |
|---|---|---|
| Pyrolysis | High bio-oil yields possible(up to 57.5% w/w for fast and flash pyrolysis [43]) | Low-moisture-content biomass required High-energy-content required to dry feedstock |
| Thermochemical liquefaction | Algal wet slurry can be used Energy (and cost) reduction High yields possible (up to 60% w/w [44]) | Reactors are complex and expensive |
| Fermentation | Co-products can be utilized Conversion of sugar to bioethanol possible | Long processing times required Biomass has to be preprocessed to be converted to sugars |
| Transesterification | Enhanced physical properties of renewable fuels Biodiesel has a current market that simplifies commercialization | Limited to conversion of lipids and does not utilize carbohydrate and protein fractions of feedstock |
1.2. Relevance of Individual Algal Cellular Components for Biofuels Production
2. Effect of Environmental Factors
2.1. Temperature
2.2. Light
2.3. pH
2.4. Salinity
2.5. Nutrients
3. Interaction among Environmental Factors
| Factor | Organism | Conditions | Biochemical changes observed | References |
|---|---|---|---|---|
| Temperature | Botryccoccus braunii | Increased from 25 to 32 °C | Decrease in intracellular lipid content from 22% to 5% wt. Accumulation of polysaccharides | [66] |
| Chlorella vulgaris | Increased from 20 to 38 °C | Decrease in starch resulting in increase in sucrose | [65] | |
| Increased from 10 to 38 °C | Transformation of L starch (high molecular weight) to S starch (low molecular weight) Reversible with temperature | [89] | ||
| Haematococcus pluvialis | Increased from 20 to 30 °C | 3-fold increase in astaxanthin formation | [91] | |
| Chlorococcum sp. | Temperature increase from 20 to 35 °C under nitrogen deprivation | Two fold increase in total carotenoid content | [92] | |
| Nitella mucronata Miquel | Increased from 5 to 20 °C | Increase in velocity of cytoplasmic streaming | [73] | |
| Light | Dunaliella virdis | Darkness (No light) | Increase in total lipid content Decrease in free fatty acids, alcohol, sterol | [30] |
| Nannochloropsis sp. | Light limited conditions | Increase in lipid content Increase in EPA * proportions | [26] | |
| Porphyridium cruentum | Red light | Enhanced Photosystem II relative to Photosystem I and phycobilisome | [212] | |
| Chlorella vulgaris | Red light | Increase in sucrose and starch formation | [113] | |
| Blue light | Increase in lipid fraction and alcohol-water insoluble non carbohydrate fraction | |||
| pH | Chlamydomonas acidophila | pH 4.4 | Denaturation of V-lysin | [128] |
| Coccochloris peniocystis | pH decreased from 7.0 to 5.0 and 6.0 | Decrease in total accumulated carbon and oxygen evolution | [130] | |
| Nitrogen | Nannochloropsis oculata | 75% decrease in Nitrogen | Increase in lipid synthesis from 7.90% to 15.31% | [80] |
| Phaeodactylum tricornutum | Nitrogen limitation | Increase in lipid synthesis; Decrease in protein content | [25] | |
| Chlorella vulgaris | 75% decrease in Nitrogen | Increase in lipid synthesis from 5.90% to 16.41% | [80] | |
| Haematococcus pluvialis | Nitrogen limitation | Increase in carotenoid formation (13% w/w) | [174] | |
| Phosphorus | Chlamydomonas reinhardtii | Limitation | Decrease in phosphatidylglycerol | [181] |
| Ankistrodesmus falcatus | Limitation | Decrease in chl a and protein; Increase in carbohydrate and lipids | [29,183,184] | |
| Selenastrum minutum | Starvation | Reduced rate of respiration; Decreased photosynthetic CO2 fixation | [186] | |
| Iron | Dunaliella tertiolecta | Limitation | Decrease in cellular chlorophyll concentration | [194] |
| Chlorella vulgaris | High concentration of iron | Increase in lipid content | [195] | |
| Haematococcus pluvialis | High concentration of iron | Increase in carotenoid formation | [185] | |
| Carbon | Chlamydomonas reinhardtii | pH exceeding 9.0 | Inefficient accumulation of carbon High supply of carbonates required to maintain photosynthetic activity | [138] |
| Dunaliella salina | CO2 concentration increased from 2% to 10% for 1 day | 30% increase in amount of fatty acid (dry weight basis) | [153] | |
| CO2 concentration increased from 2% to 10% for 7 days | 2.7 fold increase in fatty acid | |||
| Spirulina platensis | Elevated CO2 concentrations | Increase in carbohydrate content; Decrease in proteins and pigments | [155] |
4. Conclusions
4.1. Algae as a Sustainable Biofuel Source
4.2. Future Outlook
Conflicts of Interest
References
- Nichols, N.N.; Bothast, R.J. Production of Ethanol from Grain. In Genetic Improvement of Bioenergy Crops; Vermerris, W., Ed.; Springer: New York, NY, USA, 2008; pp. 75–88. [Google Scholar]
- Goldemberg, J.; Coelho, S.T.; Guardabassi, P. The sustainability of ethanol production from sugarcane. Energy Policy 2008, 36, 2086–2097. [Google Scholar] [CrossRef]
- Samson, R.A.; Omielan, J.A. Switchgrass: A Potential Biomass Energy Crop for Ethanol Production. In Proceedings of the Thirteenth North American Prarie Conference, Windsor, ON, Canada, 6–9 August 1992; pp. 253–258.
- Schmer, M.R.; Vogel, K.P.; Mitchell, R.B.; Perrin, R.K. Net energy of cellulosic ethanol from switchgrass. Proc. Natl. Acad. Sci. USA 2008, 105, 464–469. [Google Scholar] [CrossRef]
- Sheehan, J.; Aden, A.; Paustian, K.; Killian, K.; Brenner, J.; Walsh, M.; Nelson, R. Energy and environmental aspects of using corn stover for fuel ethanol. J. Ind. Ecol. 2003, 7, 117–146. [Google Scholar] [CrossRef]
- Ballesteros, I.; Negro, M.J.; Oliva, J.M.; Cabañas, A.; Manzanares, P.; Ballesteros, M. Ethanol production from steam-explosion pretreated wheat straw. Appl. Biochem. Biotechnol. 2006, 129–132, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Murthy, G.S. Pretreatments and enzymatic hydrolysis of grass straws for ethanol production in the Pacific Northwest U.S. Biol. Eng. 2010, 3, 97–110. [Google Scholar] [CrossRef]
- Kumar, D.; Murthy, G.S. Impact of pretreatment and downstream processing technologies on economics and energy in cellulosic ethanol production. Biotechnol. Biofuels 2011, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, O.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, I.; Sahajpal, R.; Zhang, X.; Izaurralde, R.C.; Gross, K.L.; Robertson, G.P. Sustainable bioenergy production from marginal lands in the U.S. Midwest. Nature 2013, 493, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Gu, S. Commercialization potential of microalgae for biofuels production. Renew. Sustain. Energy Rev. 2010, 14, 2596–2610. [Google Scholar] [CrossRef]
- Amin, S. Review on biofuel oil and gas production processes from microalgae. Energy Convers. Manag. 2009, 50, 1834–1840. [Google Scholar] [CrossRef]
- Nakas, J.; Schaedle, M.; Parkinson, C.; Coonley, C.; Tanenbaum, S. System development for linked-fermentation production of solvents from algal biomass. Appl. Environ. Microbiol. 1983, 46, 1017–1023. [Google Scholar] [PubMed]
- Sander, K.; Murthy, G.S. Life cycle analysis of algae biodiesel. Int. J. Life Cycle Assess. 2010, 15, 704–714. [Google Scholar] [CrossRef]
- Georgianna, D.R.; Mayfield, S.P. Exploiting diversity and synthetic biology for the production of algal biofuels. Nature 2012, 488, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J. A Look Back at the U.S. Department of Energy’s Aquatic Species Program: Biodiesel from Algae; National Renewable Energy Laboratory: Golden, CO, USA, 1998. [Google Scholar]
- Ahmad, A.; Yasin, N.; Derek, C.; Lim, J. Microalgae as a sustainable energy source for biodiesel production: A review. Renew. Sustain. Energy Rev. 2011, 15, 584–593. [Google Scholar] [CrossRef]
- Deng, X.; Li, Y.; Fei, X. Microalgae: A promising feedstock for biodiesel. Afr. J. Microbiol. Res. 2009, 3, 1008–1014. [Google Scholar]
- Khan, S.A.; Hussain, M.Z.; Prasad, S.; Banerjee, U. Prospects of biodiesel production from microalgae in India. Renew. Sustain. Energy Rev. 2009, 13, 2361–2372. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Eshaq, F.S.; Ali, M.N.; Mohd, M.K. Spirogyra biomass a renewable source for biofuel (bioethanol) production. Int. J. Eng. Sci. Technol. 2010, 2, 7045–7054. [Google Scholar]
- Harun, R.; Danquah, M.K.; Forde, G.M. Microalgal biomass as a fermentation feedstock for bioethanol production. J. Chem. Technol. Biotechnol. 2010, 85, 199–203. [Google Scholar]
- John, R.P.; Anisha, G.; Nampoothiri, K.M.; Pandey, A. Micro and macroalgal biomass: A renewable source for bioethanol. Bioresour. Technol. 2011, 102, 186–193. [Google Scholar] [CrossRef]
- Moen, E. Biological Degradation of Brown Seaweeds. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, November 1997. [Google Scholar]
- Morris, I.; Glover, H.; Yentsch, C. Products of photosynthesis by marine phytoplankton: The effect of environmental factors on the relative rates of protein synthesis. Mar. Biol. 1974, 27, 1–9. [Google Scholar] [CrossRef]
- Sukenik, A.; Carmeli, Y.; Berner, T. Regulation of fatty acid composition by irradiance level in the eustigmatophyte Nannochloropsis sp. J. Phycol. 1989, 25, 686–692. [Google Scholar] [CrossRef]
- Thompson, P.A.; Guo, M.; Harrison, P.J. Effects of variation in temperature. I. On the biochemical composition of eight species of marine phytoplankton. J. Phycol. 1992, 28, 481–488. [Google Scholar] [CrossRef]
- Chen, C.Y.; Durbin, E.G. Effects of pH on the growth and carbon uptake of marine phytoplankton. Mar. Ecol.-Prog. Ser. 1994, 109, 83–94. [Google Scholar] [CrossRef]
- Kilham, S.; Kreeger, D.; Goulden, C.; Lynn, S. Effects of nutrient limitation on biochemical constituents of Ankistrodesmus falcatus. Freshw. Biol. 1997, 38, 591–596. [Google Scholar] [CrossRef]
- Gordillo, F.J.L.; Goutx, M.; Figueroa, F.L.; Niell, F.X. Effects of light intensity, CO2 and nitrogen supply on lipid class composition of Dunaliella viridis. J. Appl. Phycol. 1998, 10, 135–144. [Google Scholar] [CrossRef]
- Visviki, I.; Santikul, D. The pH tolerance of Chlamydomonas applanata (Volvocales, Chlorophyta). Arch. Environ. Contam. Toxicol. 2000, 38, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Potter, A.L.; Hassid, W.Z. Starch. II. Molecular weights of amyloses and amylopectins from starches of various plant origins. J. Am. Chem. Soc. 1948, 70, 3774–3777. [Google Scholar] [CrossRef] [PubMed]
- Roessler, P.G. Environmental control of glycerolipid metabolism in microalgae: Commercial implications and future research directions. J. Phycol. 1990, 26, 393–399. [Google Scholar] [CrossRef]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High lipid induction in microalgae for biodiesel production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yeh, K.L.; Aisyah, R.; Lee, D.J.; Chang, J.S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Li, Y.; Wang, X.; Wan, Y.; Chen, Q.; Wang, C.; Lin, X.; Liu, Y.; Chen, P.; Ruan, R. Microwave-assisted pyrolysis of microalgae for biofuel production. Bioresour. Technol. 2011, 102, 4890–4896. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.P.; Wu, Y.L.; Yang, M.D.; Li, C.; Tong, J.M. Thermochemical catalytic liquefaction of the marine microalgae Dunaliella tertiolecta and characterization of bio-oils. Energy Fuels 2009, 23, 3753–3758. [Google Scholar] [CrossRef]
- Ueno, Y.; Kurano, N.; Miyachi, S. Ethanol production by dark fermentation in the marine green alga, Chlorococcum littorale. J. Ferment. Bioeng. 1998, 86, 38–43. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, B. Development of biodiesel: Current scenario. Renew. Sustain. Energy Rev. 2009, 13, 1646–1651. [Google Scholar] [CrossRef]
- Goyal, H.B.; Seal, D.; Saxena, R.C. Bio-fuels from thermochemical conversion of renewable resources: A review. Renew. Sustain. Energy Rev. 2008, 12, 504–517. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Ducey, T.; Ro, K.S.; Hunt, P.G. Livestock waste-to-bioenergy generation opportunities. Bioresour. Technol. 2008, 99, 7941–7953. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Wu, Q. High yield bio-oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides. J. Biotechnol. 2004, 110, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Jin, B.; Xu, Y.; Yang, Y.; Bai, X.; Wang, F.; Zhang, L.; Miao, J. Thermo-chemical conversion of Chlorella pyrenoidosa to liquid biofuels. Bioresour. Technol. 2013, 133, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Constraints to commercialization of algal fuels. J. Biotechnol. 2013, 167, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Pragya, N.; Pandey, K.K.; Sahoo, P. A review on harvesting, oil extraction and biofuels production technologies from microalgae. Renew. Sustain. Energy Rev. 2013, 24, 159–171. [Google Scholar] [CrossRef]
- Suali, E.; Sarbatly, R. Conversion of microalgae to biofuel. Renew. Sustain. Energy Rev. 2012, 16, 4316–4342. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, L.J.; Borowitzka, M.A. Commercial production of-carotene by Dunaliella salina in open ponds. Bull. Mar. Sci. 1990, 47, 244–252. [Google Scholar]
- Chen, F.; Zhang, Y. High cell density mixotrophic culture of Spirulina platensis on glucose for phycocyanin production using a fed-batch system. Enzyme Microb. Technol. 1997, 20, 221–224. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.L.; Boyd, E.S.; Peters, J.W.; Posewitz, M.C. Engineering algae for biohydrogen and biofuel production. Curr. Opin. Biotechnol. 2009, 20, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Benemann, J. Feasibility analysis of photobiological hydrogen production. Int. J. Hydrog. Energy 1997, 22, 979–987. [Google Scholar] [CrossRef]
- Hempel, F.; Bozarth, A.S.; Lindenkamp, N.; Klingl, A.; Zauner, S.; Linne, U.; Steinbüchel, A.; Maier, U.G. Microalgae as bioreactors for bioplastic production. Microb. Cell Fact. 2011, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.L.; Gillis, J.M.; Hwang, J.Y. Carbon dioxide mitigation by microalgal photosynthesis. Bull. Korean Chem. Soc. 2003, 24, 1763–1766. [Google Scholar] [CrossRef]
- Keffer, J.E.; Kleinheinz, G.T. Use of Chlorella vulgaris for CO2 mitigation in a photobioreactor. J. Ind. Microbiol. Biotechnol. 2002, 29, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q. Environmental Effects on Cell Composition. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell: Oxford, UK, 2004; pp. 83–93. [Google Scholar]
- Hirano, A.; Ueda, R.; Hirayama, S.; Ogushi, Y. CO2 fixation and ethanol production with microalgal photosynthesis and intracellular anaerobic fermentation. Energy 1997, 22, 137–142. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Choi, S.P.; Lee, J.; Lee, J.H.; Sim, S.J. Hydrothermal acid pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. J. Microbiol. Biotechnol. 2009, 19, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, M.R.; Chakrabarti, R. Use of Algae and Aquatic Macrophytes as Feed in Small-Scale Aquaculture: A Review; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2009. [Google Scholar]
- Zepka, L.Q.; Jacob-Lopes, E.; Goldbeck, R.; Souza-Soares, L.A.; Queiroz, M.I. Nutritional evaluation of single-cell protein produced by Aphanothece microscopica Nägeli. Bioresour. Technol. 2010, 101, 7107–7111. [Google Scholar] [CrossRef]
- Walker, J.B. Inorganic micronutrient requirements of Chlorella. II. Quantitative requirements for iron, manganese, and zinc. Arch. Biochem. Biophys. 1954, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Miyachi, S. Effect of temperature on starch degradation in Chlorella vulgaris 11 h cells. Plant Cell Physiol. 1982, 23, 333–341. [Google Scholar]
- Kalacheva, G.; Zhila, N.; Volova, T.; Gladyshev, M. The effect of temperature on the lipid composition of the green alga Botryococcus. Microbiology 2002, 71, 286–293. [Google Scholar] [CrossRef]
- Fábregas, J.; Maseda, A.; Domínguez, A.; Otero, A. The cell composition of Nannochloropsis sp. changes under different irradiances in semicontinuous culture. World J. Microbiol. Biotechnol. 2004, 20, 31–35. [Google Scholar] [CrossRef]
- Renaud, S.M.; Thinh, L.V.; Lambrinidis, G.; Parry, D.L. Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 2002, 211, 195–214. [Google Scholar] [CrossRef]
- Rhee, G.Y. Effects of environmental factors and their interactions on phytoplankton growth. Adv. Microb. Ecol. 1982, 6, 33–74. [Google Scholar]
- Harris, G.P. Phytoplankton Ecology: Structure, Function and Fluctuation; Chapman and Hall: New York, NY, USA, 1986. [Google Scholar]
- Darley, W.M. Algal Biology: A Physiological Approach; Blackwell: Oxford, UK, 1982; Volume 9, pp. 1–168. [Google Scholar]
- Hope, A.B.; Walker, N.A. The Physiology of Giant Algal Cells; Cambridge University Press: London, UK, 1975. [Google Scholar]
- Raven, J.A.; Geider, R.J. Temperature and algal growth. New Phytol. 1988, 110, 441–461. [Google Scholar] [CrossRef]
- Vonshak, A.; Torzillo, G. Environmental Stress Physiology. In Handbook of Microalgal Culture; Richmond, A., Ed.; Blackwell: Oxford, UK, 2004; pp. 57–82. [Google Scholar]
- Thompson, G.A., Jr. Lipids and membrane function in green algae. Biochim. Biophys. Acta 1996, 1302, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Harwood, J.L. Involvement of Chloroplast Lipids in the Reaction of Plants Submitted to Stress. In Lipids in Photosynthesis: Structure, Function and Genetics; Siegenthaler, P.A., Murata, N., Eds.; Springer: Berlin, Germany, 2004; Volume 6, pp. 287–302. [Google Scholar]
- Guschina, I.A.; Harwood, J.L. Algal Lipids and Effect of the Environment on Their Biochemistry. In Lipids in Aquatic Ecosystems; Springer: Berlin, Germany, 2009; pp. 1–24. [Google Scholar]
- Lynch, D.V.; Thompson, G.A., Jr. Low temperature-induced alterations in the chloroplast and microsomal membranes of Dunaliella salina. Plant Physiol. 1982, 69, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Nishida, I.; Murata, N. Chilling sensitivity in plants and cyanobacteria: The crucial contribution of membrane lipids. Annu. Rev. Plant Biol. 1996, 47, 541–568. [Google Scholar] [CrossRef]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; del Borghi, M. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Konopka, A.; Brock, T.D. Effect of temperature on blue-green algae (cyanobacteria) in Lake Mendota. Appl. Environ. Microbiol. 1978, 36, 572–576. [Google Scholar] [PubMed]
- Cuhel, R.L.; Ortner, P.B.; Lean, D.R.S. Night synthesis of protein by algae. Limnol. Oceanogr. 1984, 29, 731–744. [Google Scholar] [CrossRef]
- Rhee, G.Y.; Gotham, I.J. The effect of environmental factors on phytoplankton growth: Temperature and the interactions of temperature with nutrient limitation. Limnol. Oceanogr. 1981, 26, 635–648. [Google Scholar] [CrossRef]
- Kakinuma, M.; Coury, D.; Kuno, Y.; Itoh, S.; Kozawa, Y.; Inagaki, E.; Yoshiura, Y.; Amano, H. Physiological and biochemical responses to thermal and salinity stresses in a sterile mutant of Ulva pertusa (Ulvales, Chlorophyta). Mar. Biol. 2006, 149, 97–106. [Google Scholar] [CrossRef]
- Emerson, R.; Stauffer, J.; Umbreit, W. Relationships between phosphorylation and photosynthesis in Chlorella. Am. J. Bot. 1944, 31, 107–120. [Google Scholar] [CrossRef]
- Mitsui, A.; United States-Japan Cooperative Science Program; National Science Foundation (U.S.). Biological Solar Energy Conversion; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Nakamura, Y. Change in molecular weight distribution in starch when degraded at different temperatures in Chlorella vulgaris. Plant Sci. Lett. 1983, 30, 259–265. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyachi, S. Change in starch photosynthesized at different temperatures in Chlorella. Plant Sci. Lett. 1982, 27, 1–6. [Google Scholar] [CrossRef]
- Nakamura, Y.; Imamura, M. Change in properties of starch when photosynthesized at different temperatures in Chlorella vulgaris. Plant Sci. Lett. 1983, 31, 123–131. [Google Scholar] [CrossRef]
- Armstrong, G.A.; Hearst, J.E. Carotenoids 2: Genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J. 1996, 10, 228–237. [Google Scholar] [PubMed]
- Tjahjono, A.E.; Hayama, Y.; Kakizono, T.; Terada, Y.; Nishio, N.; Nagai, S. Hyper-accumulation of astaxanthin in a green alga Haematococcus pluvialis at elevated temperatures. Biotechnol. Lett. 1994, 16, 133–138. [Google Scholar] [CrossRef]
- Liu, B.H.; Lee, Y.K. Secondary carotenoids formation by the green alga Chlorococcum sp. J. Appl. Phycol. 2000, 12, 301–307. [Google Scholar] [CrossRef]
- Tripathi, U.; Sarada, R.; Ravishankar, G. Effect of culture conditions on growth of green alga—Haematococcus pluvialis and astaxanthin production. Acta Physiol. Plant. 2002, 24, 323–329. [Google Scholar] [CrossRef]
- NREL Web Page. Dynamic Maps, GIS Data, and Analysis Tools—Solar Maps. Available online: http://www.nrel.gov/gis/solar.html (accessed on 9 May 2013).
- Stockenreiter, M.; Haupt, F.; Graber, A.K.; Seppälä, J.; Spilling, K.; Tamminen, T.; Stibor, H. Functional group richness: Implications of biodiversity for light use and lipid yield in microalgae. J. Phycol. 2013, in press. [Google Scholar]
- Sorokin, C.; Krauss, R.W. The Effects of light intensity on the growth rates of green algae. Plant Physiol. 1958, 33, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky, Z.; Matsukawa, R.; Karube, I. Photobiological aspects of algal mass culture. J. Mar. Biotechnol. 1995, 2, 61–65. [Google Scholar]
- Berner, T.; Dubinsky, Z.; Wyman, K.; Falkowski, P.G. Photoadaptation and the “package” effect in Dunaliella tertiolecta (chlorophycae). J. Phycol. 1989, 25, 70–78. [Google Scholar] [CrossRef]
- Mock, T.; Kroon, B.M.A. Photosynthetic energy conversion under extreme conditions—II: The significance of lipids under light limited growth in Antarctic sea ice diatoms. Phytochemistry 2002, 61, 53–60. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Barnett, S.M. Effect of light quality on production of extracellular polysaccharides and growth rate of Porphyridium cruentum. Biochem. Eng. J. 2004, 19, 251–258. [Google Scholar] [CrossRef]
- Brody, M.; Vatter, A.E. Observations on cellular structures of Porphyridium cruentum. J. Biophys. Biochem. Cytol. 1959, 5, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Zafar, S. Effects of photon flux density, CO2, aeration rate, and inoculum density on growth and extracellular polysaccharide production by Porphyridium cruentum. Folia Microbiol. 1993, 38, 509–514. [Google Scholar] [CrossRef]
- Smith, R.; Cavaletto, J.; Eadie, B.; Gardner, W. Growth and lipid composition of high Arctic ice algae during the spring bloom at Resolute, Northwest Territories, Canada. Mar. Ecol. Prog. Ser. 1993, 97, 19–29. [Google Scholar] [CrossRef]
- Cohen, Z. Porphyridium Cruentum. In Chemicals from Microalgae; Cohen, Z., Ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 1–24. [Google Scholar]
- Renaud, S.; Parry, D.; Thinh, L.V.; Kuo, C.; Padovan, A.; Sammy, N. Effect of light intensity on the proximate biochemical and fatty acid composition of Isochrysis sp. and Nannochloropsis oculata for use in tropical aquaculture. J. Appl. Phycol. 1991, 3, 43–53. [Google Scholar] [CrossRef]
- Orcutt, D.M.; Patterson, G.W. Effect of light intensity upon lipid composition of Nitzschia closterium (Cylindrotheca fusiformis). Lipids 1974, 9, 1000–1003. [Google Scholar] [CrossRef]
- Molina Grima, E.; Garcia Camacho, F.; Acien Fernandez, F. Production of EPA from Phaeodactylum Tricornumtum. In Chemicals from Microalgae; Cohen, Z., Ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 57–92. [Google Scholar]
- Wu, X.; Merchuk, J.C. A model integrating fluid dynamics in photosynthesis and photoinhibition processes. Chem. Eng. Sci. 2001, 56, 3527–3538. [Google Scholar] [CrossRef]
- Long, S.; Humphries, S.; Falkowski, P.G. Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Biol. 1994, 45, 633–662. [Google Scholar] [CrossRef]
- Voigt, J.; Münzner, P. Blue light-induced lethality of a cell wall-deficient mutant of the unicellular green alga Chlamydomonas reinhardtii. Plant Cell Physiol. 1994, 35, 99–106. [Google Scholar]
- Borodin, V.B. Effect of red and blue light on acclimation of Chlamydomonas reinhardtii to CO2-limiting conditions. Rus. J. Plant Physiol. 2008, 55, 441–448. [Google Scholar] [CrossRef]
- Emerson, R.; Lewis, C.M. The dependence of the quantum yield of Chlorella photosynthesis on wave lenghth of light. Am. J. Bot. 1943, 30, 165–178. [Google Scholar] [CrossRef]
- Miyachi, S.; Kamiya, A. Wavelength effects on photosynthetic carbon metabolism in Chlorella. Plant Cell Physiol. 1978, 19, 277–288. [Google Scholar]
- Fernanda Pessoa, M. Harmful effects of UV radiation in algae and aquatic macrophytes—A review. Emir. J. Food Agric. 2012, 24, 510–526. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, Y.; Zhang, T.; An, L.; Wang, X. Effects of enhanced ultraviolet-B radiation on algae and cyanobacteria. Crit. Revi. Microbiol. 2005, 31, 79–89. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Incharoensadki, A. UV radiation-induced accumulation of photoprotective compounds in the green alga Tetraspora sp. CU2551. Plant Physiol. Biochem. 2013, 70, 7–13. [Google Scholar] [CrossRef]
- Goldman, J.C. Letter: Carbon dioxide and pH: Effect on species succession of algae. Science 1973, 182, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.J. Effect of high pH on the growth and survival of marine phytoplankton: Implications for species succession. Aquat. Microb. Ecol. 2002, 28, 279–288. [Google Scholar] [CrossRef]
- Goldman, J.C.; Azov, Y.; Riley, C.B.; Dennett, M.R. The effect of pH in intensive microalgal cultures. I. biomass regulation. J. Exp. Mar. Biol. Ecol. 1982, 57, 1–13. [Google Scholar] [CrossRef]
- Pruder, G.D.; Bolton, E.T. The role of CO2 enrichment of aerating gas in the growth of an estuarine diatom. Aquaculture 1979, 17, 1–15. [Google Scholar] [CrossRef]
- Azov, Y. Effect of pH on inorganic carbon uptake in algal cultures. Appl. Environ. Microbiol. 1982, 43, 1300–1306. [Google Scholar] [PubMed]
- Nielsen, E.S. Marine Photosynthesis: With Special Emphasis on the Ecological Aspects; Elsevier: Amsterdam, The Netherlands, 1975; Volume 13. [Google Scholar]
- Rotatore, C.; Colman, B. The acquisition and accumulation of inorganic carbon by the unicellular green alga Chlorella ellipsoidea. Plant Cell Environ. 1991, 14, 377–382. [Google Scholar] [CrossRef]
- Guckert, J.B.; Cooksey, K.E. Triglyceride accumulation and fatty acid profile changes in Chlorella (Chlorophyta) during high pH induced cell cycle inhibition. J. Phycol. 1990, 26, 72–79. [Google Scholar] [CrossRef]
- Gensemer, R.W.; Smith, R.E.H.; Duthie, H.C. Comparative effects of pH and aluminum on silica limited growth and nutrient uptake in Asterionella ralfsii var. Americana (Bacillariophyceae). J. Phycol. 1993, 29, 36–44. [Google Scholar] [CrossRef]
- Sunda, W. The Relationship between Cupric Ion Activity and the Toxicity of Copper to Phytoplankton. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, April 1975. [Google Scholar]
- Anderson, D.M.; Morel, F.M.M. Copper sensitivity of Gonyaulax tamarensis. Limnol. Oceanogr. 1978, 23, 283–295. [Google Scholar] [CrossRef]
- Visviki, I.; Palladino, J. Growth and cytology of Chlamydomonas acidophila under acidic stress. Bull. Environ. Contam. Toxicol. 2001, 66, 623–630. [Google Scholar] [PubMed]
- Hargreaves, J.; Whitton, B. Effect of pH on growth of acid stream algae. Eur. J. Phycol. 1976, 11, 215–223. [Google Scholar] [CrossRef]
- Coleman, J.R.; Colman, B. Inorganic carbon accumulation and photosynthesis in a blue-green alga as a function of external pH. Plant Physiol. 1981, 67, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Terry, N.; Abadía, J. Function of iron in chloroplasts. J. Plant Nutr. 1986, 9, 609–646. [Google Scholar] [CrossRef]
- Gehl, K.A.; Colman, B. Effect of external pH on the internal pH of Chlorella saccharophila. Plant Physiol. 1985, 77, 917–921. [Google Scholar] [CrossRef]
- Lane, A.E.; Burris, J.E. Effects of environmental pH on the internal pH of Chlorella pyrenoidosa, Scenedesmus quadricauda, and Euglena mutabilis. Plant Physiol. 1981, 68, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Fuggi, A.; Pinto, G.; Pollio, A.; Taddei, R. The role of glycerol in osmoregulation of the acidophilic alga Dunaliella acidophila (Volvocales, Chlorophyta): Effect of solute stress on photosynthesis, respiration and glycerol synthesis. Phycologia 1988, 27, 439–446. [Google Scholar] [CrossRef]
- Tatsuzawa, H.; Takizawa, E.; Wada, M.; Yamamoto, Y. Fatty acid and lipid composition of the acidophilic green alga Chlamydomonas sp. 1. J. Phycol. 1996, 32, 598–601. [Google Scholar] [CrossRef]
- Poerschmann, J.; Spijkerman, E.; Langer, U. Fatty acid patterns in Chlamydomonas sp. as a marker for nutritional regimes and temperature under extremely acidic conditions. Microb. Ecol. 2004, 48, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Moazami-Goudarzi, M.; Colman, B. Changes in carbon uptake mechanisms in two green marine algae by reduced seawater pH. J. Exp. Mar. Biol. Ecol. 2012, 413, 94–99. [Google Scholar] [CrossRef]
- Moroney, J.V.; Tolbert, N.E. Inorganic carbon uptake by Chlamydomonas reinhardtii. Plant Physiol. 1985, 77, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Colman, B.; Huertas, I.E.; Bhatti, S.; Dason, J.S. The diversity of inorganic carbon acquisition mechanisms in eukaryotic microalgae. Funct. Plant Biol. 2002, 29, 261–270. [Google Scholar] [CrossRef]
- Ramanan, R.; Vinayagamoorthy, N.; Sivanesan, S.D.; Kannan, K.; Chakrabarti, T. Influence of CO2 concentration on carbon concentrating mechanisms in cyanobacteria and green algae: A proteomic approach. Algae 2012, 27, 295–301. [Google Scholar] [CrossRef]
- Zhila, N.O.; Kalacheva, G.S.; Volova, T.G. Effect of salinity on the biochemical composition of the alga Botryococcus braunii Kütz IPPAS H-252. J. Appl. Phycol. 2011, 23, 47–52. [Google Scholar] [CrossRef]
- Renaud, S.; Parry, D. Microalgae for use in tropical aquaculture II: Effect of salinity on growth, gross chemical composition and fatty acid composition of three species of marine microalgae. J. Appl. Phycol. 1994, 6, 347–356. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Tornabene, T.G.; Thomas, W.H. Chemical profile of selected species of microalgae with emphasis on lipids. J. Phycol. 1985, 21, 72–81. [Google Scholar] [CrossRef]
- Fabregas, J.; Abalde, J.; Herrero, C.; Cabezas, B.; Veiga, M. Growth of the marine microalga Tetraselmis suecica in batch cultures with different salinities and nutrient concentrations. Aquaculture 1984, 42, 207–215. [Google Scholar] [CrossRef]
- Xu, X.Q.; Beardall, J. Effect of salinity on fatty acid composition of a green microalga from an antarctic hypersaline lake. Phytochemistry 1997, 45, 655–658. [Google Scholar] [CrossRef]
- Takagi, M.; Yoshida, T. Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J. Biosci. Bioeng. 2006, 101, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Dayananda, C.; Sarada, R.; Shamala, T.; Ravishankar, G. Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour. Technol. 2007, 98, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Duhalt, R.; Arredondo-Vega, B.O. Haloadaptation of the green alga Botryococcus braunii (race A). Phytochemistry 1991, 30, 2919–2925. [Google Scholar] [CrossRef]
- Titman, D. Ecological competition between algae: Experimental confirmation of resource-based competition theory. Science 1976, 192, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Berman-Frank, I.; Dubinsky, Z. Balanced growth and aquatic plants: Myth or reality? Phytoplankton use the imbalance between carbon assimilation and biomass production to their strategic advantage. Bioscience 1999, 49, 29–37. [Google Scholar] [CrossRef]
- Moss, B. The influence of environmental factors on the distribution of freshwater algae: An experimental study: II. The role of pH and the carbon dioxide-bicarbonate system. J. Ecol. 1973, 61, 157–177. [Google Scholar] [CrossRef]
- Riebesell, U.; Revill, A.T.; Holdsworth, D.G.; Volkman, J.K. The effects of varying CO2 concentration on lipid composition and carbon isotope fractionation in Emiliania huxleyi. Geochim. Cosmochim. Acta 2000, 64, 4179–4192. [Google Scholar] [CrossRef]
- Muradyan, E.; Klyachko-Gurvich, G.; Tsoglin, L.; Sergeyenko, T.; Pronina, N. Changes in lipid metabolism during adaptation of the Dunaliella salina photosynthetic apparatus to high CO2 concentration. Rus. J. Plant Physiol. 2004, 51, 53–62. [Google Scholar] [CrossRef]
- Tsuzuki, M.; Ohnuma, E.; Sato, N.; Takaku, T.; Kawaguchi, A. Effects of CO2 concentration during growth on fatty acid composition in microalgae. Plant Physiol. 1990, 93, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Gordillo, F.J.L.; Jiménez, C.; Figueroa, F.L.; Niell, F.X. Effects of increased atmospheric CO2 and N supply on photosynthesis, growth and cell composition of the cyanobacterium Spirulina platensis (Arthrospira). J. Appl. Phycol. 1998, 10, 461–469. [Google Scholar] [CrossRef]
- Fujita, R.M.; Wheeler, P.A.; Edwards, R.L. Metabolic regulation of ammonia uptake by Ulva rigida (Chlorophyta): A compartmental analysis of the rate limiting step for uptake. J. Phycol. 1988, 24, 560–566. [Google Scholar] [CrossRef]
- Vergara, J.; Bird, K.; Niell, F. Nitrogen assimilation following NH4+ pulses in the red alga Gracilariopsis lemaneiformis: Effect on C metabolism. Mar. Ecol. Prog. Ser. 1995, 122, 253–263. [Google Scholar] [CrossRef]
- Shifrin, N.S.; Chisholm, S.W. Phytoplankton lipids: Interspecific differences and effects of nitrate, silicate and light-dark cycles. J. Phycol. 1981, 17, 374–384. [Google Scholar] [CrossRef]
- Wang, Z.T.; Ullrich, N.; Joo, S.; Waffenschmidt, S.; Goodenough, U. Algal lipid bodies: Stress induction, purification, and biochemical characterization in wild-type and starchless Chlamydomonas reinhardtii. Eukaryot. Cell 2009, 8, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Use of algae as biofuel sources. Energy Convers. Manag. 2010, 51, 2738–2749. [Google Scholar] [CrossRef]
- Takagi, M.; Watanabe, K.; Yamaberi, K.; Yoshida, T. Limited feeding of potassium nitrate for intracellular lipid and triglyceride accumulation of Nannochloris sp. UTEX LB1999. Appl. Microbiol. Biotechnol. 2000, 54, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.L.; Dennis, J.S.; Howe, C.J.; Scott, S.A.; Smith, A.G. Influence of nitrogen-limitation regime on the production by Chlorella vulgaris of lipids for biodiesel feedstocks. Biofuels 2010, 1, 47–58. [Google Scholar] [CrossRef]
- Fogg, G. Photosynthesis and formation of fats in a diatom. Ann. Bot. 1956, 20, 265–285. [Google Scholar]
- Holm-Hansen, O.; Nishida, K.; Moses, V.; Calvin, M. Effects of mineral salts on short-term incorporation of carbon dioxide in Chlorella. J. Exp. Bot. 1959, 10, 109–124. [Google Scholar] [CrossRef]
- Lynn, S.G.; Kilham, S.S.; Kreeger, D.A.; Interlandi, S.J. Effect of nutrient availability on the biochemical and elemental stoichiometry in the freshwater diatom Stephanodiscus minutulus (Bacillariophyceae). J. Phycol. 2000, 36, 510–522. [Google Scholar] [CrossRef]
- Heraud, P.; Wood, B.R.; Tobin, M.J.; Beardall, J.; McNaughton, D. Mapping of nutrient-induced biochemical changes in living algal cells using synchrotron infrared microspectroscopy. FEMS Microbiol. Lett. 2005, 249, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Metting, F.B., Jr. Biodiversity and application of microalgae. J. Ind. Microbiol. 1996, 17, 477–489. [Google Scholar] [CrossRef]
- Kolber, Z.; Zehr, J.; Falkowski, P. Effects of growth irradiance and nitrogen limitation on photosynthetic energy conversion in photosystem II. Plant Physiol. 1988, 88, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.; Orcutt, D.; Schwertner, H.; Martinez, C.L.; Wickline, H.E. Effects of nitrogen limitation on the growth and composition of unicellular algae in continuous culture. Appl. Microbiol. 1969, 18, 245–250. [Google Scholar] [PubMed]
- Collier, J.L.; Grossman, A. Chlorosis induced by nutrient deprivation in Synechococcus sp. strain PCC 7942: Not all bleaching is the same. J. Bacteriol. 1992, 174, 4718–4726. [Google Scholar] [PubMed]
- Round, F.E. The Ecology of Algae; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Ben-Amotz, A.; Avron, M. The biotechnology of cultivating the halotolerant alga Dunaliella. Trends Biotechnol. 1990, 8, 121–126. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Huisman, J.M.; Osborn, A. Culture of the astaxanthin-producing green alga Haematococcus pluvialis. Effects of nutrients on growth and cell type. J. Appl. Phycol. 1991, 3, 295–304. [Google Scholar] [CrossRef]
- Harker, M.; Tsavalos, A.J.; Young, A.J. Factors responsible for astaxanthin formation in the chlorophyte Haematococcus pluvialis. Bioresour. Technol. 1996, 55, 207–214. [Google Scholar] [CrossRef]
- Zhekisheva, M.; Boussiba, S.; Khozin Goldberg, I.; Zarka, A.; Cohen, Z. Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high ligh is correlated with that of astaxanthin esters. J. Phycol. 2002, 38, 325–331. [Google Scholar] [CrossRef]
- Larned, S. Nitrogen-versus phosphorus-limited growth and sources of nutrients for coral reef macroalgae. Mar. Biol. 1998, 132, 409–421. [Google Scholar] [CrossRef]
- Borchardt, J.A.; Azad, H.S. Biological extraction of nutrients. J. Water Pollut. Control Fed. 1968, 40, 1739–1754. [Google Scholar]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Li, X.; Hu, H.Y.; Gan, K.; Sun, Y.X. Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour. Technol. 2010, 101, 5494–5500. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Hagio, M.; Wada, H.; Tsuzuki, M. Environmental effects on acidic lipids of thylakoid membranes. Biochem. Soc. Trans. 2000, 28, 912–914. [Google Scholar] [CrossRef] [PubMed]
- Reitan, K.I.; Rainuzzo, J.R.; Olsen, Y. Effect of nutrient limitation on fatty acid and lipid content of marine microalgae. J. Phycol. 1994, 30, 972–979. [Google Scholar] [CrossRef]
- Healey, F.P. Phosphate. Biol. Cyanobacteria 1982, 19, 105–124. [Google Scholar]
- Healey, F.P.; Hendzel, L.L. Indicators of phosphorus and nitrogen deficiency in five algae in culture. J. Fish. Board Can. 1979, 36, 1364–1369. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kakizono, T.; Nagai, S. Enhanced carotenoid biosynthesis by oxidative stress in acetate-induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl. Environ. Microbiol. 1993, 59, 867–873. [Google Scholar] [PubMed]
- Theodorou, M.E.; Elrifi, I.R.; Turpin, D.H.; Plaxton, W.C. Effects of phosphorus limitation on respiratory metabolism in the green alga Selenastrum minutum. Plant Physiol. 1991, 95, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Bruland, K.W.; Donat, J.R.; Hutchins, D.A. Interactive influences of bioactive trace metals on biological production in oceanic waters. Limnol. Oceanogr. 1991, 36, 1555–1577. [Google Scholar] [CrossRef]
- Parent, L.; Twiss, M.R.; Campbell, P.G.C. Influences of natural dissolved organic matter on the interaction of aluminum with the microalga Chlorella: A test of the free-ion model of trace metal toxicity. Environ. Sci. Technol. 1996, 30, 1713–1720. [Google Scholar] [CrossRef]
- McKay, R.M.L.; La Roche, J.; Yakunin, A.F.; Durnford, D.G.; Geider, R.J. Accumulation of ferredoxin and flavodoxin in a marine diatom in response to Fe. J. Phycol. 1999, 35, 510–519. [Google Scholar] [CrossRef]
- Sandmann, G.; Malkin, R. Iron-sulfur centers and activities of the photosynthetic electron transport chain in iron-deficient cultures of the blue-green alga Aphanocapsa. Plant Physiol. 1983, 73, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Geider, R.J.; Graziano, L.M.; Murray, H.; Lewis, K. Induction of specific proteins in eukaryotic algae grown under iron-, phosphorus-, or nitrogen-deficient conditions. J. Phycol. 1993, 29, 767–777. [Google Scholar] [CrossRef]
- Straus, N.A. Iron Deprivation: Physiology and Gene Regulation. In The Molecular Biology of Cyanobacteria; Bryant, D.A., Ed.; Springer: Berlin, Germany, 2004; Volume 1, pp. 731–750. [Google Scholar]
- Raven, J.A. Energetics and Transport in Aquatic Plants; Alan R. Liss: New York, NY, USA, 1984. [Google Scholar]
- Greene, R.M.; Geider, R.J.; Kolber, Z.; Falkowski, P.G. Iron-induced changes in light harvesting and photochemical energy conversion processes in eukaryotic marine algae. Plant Physiol. 1992, 100, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Wang, G.C.; Zhou, B.C. Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour. Technol. 2008, 99, 4717–4722. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwe, M.A.; Stefels, J. Effects of iron and light stress on the biochemical composition of Antarctic Phaeocystis sp. (Prymnesiophyceae). II. Pigment composition. J. Phycol. 1998, 34, 496–503. [Google Scholar] [CrossRef]
- Kennish, M.J. Ecology of Estuaries: Anthropogenic Effects; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Campanella, L.; Cubadda, F.; Sammartino, M.; Saoncella, A. An algal biosensor for the monitoring of water toxicity in estuarine environments. Water Res. 2001, 35, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Crist, R.H.; Martin, J.R.; Guptill, P.W.; Eslinger, J.M.; Crist, D.L.R. Interaction of metals and protons with algae. 2. Ion exchange in adsorption and metal displacement by protons. Environ. Sci. Technol. 1990, 24, 337–342. [Google Scholar] [CrossRef]
- Rai, L.; Mallick, N. Heavy metal toxicity to algae under synthetic microcosm. Ecotoxicology 1993, 2, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Stauber, J.; Florence, T. Mechanism of toxicity of ionic copper and copper complexes to algae. Mar. Biol. 1987, 94, 511–519. [Google Scholar] [CrossRef]
- Peterson, H.G.; Healey, F.P.; Wagemann, R. Metal toxicity to algae: A highly pH dependent phenomenon. Can. J. Fish. Aquat. Sci. 1984, 41, 974–979. [Google Scholar] [CrossRef]
- Wong, P.; Burnison, G.; Chau, Y. Cadmium toxicity to freshwater algae. Bull. Environ. Contam. Toxicol. 1979, 23, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Chau, Y. Zinc toxicity to freshwater algae. Toxic. Assess. 1990, 5, 167–177. [Google Scholar] [CrossRef]
- Sterner, R.W.; Grover, J.P. Algal growth in warm temperate reservoirs: Kinetic examination of nitrogen, temperature, light, and other nutrients. Water Res. 1998, 32, 3539–3548. [Google Scholar] [CrossRef]
- Smith, A.; Morris, I. Pathways of carbon assimilation in phytoplankton from the Antarctic Ocean. Limnol. Oceanogr. 1980, 25, 865–872. [Google Scholar] [CrossRef]
- Kudo, I.; Miyamoto, M.; Noiri, Y.; Maita, Y. Combined effects of temperature and iron on the growth and physiology of the marine diatom Phaeodactylum tricornutum (Bacillariophyceae). J. Phycol. 2000, 36, 1096–1102. [Google Scholar] [CrossRef]
- Cloern, J.E.; Grenz, C.; Vidergar-Lucas, L. An empirical model of the phytoplankton chrlorphyll: Carbon ratio—the conversion factor between productivity and growth rate. Limnol. Oceanogr. 1995, 40, 1313–1321. [Google Scholar] [CrossRef]
- Morgan, K.C.; Kalff, J. Effect of light and temperature interactions on growth of Cryptomonas erosa (Cryptophyceae). J. Phycol. 1979, 15, 127–134. [Google Scholar] [CrossRef]
- Sorokin, C.; Krauss, R.W. Effects of temperature & illuminance on chlorella growth uncoupled from cell division. Plant Physiol. 1962, 37, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.S.; Armbrust, E. Synergistic effects of light, temperature, and nitrogen source on transcription of genes for carbon and nitrogen metabolism in the centric diatom Thalassiosira pseudonana (Bacillariophyceae). J. Phycol. 2005, 41, 1142–1153. [Google Scholar] [CrossRef]
- Cunningham, F.X., Jr.; Dennenberg, R.J.; Jursinic, P.A.; Gantt, E. Growth under red light enhances photosystem II relative to photosystem I and phycobilisomes in the red alga Porphyridium cruentum. Plant Physiol. 1990, 93, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Yang, P.Y.; Duerr, E.O. Bio-process of anaerobically digested pig manure for production of Spirulina sp. Am. Soc. Agric. Eng. 1987. fiche no. 87-6056. [Google Scholar]
- Wilkie, A.C.; Mulbry, W.W. Recovery of dairy manure nutrients by benthic freshwater algae. Bioresour. Technol. 2002, 84, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.C. Algae production and harvesting from animal wastewaters. Agric. Wastes 1979, 1, 23–37. [Google Scholar] [CrossRef]
- An, J.Y.; Sim, S.J.; Lee, J.S.; Kim, B.W. Hydrocarbon production from secondarily treated piggery wastewater by the green alga Botryococcus braunii. J. Appl. Phycol. 2003, 15, 185–191. [Google Scholar] [CrossRef]
- Aslan, S.; Kapdan, I.K. Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecol. Eng. 2006, 28, 64–70. [Google Scholar] [CrossRef]
- Bich, N.N.; Yaziz, M.I.; Bakti, N.A.K. Combination of Chlorella vulgaris and Eichhornia crassipes for wastewater nitrogen removal. Water Res. 1999, 33, 2357–2362. [Google Scholar] [CrossRef]
- González, L.E.; Cañizares, R.O.; Baena, S. Efficiency of ammonia and phosphorus removal from a colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and Scenedesmus dimorphus. Bioresour. Technol. 1997, 60, 259–262. [Google Scholar] [CrossRef]
- Martınez, M.E.; Sánchez, S.; Jiménez, J.M.; El Yousfi, F.; Muñoz, L. Nitrogen and phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus. Bioresour. Technol. 2000, 73, 263–272. [Google Scholar] [CrossRef]
- Hoffmann, J.P. Wastewater treatment with suspended and nonsuspended algae. J. Phycol. 1998, 34, 757–763. [Google Scholar] [CrossRef]
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Maréchal-Drouard, L. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 2007, 318, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Blanc, G.; Duncan, G.; Agarkova, I.; Borodovsky, M.; Gurnon, J.; Kuo, A.; Lindquist, E.; Lucas, S.; Pangilinan, J.; Polle, J. The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell Online 2010, 22, 2943–2955. [Google Scholar] [CrossRef]
- Smith, D.; Lee, R.; Cushman, J.; Magnuson, J.; Tran, D.; Polle, J. The Dunaliella salina organelle genomes: Large sequences, inflated with intronic and intergenic DNA. BMC Plant Biol. 2010, 10, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.L.; Ghamsari, L.; Manichaikul, A.; Hom, E.F.; Balaji, S.; Fu, W.; Shen, Y.; Hao, T.; Palsson, B.Ø.; Salehi-Ashtiani, K.; et al. Metabolic network reconstruction of Chlamydomonas offers insight into light-driven algal metabolism. Mol. Syst. Biol. 2011, 7. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of Environmental Factors and Nutrient Availability on the Biochemical Composition of Algae for Biofuels Production: A Review. Energies 2013, 6, 4607-4638. https://doi.org/10.3390/en6094607
Juneja A, Ceballos RM, Murthy GS. Effects of Environmental Factors and Nutrient Availability on the Biochemical Composition of Algae for Biofuels Production: A Review. Energies. 2013; 6(9):4607-4638. https://doi.org/10.3390/en6094607
Chicago/Turabian StyleJuneja, Ankita, Ruben Michael Ceballos, and Ganti S. Murthy. 2013. "Effects of Environmental Factors and Nutrient Availability on the Biochemical Composition of Algae for Biofuels Production: A Review" Energies 6, no. 9: 4607-4638. https://doi.org/10.3390/en6094607




