Non-Destructive Analysis of Degradation Mechanisms in Cycle-Aged Graphite/LiCoO2 Batteries
Abstract
:1. Introduction
2. Experimental Section
| Specification | Description | Value |
|---|---|---|
| Capnom | Nominal capacity | 750 mA·h |
| Umax-ch | Maximum charge voltage | 4.2 V |
| Imax-cc | Maximum continuous current | 2C (1500 mA) |
| Ucut-dis | Discharge cut-off voltage | 2.75 V |
| Trange | Temperature range | From −20 °C to 60 °C |
| Dcell | Diameter | 14.1 ± 0.2 mm |
| Hcell | Height | 48.5 ± 0.5 mm |
| mcell | Mass | 21 g |



3. Modeling and Parameterization
3.1. Multi-Physics Model of Cylindrical Batteries

| Mechanisms | Equations | Number | Boundary conditions |
|---|---|---|---|
| Solid phase diffusion | (2) | , | |
| Electrolyte phase diffusion | (3) | , , | |
| Solid phase charge balance | (4) | , , | |
| Electrolyte phase charge balance | (5) | , , | |
| Electrochemical kinetics (Butler–Volmer equation) | (6) | - | |
| Reaction overpotential | (7) | - | |
| Exchange current density | (8) | - | |
| Terminal voltage | (9) | - |
| Coefficients | i = 1 | i = 2 | i = 3 | i = 4 | i = 5 | i = 6 |
|---|---|---|---|---|---|---|
| ai | 4.728 | 3.195 | 0.5112 | 0.0885 | 0.4989 | 0.1562 |
| bi | 0.06537 | 0.7397 | 0.9304 | 0.9124 | 0.8638 | 0.9839 |
| ci | 0.4462 | 0.4066 | 0.08774 | 0.01929 | 0.1397 | 0.02694 |
| Mechanisms | Equations | Number |
|---|---|---|
| Energy balance | (11) | |
| Electrochemical reaction heat | (12) | |
| Entropic heat | (13) | |
| Ohmic heat | (14) | |
| Heat exchange (convection) | (15) | |
| Heat exchange (radiation) | (16) | |
| Arrhenius’ law | (17) | |
| Nernst equation | (18) |

3.2. Parameter Identification

| Parameter | Unit | Lower boundary | Upper boundary | Result |
|---|---|---|---|---|
| Ce | mol·m−3 | 750 | 1100 | 868.06 |
| x0 | - | 0.50 | 0.82 | Variable |
| y0 | - | 0.36 | 0.46 | 0.4058 |
| Ds,a | m2·s−1 | 0.1 × 10−14 | 10 × 10−14 | Variable |
| Ds,c | m2·s−1 | 0.1 × 10−14 | 10 × 10−14 | Variable |
| Rs,a | μm | 5.0 | 15 | 11.41 |
| Rs,c | μm | 2.0 | 12 | 4.85 |
| εe,a | - | 0.30 | 0.45 | 0.3245 |
| εs,a | - | 0.30 | 0.60 | Variable |
| εe,c | - | 0.2 | 0.35 | 0.2418 |
| εs,c | - | 0.3 | 0.70 | Variable |
| ks,a | m2.5·mol−0.5·s−1 | 0.1 × 10−11 | 10 × 10−11 | Variable |
| ks,c | m2.5·mol−0.5·s−1 | 0.1 × 10−11 | 10 × 10−11 | Variable |
| Rfilm,a | Ω·m2 | 0 | 0.010 | Variable |
| ĒDs,a | kJ·mol−1 | 10 | 60 | 27.47 |
| ĒDs,c | kJ·mol−1 | 10 | 60 | 44.23 |
| Ēκe | kJ·mol−1 | 5 | 50 | 24.43 |
| ĒDe | kJ·mol−1 | 5 | 50 | 19.62 |
| Ēks,a | kJ·mol−1 | 10 | 60 | 45.04 |
| Ēks,c | kJ·mol−1 | 10 | 60 | 38.58 |
| κe | S·m−1 | 0.10 | 2.5 | 0.61 |
| De | m2 s−1 | 0.1 × 10−11 | 10 × 10−10 | Variable |
| Rext | Ω m2 | 0 | 0.010 | 0.0016 |
| λr | W·m−1 K−1 | 0.10 | 2 | 1.01 |
| h | W·m−2·K−1 | 20 | 30 | 25.26 |
4. Results and Discussion
4.1. Degradation of Battery Performance


4.2. Degradation of Key Parameters
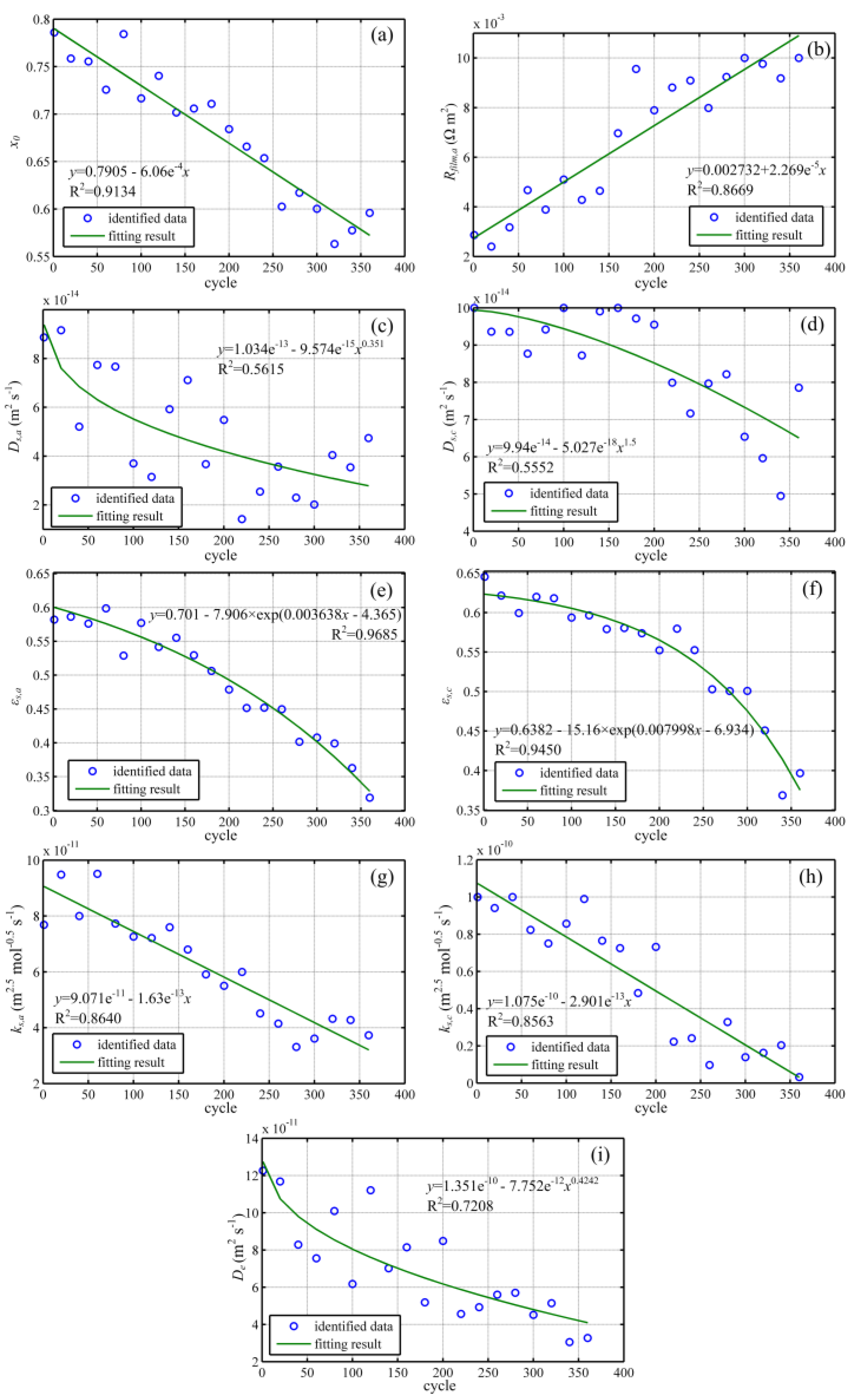
4.3. Degradation Analysis
4.3.1. Analysis of Capacity Fade
| Parameter | Description | Anode | Cathode |
|---|---|---|---|
| Acell (m2) | Area of the electrode | 0.0284 | 0.0284 |
| L (m) | Thickness of the electrode | 7.2 × 10−5 | 6.1 × 10−5 |
| M (mA·h·g−1) | Specific capacity | 372 | 274 |
| ρ (kg·m−3) | Density | 2260 | 5010 |


- (1)
- The anode is always the control electrode during the whole cycle life, because the position of yend is always on the voltage plateau and the terminal voltage of the battery at the end of discharging is always determined by the anode.
- (2)
- The terminal voltage of the battery at the beginning of discharging stays at 4.2 V. The battery is fully charged in every cycle, so there is no capacity fade caused by under-charge (UC).
- (3)
- Observing the equilibrium potential of the battery at the end of discharging, the value of a fresh one is = 3.18 V, while the value of a cycled battery after 360 cycles is = 3.71 V; considering that the discharge cutoff voltage is 2.75 V, the total overpotential increases from 430 mV to 960 mV during the cycle life.
- (4)
- After 200 cycles, when the terminal voltage reaches the discharge cutoff voltage, the anode has not been fully discharged, and the stoichiometric number is still 0.05–0.1, meaning there are some lithium ions that are underutilized. Therefore, in this stage, the under-discharge (UD) becomes another factor of capacity fade.
4.3.2. Analysis of Overpotential Increase

- (1)
- Parts ③-1 and ⑤ have no contributions to the overpotential degradation, because the related key parameters κe, εe and Rext do not vary with cycles, so that the values of these two parts are almost constants.
- (2)
- When substituting the solid phase charge balance Equation (4) into Part ③-2 of Equation (32), the following equation is obtained:where and represent the total current density of electrons in the cathode and anode, respectively, and they are determined by the applied current only. Therefore, this part is mostly related to the key parameters κe, εe, and, thus, is almost a constant.
- (3)
- Part ④ is determined by the key parameter Rfilm directly, and the electrochemical reaction current density of the anode (is,a) changes very little. Therefore, this part increases linearly with the cycle number, the same as the degradation trend of Rfilm.
- (4)
- Part ② is mostly related to the key parameters ks as in Equation (6) and (8), while other parameters change little during the cycle life. Therefore, this part also increases linearly with the cycle number.

4.4. Summary of Degradation Mechanisms
5. Conclusions
List of Symbols
| Ce | Electrolyte concentration (mol·m−3) |
| Cs | Concentration of Li+ in the intercalation particle (mol·m−3) |
| Ce/s | Concentration of Li+ at the surface of intercalation particle (mol·m−3) |
| De | Electrolyte diffusion coefficient (m2·s−1) |
| Ds | Li+ diffusion coefficient in active material (m2·s−1) |
| Ē | Activation energy (kJ·mol−1) |
| Eocv | Open circuit potential of electrode (V) |
| F | Faraday’s constant (=96,487 C·mol−1) |
| h | Heat transfer coefficient (W·m−2·K−1) |
| iapp | Applied current (A) |
| i0 | Exchange current density (A·m−2) |
| i1 | Solid phase current density (A·m−2) |
| i2 | Solution phase current density (A·m−2) |
| is | Electrochemical reaction current density (A·m−2) |
| jLi | Wall flux of Li+ on the intercalation particle of electrode (mol·m−2·s−1) |
| ks | Electrochemical reaction rate (m2.5·mol−0.5·s−1) |
| L | Thickness of the electrode or separator (m) |
Heat exchange rate (W·m−2) | |
Heat generation rate (W·m−3) | |
| R | Ideal gas constant (=8.314·J mol−1·K−1) |
| Rext | Contact resistance (Ω·m2) |
| Rfilm | Solid electrolyte interface (SEI) film resistance (Ω·m2) |
| Rs | Intercalation particle radius of electrode (m) |
Li+ transference number in the electrolyte | |
| T | Battery temperature (K) |
| Tam | Ambient temperature (K) |
| Tsh | Surface temperature of the battery (K) |
| Uapp | Applied potential (V) |
Greek Symbols
| α | Transfer coefficient of the electrochemical reactions |
| ε | Emissivity |
| εe | Volume fraction of electrolyte in electrode or separator |
| εs | Volume fraction of active material in electrode |
| η | Reaction overpotential (V) |
| κe | Ionic conductivity of the electrolyte (S m−1) |
| λr | Thermal conductivity in the radial direction (W m−1 K−1) |
| σ | Stefan-Boltzmann constant (=5.6704 × 10−8 W m−2 K−4) |
| σs | Solid phase conductivity (S m−1) |
| ϕs | Solid phase potential (V) |
| ϕe | Solution phase potential (V) |
Subscript
| a | Anode |
| c | Cathode |
| s | Separator |
| 0 | Initial value |
| ref | Reference value |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- He, W.; Williard, N.; Osterman, M.; Pecht, M. Prognostics of lithium-ion batteries based on Dempster–Shafer theory and the Bayesian Monte Carlo method. J. Power Sources 2011, 196, 10314–10321. [Google Scholar]
- Saha, B.; Goebel, K.; Poll, S.; Christophersen, J. Prognostics methods for battery health monitoring using a Bayesian framework. IEEE Trans. Instrum. Meas. 2009, 58, 291–296. [Google Scholar]
- Stiaszny, B.; Ziegler, J.C.; Krauß, E.E.; Schmidt, J.P.; Ivers-Tiffée, E. Electrochemical characterization and post-mortem analysis of aged LiMn2O4-Li(Ni0.5Mn0.3Co0.2)O2/graphite lithium ion batteries. Part I: Cycle aging. J. Power Sources 2014, 251, 439–450. [Google Scholar]
- Stiaszny, B.; Ziegler, J.C.; Krauß, E.E.; Zhang, M.; Schmidt, J.P.; Ivers-Tiffée, E. Electrochemical characterization and post-mortem analysis of aged LiMn2O4-Li(Ni0.5Mn0.3Co0.2)O2/graphite lithium ion batteries. Part II: Calendar aging. J. Power Sources 2014, 258, 61–75. [Google Scholar]
- Waldmann, T.; Wilka, M.; Kasper, M.; Fleischhammer, M.; Wohlfahrt-Mehrens, M. Temperature dependent ageing mechanisms in Lithium-ion batteries—A Post-Mortem study. J. Power Sources 2014, 262, 129–135. [Google Scholar]
- Bodenes, L.; Naturel, R.; Martinez, H.; Dedryvère, R.; Menetrier, M.; Croguennec, L.; Pérès, J.P.; Tessier, C.; Fischer, F. Lithium secondary batteries working at very high temperature: Capacity fade and understanding of aging mechanisms. J. Power Sources 2013, 236, 265–275. [Google Scholar]
- Williard, N.; He, W.; Osterman, M.; Pecht, M. Comparative analysis of features for determining state of health in lithium-ion batteries. Int. J. Progn. Health Manag. 2013, 4, 1–7. [Google Scholar]
- Dubarry, M.; Liaw, B.Y. Identify capacity fading mechanism in a commercial LiFePO4 cell. J. Power Sources 2009, 194, 541–549. [Google Scholar]
- Dubarry, M.; Truchot, C.; Liaw, B.Y. Synthesize battery degradation modes via a diagnostic and prognostic model. J. Power Sources 2012, 219, 204–216. [Google Scholar]
- Dubarry, M.; Truchot, C.; Liaw, B.Y. Cell degradation in commercial LiFePO4 cells with high-power and high-energy designs. J. Power Sources 2014, 258, 408–419. [Google Scholar]
- Ecker, M.; Gerschler, J.B.; Vogel, J.; Käbitz, S.; Hust, F.; Dechent, P.; Sauer, D.U. Development of a lifetime prediction model for lithium-ion batteries based on extended accelerated aging test data. J. Power Sources 2012, 215, 248–257. [Google Scholar]
- Käbitz, S.; Gerschler, J.B.; Ecker, M.; Yurdagel, Y.; Emmermacher, B.; André, D.; Mitsch, T.; Sauer, D.U. Cycle and calendar life study of a graphite| LiNi1/3Mn1/3Co1/3O2 Li-ion high energy system. Part A: Full cell characterization. J. Power Sources 2013, 239, 572–583. [Google Scholar]
- Ecker, M.; Nieto, N.; Käbitz, S.; Schmalstieg, J.; Blanke, H.; Warnecke, A.; Sauer, D.U. Calendar and cycle life study of Li(NiMnCo)O2-based 18650 lithiumion batteries. J. Power Sources 2014, 248, 839–851. [Google Scholar]
- Schmalstieg, J.; Käbitz, S.; Ecker, M.; Sauer, D.U. A holistic aging model for Li(NiMnCo)O2 based 18650 lithium-ion batteries. J. Power Sources 2014, 257, 325–334. [Google Scholar]
- Honkura, K.; Horiba, T. Study of the deterioration mechanism of LiCoO2/graphite cells in charge/discharge cycles using the discharge curve analysis. J. Power Sources 2014, 264, 140–146. [Google Scholar]
- He, H.; Qin, H.; Sun, X.; Shui, Y. Comparison study on the battery SoC estimation with EKF and UKF algorithms. Energies 2013, 6, 5088–5100. [Google Scholar]
- Feng, F.; Lu, R.; Zhu, C. A combined state of charge estimation method for lithium-ion batteries used in a wide ambient temperature range. Energies 2014, 7, 3004–3032. [Google Scholar]
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J. Electrochem. Soc. 1993, 140, 1526–1533. [Google Scholar]
- Doyle, M.; Newman, J.; Gozdz, A.S.; Schmutz, C.N.; Tarascon, J.M. Comparison of modeling predictions with experimental data from plastic lithium ion cells. J. Electrochem. Soc. 1996, 143, 1890–1903. [Google Scholar]
- Safari, M.; Delacourt, C. Modeling of a commercial graphite/LiFePO4 cell. J. Electrochem. Soc. 2011, 158, A562–A571. [Google Scholar]
- Ye, Y.; Shi, Y.; Tay, A.O. Electro-thermal cycle life model for lithium iron phosphate battery. J. Power Sources 2012, 217, 509–518. [Google Scholar]
- Zhang, Q.; White, R.E. Calendar life study of Li-ion pouch cells: Part 2: Simulation. J. Power Sources 2008, 179, 785–792. [Google Scholar]
- Zhang, Q.; White, R.E. Capacity fade analysis of a lithium ion cell. J. Power Sources 2008, 179, 793–798. [Google Scholar]
- Delacourt, C.; Safari, M. Life simulation of a graphite/LiFePO4 cell under cycling and storage. J. Electrochem. Soc. 2012, 159, A1283–A1291. [Google Scholar]
- Schmidt, A.P.; Bitzer, M.; Imre, Á.W.; Guzzella, L. Model-based distinction and quantification of capacity loss and rate capability fade in Li-ion batteries. J. Power Sources 2010, 195, 7634–7638. [Google Scholar]
- Ramadesigan, V.; Chen, K.; Burns, N.A.; Boovaragavan, V.; Braatz, R.D.; Subramanian, V.R. Parameter estimation and capacity fade analysis of lithium-ion batteries using reformulated models. J. Electrochem. Soc. 2011, 158, A1048–A1054. [Google Scholar]
- Fu, R.; Choe, S.Y.; Agubra, V.; Fergus, J. Modeling of degradation effects considering side reactions for a pouch type Li-ion polymer battery with carbon anode. J. Power Sources 2014, 261, 120–135. [Google Scholar]
- Zhang, L.; Lyu, C.; Hinds, G.; Wang, L.; Luo, W.; Zheng, J.; Ma, K. Paremeter sensitivity analysis of cylindrical LiFePO4 battery performance using multi-physics modeling. J. Electrochem. Soc. 2014, 161, A762–A776. [Google Scholar]
- Zhang, L.; Wang, L.; Hinds, G.; Lyu, C.; Zheng, J.; Li, J. Multi-objective optimization of lithium-ion battery model using genetic algorithm approach. J. Power Sources 2014, 270, 367–378. [Google Scholar]
- Yang, H.; Prakash, J. Determination of the reversible and irreversible heats of a LiNi0.8Co0.15Al0.05O2/natural graphite cell using electrochemical-calorimetric technique. J. Electrochem. Soc. 2004, 151, A1222–A1229. [Google Scholar]
- Ohzuku, T.; Iwakoshi, Y.; Sawai, K. Formation of lithium-graphite intercalation compounds in nonaqueous electrolytes and their application as a negative electrode for a lithium ion (shuttlecock) cell. J. Electrochem. Soc. 1993, 140, 2490–2498. [Google Scholar]
- Guo, M.; Sikha, G.; White, R.E. Single-particle model for a lithium-ion cell: Thermal behavior. J. Electrochem. Soc. 2011, 158, A122–A132. [Google Scholar]
- Drake, S.J.; Wetz, D.A.; Ostanek, J.K.; Miller, S.P.; Heinzel, J.M.; Jain, A. Measurement of anisotropic thermophysical properties of cylindrical Li-ion cells. J. Power Sources 2014, 252, 298–304. [Google Scholar]
- Newman, J. FORTRAN Programs for the Simulation of Electrochemical Systems. Available online: http://www.cchem.berkeley.edu/jsngrp/ (accessed on 10 September 2014).
- Zhang, L.; Lyu, C.; Wang, L.; Luo, W.; Ma, K. Thermal-electrochemical modeling and parameter sensitivity analysis of lithium-ion battery. Chem. Eng. Trans. 2013, 33, 943–948. [Google Scholar]
- Zhang, L.; Lyu, C.; Wang, L.; Zheng, J.; Luo, W.; Ma, K. Parallelized genetic identification of the thermal-electrochemical model for lithium-ion battery. Adv. Mech. Eng. 2013, 2013. [Google Scholar] [CrossRef]
- Wang, J.; Liu, P.; Hicks-Garner, J.; Sherman, E.; Soukiazian, S.; Verbrugge, M.; Tataria, H.; Musser, J.; Finamore, P. Cycle-life model of graphite-LiFePO4 cells. J. Power Sources 2011, 196, 3942–3948. [Google Scholar]
- Stevens, D.A.; Ying, R.Y.; Fathi, R.; Reimers, J.N.; Harlow, J.E.; Dahn, J.R. Using high precision coulometry measurements to compare the degradation mechanisms of NMC/LMO and NMC-only automotive scale pouch cells. J. Electrochem. Soc. 2014, 161, A1364–A1370. [Google Scholar]
- Fuller, T.F.; Doyle, M.; Newman, J. Relaxation phenomena in lithium-ion-insertion cells. J. Electrochem. Soc. 1994, 141, 982–990. [Google Scholar]
- Chiu, K.C.; Lin, C.H.; Yeh, S.F.; Lin, Y.H.; Huang, C.S.; Chen, K.C. Cycle life analysis of series connected lithium-ion batteries with temperature difference. J. Power Sources 2014, 263, 75–84. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, L.; Lyu, C.; Li, J.; Zheng, J. Non-Destructive Analysis of Degradation Mechanisms in Cycle-Aged Graphite/LiCoO2 Batteries. Energies 2014, 7, 6282-6305. https://doi.org/10.3390/en7106282
Zhang L, Wang L, Lyu C, Li J, Zheng J. Non-Destructive Analysis of Degradation Mechanisms in Cycle-Aged Graphite/LiCoO2 Batteries. Energies. 2014; 7(10):6282-6305. https://doi.org/10.3390/en7106282
Chicago/Turabian StyleZhang, Liqiang, Lixin Wang, Chao Lyu, Junfu Li, and Jun Zheng. 2014. "Non-Destructive Analysis of Degradation Mechanisms in Cycle-Aged Graphite/LiCoO2 Batteries" Energies 7, no. 10: 6282-6305. https://doi.org/10.3390/en7106282




