Aqueous Hybrids of Silica Nanoparticles and Hydrophobically Associating Hydrolyzed Polyacrylamide Used for EOR in High-Temperature and High-Salinity Reservoirs
Abstract
:1. Introduction
2. Results and Discussion
2.1. Rheological Behaviors of HAHPAM Solution and HAHPAM/Silica Hybrids
2.1.1. Concentration Dependence of HAPAM

2.1.2. Effect of Nanoparticle Loading on Steady Rheological Behaviors of HAHPAM/Silica Hybrids
 = 10 s−1, Cp = 0.5 wt%).
= 10 s−1, Cp = 0.5 wt%).
 = 10 s−1, Cp = 0.5 wt%).
= 10 s−1, Cp = 0.5 wt%).


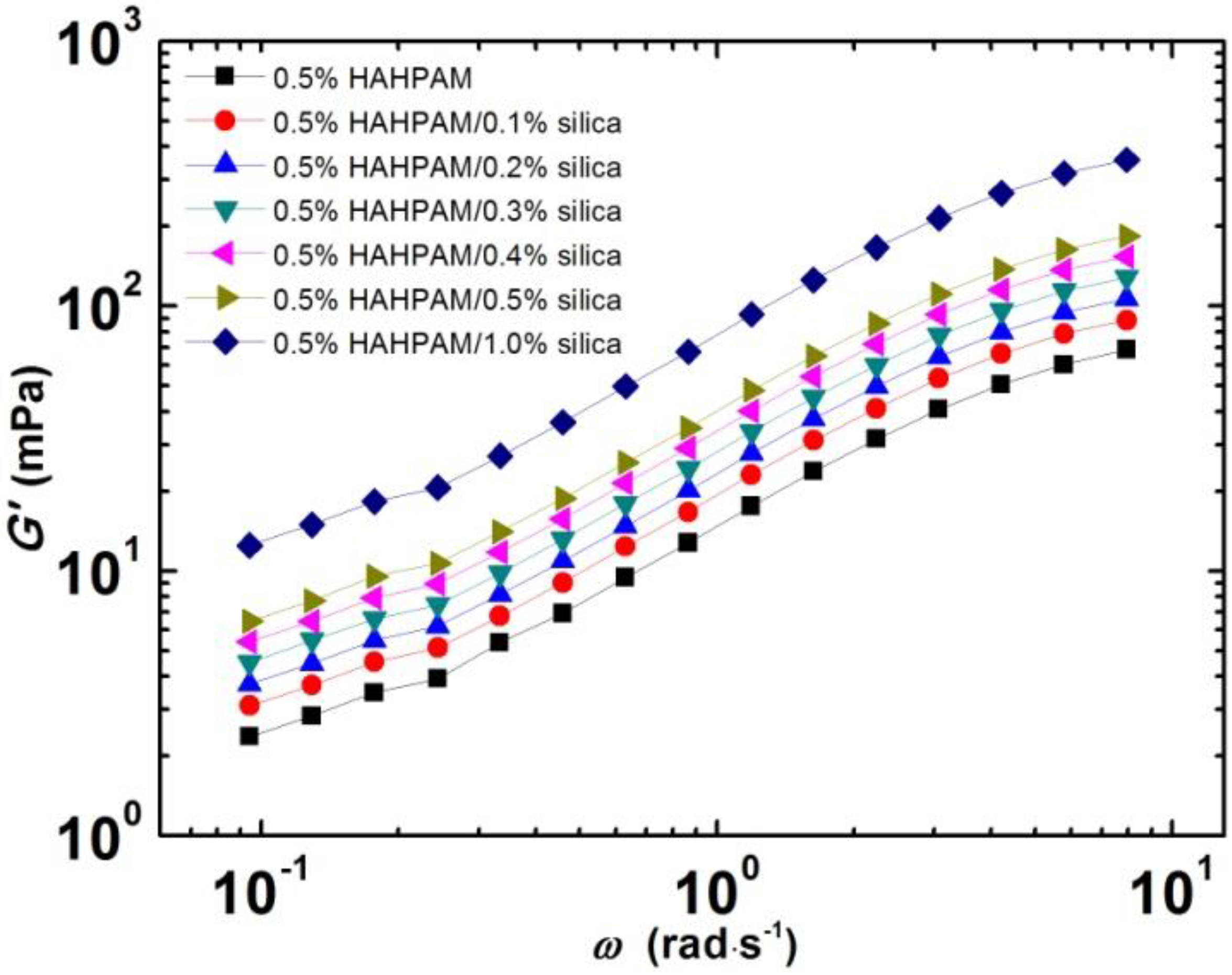
2.1.3. Effect of Nanoparticle Loading on Dynamic Rheological Behaviors of HAHPAM/Silica Hybrids
2.2. Long-Term Thermal Stability
 = 10 s−1). Both the aging and measuring temperature is 85 °C.
= 10 s−1). Both the aging and measuring temperature is 85 °C.
 = 10 s−1). Both the aging and measuring temperature is 85 °C.
= 10 s−1). Both the aging and measuring temperature is 85 °C.
2.3. Oil Displacement Test

| Core No. | Permeability (mDarcy) | Pore volume (cm3) | Saturated oil (cm3) | Slug | Slug injected (PV) | Water flooding recovery (%) | Ultimate recovery (%) | Oil recovery factor (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1497 | 50.2 | 42.0 | HAHPAM | 0.3 | 52.42 | 57.86 | 5.44 |
| 2 | 1498 | 47.3 | 38.0 | Hybrid | 0.3 | 55.48 | 66.05 | 10.57 |
3. Experimental Section
3.1. Materials

3.2. Preparation of Hybrid Samples
3.3. Infrared Spectroscopy
3.4. Rheological Measurements
3.5. Long-Term Thermal Stability Measurement
3.6. Core Flooding Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sorbie, K.S. Polymer-Improved Oil Recovery; Springer: Boca Raton, FL, USA, 1991; pp. 82–125. [Google Scholar]
- Needham, R.B.; Doe, P.H. Polymer flooding review. J. Pet. Technol. 1987, 39, 1503–1507. [Google Scholar]
- Taylor, K.C.; Nasr-El-Din, H.A. Water-soluble hydrophobically associating polymers for improved oil recovery: A literature review. J. Pet. Sci. Eng. 1998, 19, 265–280. [Google Scholar] [CrossRef]
- Chelaru, C.; Diaconu, I.; Simionescu, I. Polyacrylamide obtained by plasma-induced polymerization for a possible application in enhanced oil recovery. Polym. Bull. 1998, 40, 757–764. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Gu, Y. Application of a novel polymer system in chemical enhanced oil recovery (EOR). Colloid Polym. Sci. 2003, 281, 1046–1054. [Google Scholar] [CrossRef]
- Jung, J.C.; Zhang, K.; Chon, B.H.; Choi, H.J. Rheology and polymer flooding characteristics of partially hydrolyzed polyacrylamide for enhanced heavy oil recovery. J. Appl. Polym. Sci. 2013, 127, 4833–4839. [Google Scholar] [CrossRef]
- Mothé, C.G.; Correia, D.Z.; de Fran, F.P.; Riga, A.T. Thermal and rheological study of polysaccharides for enhanced oil recovery. J. Therm. Anal. Calorim. 2006, 85, 31–36. [Google Scholar] [CrossRef]
- Zaitoun, A.; Poitie, B. Limiting conditions for the use of hydrolysed polyacrylamides in brines containing divalent ions. In Proceedings of the SPE International Oilfield and Geothermal Chemistry Symposium, Denver, CO, USA, 1–3 June 1983.
- Leung, W.M.; Axelson, D.E. Thermal degradation of polyacrylamide and poly(acrylamide-co-acrylate). J. Polym. Sci. Part A Polym. Chem. 1987, 25, 1852–1864. [Google Scholar]
- Yang, M.H. The rheological behavior of polyacrylamide solution. J. Polym. Eng. 1999, 19, 371–381. [Google Scholar]
- Sabhapondit, A.; Borthakur, A.; Haque, I. Characterization of acrylamide polymers for enhanced oil recovery. J. Appl. Polym. Sci. 2003, 87, 1869–1878. [Google Scholar] [CrossRef]
- Kheradmand, H.; François, J.; Plazanet, V. Hydrolysis of polyacrylamide and acrylic acid-acrylamide copolymers at neutral pH and high temperature. Polymer 1988, 29, 860–870. [Google Scholar] [CrossRef]
- François, J.; Truong, N.D.; Medjahdi, G.; Mestdagh, M.M. Aqueous solutions of acrylamide-acrylic acid copolymers: stability in the presence of alkalinoearth cations. Polymer 1997, 38, 6115–6127. [Google Scholar] [CrossRef]
- Chauveteau, G.; Sorbie, K.S. Mobility control by polymers. In Basic Concepts in Enhanced Oil Recovery Process; Bavière, M., Ed.; Elsevier: London, UK, 1991; Volume 30, pp. 44–87. [Google Scholar]
- Mungan, N.; Smith, F.W.; Thompson, J.L.; Sinclair, O.; Gas, C. Some aspects of polymer floods. J. Petrol. Technol. 1996, 18, 1143–1150. [Google Scholar]
- Chang, S.H.; Chung, I.J. Effect of shear flow on polymer desorption and latex dispersion stability in the presence of adsorbed polymer. Macromolecules 1991, 24, 567–571. [Google Scholar] [CrossRef]
- Xue, L.; Agarwal, U.S.; Lemstra, P.J. Shear degradation resistance of star polymers during elongational flow. Macromolecules 2005, 38, 8825–8832. [Google Scholar] [CrossRef]
- Evani, S.; Rose, G.D. Water soluble hydrophobe association polymers. Polym. Mater. Sci. Eng. 1987, 57, 477–481. [Google Scholar]
- Lu, H.; Feng, Y.; Huang, Z. Association and effective hydrodynamic thickness of hydrophobically associating polyacrylamide through porous media. J. Appl. Polym. Sci. 2008, 110, 1837–1843. [Google Scholar] [CrossRef]
- Lu, H.; Feng, Y. Study on associative polymerizable inverse microemulsion. J. Macromol. Sci. Pure Appl. Chem. 2008, 45, 372–380. [Google Scholar] [CrossRef]
- Feng, Y.; Billon, L.; Grassl, B.; Khoukh, A.; François, J. Hydrophobically associating polyacrylamides and their partially hydrolyzed derivatives prepared by post-modification 1. Synthesis and characterization. Polymer 2002, 43, 2055–2064. [Google Scholar]
- Tokarev, I.; Tokareva, I.; Minko, S. Gold-nanoparticle-enhanced plasmonic effects in a responsive polymer gel. Adv. Mater. 2008, 20, 2730–2734. [Google Scholar] [CrossRef]
- Xiang, Y.; Chen, D. Preparation of a novel pH-responsive silver nanoparticle/poly (HEMA-PEGMA-MAA) composite hydrogel. Eur. Polym. J. 2007, 43, 4178–4187. [Google Scholar] [CrossRef]
- Xia, H.S.; Wang, Q. Preparation of conductive polyaniline/nanosilica particle composites through ultrasonic irradiation. J. Appl. Polym. Sci. 2003, 87, 1811–1817. [Google Scholar] [CrossRef]
- Bershtein, V.A.; Egorova, L.M.; Yakushev, P.N.; Pissis, P.; Sysel, P.; Brozova, L. Molecular dynamics in nanostructured polyimide–silica hybrid materials and their thermal stability. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1056–1069. [Google Scholar] [CrossRef]
- Walldal, C.; Wall, S. Coil-to-globule-type transition of poly (N-isopropylacrylamide) adsorbed on colloidal silica particles. Colloid Polym. Sci. 2002, 278, 936–945. [Google Scholar] [CrossRef]
- Portehault, D.; Petit, L.; Pantoustier, N.; Ducouret, G.; Lafuma, F.; Hourdet, D. Hybrid thickeners in aqueous media. Colloids Surf. A Physicochem. Eng. Asp. 2006, 278, 26–32. [Google Scholar] [CrossRef]
- Petit, L.; Bouteiller, L.; Brûlet, A.; Lafuma, F.; Hourdet, D. Responsive hybrid self assemblies in aqueous media. Langmuir 2007, 23, 147–158. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Zhou, J. Microscopic roles of “viscoelasticity” in HPMA polymer flooding for EOR. Transport Porous Med. 2011, 86, 199–214. [Google Scholar] [CrossRef]
- Kotlar, H.K.; Selle, O.; Torsaeter, O. Enhanced oil recovery by comb flow: Polymer floods revitalized. In Proceedings of the SPE Oilfield and Geothermal Chemistry Symposium, Houston, TX, USA, 28 February–2 March 2007.
- Moradi-Araghi, A.; Ahmed, I. Water-Soluble Polymers (Oil Recovery Applications) 1996, 8638–8655.
- Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Polymers for enhanced oil recovery: A paradigm for structure-property relationship in aqueous solution. Prog. Polym. Sci. 2011, 36, 1558–1628. [Google Scholar] [CrossRef]
- Zhang, L.J.; Yue, X.A. Mechanism for viscoelastic polymer solution percolating through porous media. J. Hydrodynamics Ser. B 2007, 19, 241–248. [Google Scholar] [CrossRef]
- Cayias, J.L.; Hayes, M.E.; Schechter, R.S.; Wade, W.H. Surfactant aging-possible detriment to the tertiary oil recovery. J. Petrol. Technol. 1976, 28, 985–988. [Google Scholar] [CrossRef]
- Portehault, D.; Petit, L.; Hourdet, D. Synthesis and self assembly processes of aqueous thermoresponsive hybrid formulations. Soft Matter 2010, 6, 2178–2186. [Google Scholar] [CrossRef]
- Wang, H.Y.; Cao, X.L.; Zhang, J.C.; Zhang, A.M. Development and application of dilute surfactant-polymer flooding system for Shengli oilfield. J. Pet. Sci. Eng. 2009, 65, 45–50. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhu, D.; Wei, L.; Wang, B.; Feng, Y. Aqueous Hybrids of Silica Nanoparticles and Hydrophobically Associating Hydrolyzed Polyacrylamide Used for EOR in High-Temperature and High-Salinity Reservoirs. Energies 2014, 7, 3858-3871. https://doi.org/10.3390/en7063858
Zhu D, Wei L, Wang B, Feng Y. Aqueous Hybrids of Silica Nanoparticles and Hydrophobically Associating Hydrolyzed Polyacrylamide Used for EOR in High-Temperature and High-Salinity Reservoirs. Energies. 2014; 7(6):3858-3871. https://doi.org/10.3390/en7063858
Chicago/Turabian StyleZhu, Dingwei, Limin Wei, Biqing Wang, and Yujun Feng. 2014. "Aqueous Hybrids of Silica Nanoparticles and Hydrophobically Associating Hydrolyzed Polyacrylamide Used for EOR in High-Temperature and High-Salinity Reservoirs" Energies 7, no. 6: 3858-3871. https://doi.org/10.3390/en7063858




