New Approach to Fuelization of Herbaceous Lignocelluloses through Simultaneous Saccharification and Fermentation Followed by Photocatalytic Reforming
Abstract
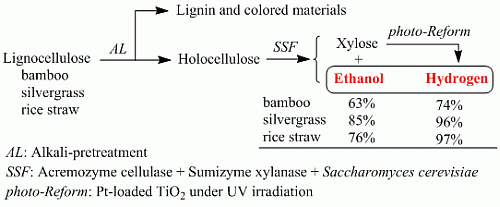
:1. Introduction



2. Results and Discussion
2.1. SSF Process
 ), rice straw (△), and silvergrass (
), rice straw (△), and silvergrass (  ); (B) Typical time-conversion plots of the total gas volume evolved from photo-Reform of xylose which was obtained from SSF of bamboo.
); (B) Typical time-conversion plots of the total gas volume evolved from photo-Reform of xylose which was obtained from SSF of bamboo.
 ), rice straw (△), and silvergrass (
), rice straw (△), and silvergrass (  ); (B) Typical time-conversion plots of the total gas volume evolved from photo-Reform of xylose which was obtained from SSF of bamboo.
); (B) Typical time-conversion plots of the total gas volume evolved from photo-Reform of xylose which was obtained from SSF of bamboo.
| Lignocellulose | Components (wt %) | SSF process (a) | ||||
|---|---|---|---|---|---|---|
| Holocellulose (b) (glucan and xylan) | Lignin | Others | Time (h) | Ethanol (g) (yield (%)) | Xylose (g) (c) (yield (%)) | |
| Bamboo | 60.4 (35.9, 23.9) | 17.1 | 22.5 | 72 | 1.29 ± 0.1 (63) | 2.02 ± 0.2 (74) |
| Rice straw | 39.0 (29.5, 9.3) | 21.3 | 39.7 | 144 | 1.42 ± 0.0 (85) | 1.01 ± 0.1 (96) |
| Silvergrass | 37.5 (28.1, 9.5) | 23.0 | 39.5 | 59 | 1.21 ± 0.1 (76) | 1.00 ± 0.4 (97) |
2.2. Photo-Reform of Xylose
2.2.1. Determination of Limiting Mole Amount of Hydrogen Evolved from Photo-Reform
| 1 (b) (H2 (g)) | Run No. | 1 (mg) (1/catalyst) | Irradn. Time (h) (c) | Volume (mL) | Molar Ratio | H2max (e) (CO2max) | H2 (g) (f) (yield (%)) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (d) | H2 | CO2 | H2/1 | CO2/1 | ||||||
| From bamboo (0.362) | 1 | 53 (0.3) | 52 | 110 | 73 | 37 | 9.35 | 4.68 | 10.0 (5.0) | 0.269 (74) |
| 2 | 75 (0.4) | 58 | 152 | 101 | 51 | 9.05 | 4.52 | |||
| 3 | 113 (0.6) | 100 | 166 | 149 | 74 | 8.87 | 4.40 | |||
| 4 | 150 (0.8) | 147 | 230 | 193 | 97 | 8.62 | 4.33 | |||
| 5 | 188 (1.0) | 124 | 270 | 216 | 108 | 7.71 | 3.86 | |||
| From rice straw (0.141) | 6 | 38 (0.2) | 69 | 84 | 56 | 28 | 10.00 | 5.00 | 10.1 (5.1) | 0.136 (96) |
| 7 | 75 (0.4) | 74 | 155 | 103 | 52 | 9.20 | 4.64 | |||
| 8 | 113 (0.6) | 87 | 230 | 153 | 77 | 9.11 | 4.58 | |||
| 9 | 150 (0.8) | 102 | 300 | 200 | 100 | 8.93 | 4.46 | |||
| 10 | 188 (1.0) | 130 | 355 | 243 | 122 | 8.68 | 4.36 | |||
| From silvergrass (0.139) | 11 | 38 (0.2) | 49 | 80 | 53 | 27 | 9.46 | 4.82 | 10.1 (5.0) | 0.134 (97) |
| 12 | 75 (0.4) | 58 | 136 | 91 | 45 | 8.13 | 4.02 | |||
| 13 | 113 (0.6) | 70 | 170 | 113 | 57 | 6.73 | 3.39 | |||
| 14 | 150 (0.8) | 106 | 185 | 123 | 62 | 5.49 | 2.77 | |||
| 15 | 188 (1.0) | 155 | 215 | 143 | 72 | 5.11 | 2.57 | |||
 ) and CO2/xylose (
) and CO2/xylose (  ) against xylose/catalyst: (A) bamboo; (B) rice straw; and (C) silvergrass.
) against xylose/catalyst: (A) bamboo; (B) rice straw; and (C) silvergrass.
 ) and CO2/xylose (
) and CO2/xylose (  ) against xylose/catalyst: (A) bamboo; (B) rice straw; and (C) silvergrass.
) against xylose/catalyst: (A) bamboo; (B) rice straw; and (C) silvergrass.
2.2.2. Reuse of Photocatalyst
 ) and irradiation time (+) on number of cycles inphoto-Reform of xylose obtained from the SSF of bamboo.
) and irradiation time (+) on number of cycles inphoto-Reform of xylose obtained from the SSF of bamboo.
 ) and irradiation time (+) on number of cycles inphoto-Reform of xylose obtained from the SSF of bamboo.
) and irradiation time (+) on number of cycles inphoto-Reform of xylose obtained from the SSF of bamboo.

2.3. Evaluation by Combustion Energy
| Lignocellulose | Biofuels | Eff (%) (d) | |||
|---|---|---|---|---|---|
| H0 (kJ) (WG, WX) (a) | H (kJ) (b) (weight (g)) | HF (kJ) (c) | |||
| C2H5OH | H2 | ||||
| Bamboo | 104.5 (3.99, 2.72) | 38.3 (1.29) | 38.3 (0.269) | 76.6 | 73.4 |
| Rice straw | 67.6 (3.28, 1.06) | 42.2 (1.42) | 19.4 (0.136) | 61.6 | 91.1 |
| Silvergrass | 64.8 (3.12, 1.04) | 36.0 (1.21) | 19.1 (0.134) | 55.1 | 85.0 |
3. Materials and Methods
3.1. Chemical Components of Lignocellulose
3.2. Hydrolytic Enzyme and Preparation of the Inoculum Culture of S. cerevisiae
3.3. Procedures of SSF

3.4. Procedures of Photo-Reform
3.5. Preparation of Photocatalyst
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ward, O.P.; Singh, A. Bioethanol technology: Development and perspectives. Adv. Appl. Microbiol. 2002, 51, 53–80. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energy 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- Yasuda, M.; Miura, A.; Yuki, R.; Nakamura, Y.; Shiragami, T.; Ishii, Y.; Yokoi, H. The effect of TiO2-photocatalytic pretreatment on the biological production of ethanol from lignocelluloses. J. Photochem. Photobiol. A 2011, 220, 195–199. [Google Scholar] [CrossRef]
- Shiragami, T.; Tomo, T.; Tsumagari, H.; Ishii, Y.; Yasuda, M. Hydrogen evolution from napiergrass by the combination of biological treatment and a Pt-loaded TiO2-photocatalytic reaction. Catalyst 2012, 2, 56–67. [Google Scholar]
- Ohta, K.; Alterthum, F.; Ingram, L.O. Effects of environmental conditions on xylose fermentation by recombinant Escherichia coli. Appl. Environ. Microbiol. 1990, 56, 463–465. [Google Scholar]
- Ohta, K.; Beall, D.S.; Mejia, J.P.; Shanmugam, K.T.; Ingram, L.O. Genetic improvement of Escherichia coli for ethanol production: Chromosomal integration of Zymomonas mobilis genes encoding pyruvate decarboxylase and alcohol dehydrogenase II. Appl. Environ. Microbiol. 1991, 57, 893–900. [Google Scholar]
- Underwood, S.A.; Buszko, M.L.; Shanmugam, K.T.; Ingram, L.O. Flux through citrate synthase limits the growth of ethanologenic Escherichia coli KO11 during xylose fermentation. Appl. Environ. Microbiol. 2002, 68, 1071–1081. [Google Scholar]
- Brandon, S.K.; Sharma, L.N.; Hawkins, G.M.; Anderson, W.F.; Chambliss, C.K.; Doran-Peterson, J. Ethanol and co-product generation from pressurized batch hot water pretreated T85 bermudagrass and Merkeron napiergrass using recombinant Escherichia coli as biocatalyst. Biomass Bioenerg. 2011, 35, 3667–3673. [Google Scholar]
- Jin, M.; Gunawan, C.; Balan, V.; Lau, M.W.; Dale, B.E. Simultaneous saccharification and co-fermentation (SSF) of AFEXTM pretreated corn stover for ethanol production using commercial enzymes and Saccharimyces cerevisiae 424A (LNH-ST). Bioresouce Technol. 2012, 110, 587–594. [Google Scholar] [CrossRef]
- Jin, M.; Gunawan, C.; Balan, V.; Yu, X.; Dale, B.E. Continuous SSF and AFEXTM pretreated corn stover for enhanced ethanol productivity using commercial enzymes and Saccharimyces cerevisiae 424A (LNH-ST). Biotechnol. Bioeng. 2012, 110, 1302–1311. [Google Scholar]
- Ohgren, K.; Bengtsson, O.; Gorwa-Grauslund, M.F.; Galbe, M.; Hahn-Hagerdal, B.; Zacchi, G. Simultaneous saccharification and co-fermentation of glucose and xylose in steam-pretreated corn stover at high fiber content with Saccharomyces cerevisiae TMB3400. J. Biotechnol. 2006, 126, 488–498. [Google Scholar] [CrossRef]
- Matsushika, A.; Inoue, H.; Kodaki, T.; Sawayama, S. Ethanol production from xylose in engineered Saccharomyces cerevisiae strains: current state and perspectives. Appl. Micorobiol. Biotechnol. 2009, 84, 37–53. [Google Scholar]
- Shiragami, T.; Tomo, T.; Tsumagari, H.; Yuki, R.; Yamashita, T.; Yasuda, M. Pentose acting as a sacrificial multi-electron source in photocatalytic hydrogen evolution from water by Pt-doped TiO2. Chem. Lett. 2012, 41, 29–30. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Rev. 2000, 1, 1–21. [Google Scholar]
- Galinska, A.; Walendziewski, J. Photocatalytic water splitting over Pt-TiO2 in the presence of sacrificial reagents. Energy Fuels 2005, 19, 1143–1147. [Google Scholar] [CrossRef]
- Shiragami, T.; Tomo, T.; Matsumoto, T.; Yasuda, M. Structural dependence of alcoholic sacrificial agents on TiO2-photocatalytic hydrogen evolution. Bull. Chem. Soc. Jpn. 2013, 86, 382–389. [Google Scholar] [CrossRef]
- Yasuda, M.; Takeo, K.; Nagai, H.; Uto, T.; Yui, T.; Matsumoto, T.; Ishii, Y.; Ohta, K. Enhancement of ethanol production from napiergrass (Pennisetum purpureum Schumach) by a low-moisture anhydrous ammonia pretreatment. J. Sustain. Bioenergy Syst. 2013, 3, 179–185. [Google Scholar] [CrossRef]
- Atkins, P.W. Physical Chemistry, 5th ed.; Oxford University Press: Oxford, UK, 1994; pp. 922–926. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templaton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report; NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2010. [Google Scholar]
- Yasuda, M.; Miura, A.; Shiragami, T.; Matsumoto, J.; Kamei, I.; Ishii, Y.; Ohta, K. Ethanol productionfrom non-pretreated napiergrass through a simultaneous saccharification and fermentation process followed by a pentose fermentation with Escherichia coli KO11. J. Biosci. Bioeng. 2012, 114, 188–192. [Google Scholar] [CrossRef]
- Kennedy, J.C., III; Datye, A.K. Photochemical heterogeneous oxidation of ethanol over Pt/TiO2. J. Catal. 1998, 179, 375–389. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yasuda, M.; Kurogi, R.; Tsumagari, H.; Shiragami, T.; Matsumoto, T. New Approach to Fuelization of Herbaceous Lignocelluloses through Simultaneous Saccharification and Fermentation Followed by Photocatalytic Reforming. Energies 2014, 7, 4087-4097. https://doi.org/10.3390/en7074087
Yasuda M, Kurogi R, Tsumagari H, Shiragami T, Matsumoto T. New Approach to Fuelization of Herbaceous Lignocelluloses through Simultaneous Saccharification and Fermentation Followed by Photocatalytic Reforming. Energies. 2014; 7(7):4087-4097. https://doi.org/10.3390/en7074087
Chicago/Turabian StyleYasuda, Masahide, Ryo Kurogi, Hikaru Tsumagari, Tsutomu Shiragami, and Tomoko Matsumoto. 2014. "New Approach to Fuelization of Herbaceous Lignocelluloses through Simultaneous Saccharification and Fermentation Followed by Photocatalytic Reforming" Energies 7, no. 7: 4087-4097. https://doi.org/10.3390/en7074087