Understanding of Sulfurized Polyacrylonitrile for Superior Performance Lithium/Sulfur Battery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Structure of Sulfurized Polyacrylonitrile (SPAN)


| Synthesis Condition | Result, % | Ratio of Element | Source | |||||
|---|---|---|---|---|---|---|---|---|
| S | N | C | H | C/N | C/S | C/H | ||
| 280–300 °C, 6 h | 53.41 | 10.73 | 30.92 | 0.89 | 3.36 | 1.55 | 2.92 | [2] |
| 330 °C, 6 h | 41 ± 1 | 18.2 | 53 | 1.7 | 3.4 | – | 2.62 | [9] |
| 280–300 °C, time 1 | 46.3 | 14.4 | 38.1 | 1.1 | 3.09 | 2.20 | 2.91 | [7] |
| time 2 (>time 1) | 42 | 15.6 | 41.2 | 1.2 | 3.08 | 2.62 | 2.89 | – |
| time 3 (>time 2) | 33.7 | 16.5 | 43.6 | 1.3 | 3.08 | 3.45 | 2.82 | – |
| 300 °C, 8 h | 43.28 | 13.99 | 38.12 | – | 3.17 | 2.34 | – | [16] |
| 450 °C | 35.24 | 15.17 | 40.80 | – | 3.13 | 3.08 | – | – |
| 800 °C | 15.32 | 19.05 | 52.28 | – | 3.20 | 8.98 | – | – |

2.2. Electrochemical Characteristics of Li/SPAN Cell
2.2.1. Electrochemical Mechanism

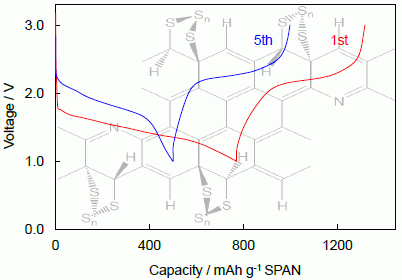
2.2.2. Cycling Characteristics of Li/SPAN Cell

| Synthesis Condition | S in SPAN wt% | CE % | Capacity at 2nd Cycle, mAh g−1 | Source | |
|---|---|---|---|---|---|
| vs. SPAN | vs. S | ||||
| 350 °C, 5 h | 42 | 81 | 726 | 1727 | [7] |
| 42 | 86 | 811 | 1930 | [8] | |
| 300 °C, 3 h, 4:1 S/PAN | 48 | 75 | 477 | 993 | [13] |
| 48 | 79 | 681 | 1419 | – | |
2.2.3. Chemical Compatibility of Electrolyte
2.2.4. Over-Discharging and Over-Charging Characteristics

3. Experimental Section
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zhang, S.S. Sulfurized carbon: A class of cathode materials for high performance lithium/sulfur batteries. Front. Energy Res. 2013, 1. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Xie, J.; Xu, N. A novel conductive polymer–sulfur composite cathode material for rechargeable lithium batteries. Adv. Mater. 2002, 14, 963–965. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Wan, C.; Du, K.; Xie, J.; Xu, N. Sulfur composite cathode materials for rechargeable lithium batteries. Adv. Funct. Mater. 2003, 13, 487–492. [Google Scholar] [CrossRef]
- He, X.; Pu, W.; Ren, J.; Wang, L.; Wang, J.; Jiang, C.; Wan, C. Charge/discharge characteristics of sulfur composite cathode materials in rechargeable lithium batteries. Electrochim. Acta 2007, 52, 7372–7376. [Google Scholar] [CrossRef]
- Pu, W.; He, X.; Wang, L.; Tian, Z.; Jiang, C.; Wan, C. Sulfur composite cathode materials: Comparative characterization of polyacrylonitrile precursor. Ionics 2007, 13, 273–276. [Google Scholar] [CrossRef]
- He, X.; Ren, J.; Wang, L.; Pu, W.; Wan, C.; Jiang, C. Electrochemical characteristics of sulfur composite cathode for reversible lithium storage. Ionics 2009, 15, 477–481. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Li, J.; Chen, M.; Gao, J.; Jiang, C. Charge/discharge characteristics of sulfurized polyacrylonitrile composite with different sulfur content in carbonate based electrolyte for lithium batteries. Electrochim. Acta 2012, 72, 114–119. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Li, J.; Gao, J.; Guo, J.; Jiang, C.; Wan, C. Analysis of the synthesis process of sulphur–poly(acrylonitrile)-based cathode materials for lithium batteries. J. Mater. Chem. 2012, 22, 22077–22081. [Google Scholar] [CrossRef]
- Fanous, J.; Wegner, M.; Grimminger, J.; Andresen, A.; Buchmeiser, M.R. Structure-related electrochemistry of sulfur–poly(acrylonitrile) composite cathode materials for rechargeable lithium batteries. Chem. Mater. 2011, 23, 5024–5028. [Google Scholar] [CrossRef]
- Fanous, J.; Wegner, M.; Grimminger, J.; Rolff, M.; Spera, M.B.M.; Tenzer, M.; Buchmeiser, M.R. Correlation of the electrochemistry of poly(acrylonitrile)–sulfur composite cathodes with their molecular structure. J. Mater. Chem. 2012, 22, 23240–23245. [Google Scholar]
- Fanous, J.; Wegner, M.; Spera, M.B.M.; Buchmeiser, M.R. High energy density poly(acrylonitrile)–sulfur composite-based lithium–sulfur batteries. J. Electrochem. Soc. 2013, 160, A1169–A1170. [Google Scholar] [CrossRef]
- Doan, T.N.L.; Ghaznavi, M.; Zhao, Y.; Zhang, Y.; Konarov, A.; Sadhu, M.; Tangirala, R.; Chen, P. Binding mechanism of sulfur and dehydrogenated polyacrylonitrile in sulfur/polymer composite cathode. J. Power Sources 2013, 241, 61–69. [Google Scholar] [CrossRef]
- Konarov, A.; Gosselink, D.; Doan, T.N.L.; Zhang, Y.; Zhao, Y.; Chen, P. Simple, scalable, and economical preparation of sulfure–PAN composite cathodes for Li/S batteries. J. Power Sources 2014, 259, 183–187. [Google Scholar] [CrossRef]
- Jeddi, K.; Ghaznavi, M.; Chen, P. A novel polymer electrolyte to improve the cycle life of high performance lithium–sulfur batteries. J. Mater. Chem. A 2013, 1, 2769–2772. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Bakenov, Z.; Babaa, M.R.; Konarov, A.; Ding, C.; Chen, P. Effect of graphene on sulfur/polyacrylonitrile nanocomposite cathode in high performance lithium/sulfur batteries. J. Electrochem. Soc. 2013, 160, A1194–A1198. [Google Scholar] [CrossRef]
- Yu, X.; Xie, J.; Yang, J.; Huang, H.; Wang, K.; Wen, Z. Lithium storage in conductive sulfur-containing polymers. J. Electroanal. Chem. 2004, 573, 121–128. [Google Scholar]
- Yu, X.; Xie, J.; Li, Y.; Huang, H.; Lai, C.; Wang, K. Stable-cycle and high-capacity conductive sulfur-containing cathode materials for rechargeable lithium batteries. J. Power Sources 2005, 146, 335–339. [Google Scholar] [CrossRef]
- Trevey, J.E.; Gilsdorf, J.R.; Stoldt, C.R.; Lee, S.H.; Liu, P. Electrochemical investigation of all-solid-state lithium batteries with a high capacity sulfur-based electrode. J. Electrochem. Soc. 2012, 159, A1019–A1022. [Google Scholar] [CrossRef]
- Wen, Z.; Lu, D. Fabrication and electrochemical performance of polyacrylonitrile-S/carbon composite as cathode for lithium ion batteries. J. Electrochem. Soc. 2013, 160, A2311–A2314. [Google Scholar] [CrossRef]
- Unemoto, A.; Gambe, Y.; Komatsu, D.; Honma, I. Development of high capacity all-solid-state lithium battery using quasi-solid-state electrolyte containing tetraglyme–Li-TFSA equimolar complexes. Solid State Ionics 2014, 262, 765–768. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Bond Dissociation Energies in Simple Molecules. Available online: http://www.nist.gov/data/nsrds/NSRDS-NBS31.pdf (assessed on 17 July 2014).
- Zhang, S.S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Sources 2013, 231, 153–162. [Google Scholar] [CrossRef]
- Ponrouch, A.; Taberna, P.; Simon, P.; Palacin, M.R. On the origin of the extra capacity at low potential in materials for Li batteries reacting through conversion reaction. Electrochim. Acta 2012, 61, 13–18. [Google Scholar] [CrossRef]
- Zhang, X.; Kostecki, R.; Richardson, T.J.; Pugh, J.K.; Ross, P.N. Electrochemical and infrared studies of the reduction of organic carbonates. J. Electrochem. Soc. 2001, 148, A1341–A1345. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, Z.; Gao, J.; Li, J.; Xu, J.; Wang, Z.; Yang, Y. Vinyl ethylene sulfite as a new additive in propylene carbonate-based electrolyte for lithium ion batteries. Energy Environ. Sci. 2009, 2, 1102–1108. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, Z.; Fu, W.; Dudney, N.J.; Liang, C. Lithium polysulfidophosphates: A family of lithium-conducting sulfur-rich compounds for lithium–sulfur batteries. Angew. Chem. Int. Ed. 2013, 52, 7460–7463. [Google Scholar] [CrossRef]
- Nagao, M.; Hayashi, A.; Tatsumisago, M. Electrochemical performance of all-solid-state Li/S batteries with sulfur-based composite electrodes prepared by mechanical milling at high temperature. Energy Technol. 2013, 1, 186–192. [Google Scholar] [CrossRef]
- Zhang, S.S.; Tran, D. How a gel polymer electrolyte affects performance oflithium/sulfur batteries. Electrochim. Acta 2013, 114, 296–302. [Google Scholar] [CrossRef]
- Gao, J.; Lowe, M.A.; Kiya, Y.; Abruna, H.D. Effects of liquid electrolytes on the charge–discharge performance of rechargeable lithium/sulfur batteries: Electrochemical and in-situ X-ray absorption spectroscopic studies. J. Phys. Chem. C 2011, 115, 25132–25137. [Google Scholar] [CrossRef]
- Yim, T.; Park, M.S.; Yu, J.S.; Kim, K.J.; Im, K.Y.; Kim, J.H.; Jeong, G.; Jo, Y.N.; Woo, S.; Kang, K.S.; et al. Effect of chemical reactivity of polysulfide toward carbonate-based electrolyte on the electrochemical performance of Li–S batteries. Electrochim. Acta 2013, 107, 454–460. [Google Scholar] [CrossRef]
- Shacklette, L.W.; Elsenbaumer, R.L.; Baughman, R.H. Electrochemical cells employing polyacetylene and poly(p-phenylene) as active materials. J. Phys. Colloq. 1983, 44. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, S.S. Understanding of Sulfurized Polyacrylonitrile for Superior Performance Lithium/Sulfur Battery. Energies 2014, 7, 4588-4600. https://doi.org/10.3390/en7074588
Zhang SS. Understanding of Sulfurized Polyacrylonitrile for Superior Performance Lithium/Sulfur Battery. Energies. 2014; 7(7):4588-4600. https://doi.org/10.3390/en7074588
Chicago/Turabian StyleZhang, Sheng S. 2014. "Understanding of Sulfurized Polyacrylonitrile for Superior Performance Lithium/Sulfur Battery" Energies 7, no. 7: 4588-4600. https://doi.org/10.3390/en7074588